Abstract
Humans are frequently exposed to various airborne allergens in the atmospheric environment. These allergens may trigger a complex network of immune responses in the airways, resulting in asthma and other chronic airway diseases. Here, we investigated the immunological mechanisms involved in the pathological changes induced by chronic exposure to multiple airborne allergens. Naïve mice were exposed intranasally to a combination of common airborne allergens, including the house dust mite, Alternaria, and Aspergillus, for up to 8 weeks. These allergens acted synergistically and induced robust eosinophilic airway inflammation, specific IgE antibody production, type 2 cytokine response and airway hyperreactivity (AHR) in 4 weeks, followed by airway remodeling in 8 weeks. Increased lung infiltration of T cells, B cells, and type 2 innate lymphoid cells (ILC2s) was observed. CD4+ T cells and ILC2s contributed to the sources of IL-5 and IL-13, suggesting involvement of both innate and adaptive immunity in this model. The lung levels of IL-33 increased quickly within several hours after allergen exposure and continued to rise throughout the chronic phase of inflammation. Mice deficient in IL-33 receptor (Il1rl1−/−) and TSLP receptor (Tslpr−/−) showed significant reduction in airway inflammation, IgE antibody levels and AHR. In contrast, mice deficient in IL-25 receptor or IL-1 receptor showed minimal differences as compared to wild-type animals. Thus, chronic exposure to natural airborne allergens triggers a network of innate and adaptive type 2 immune responses and airway pathology, and IL-33 and TSLP likely play key roles in this process.
INTRODUCTION
Exposure and sensitization to allergens are risk factors for developing asthma and allergic airway diseases (1). Allergens play a key role in triggering and exacerbating asthma and allergy symptoms (2). In particular, exposure to airborne allergens derived from animals, arthropods, and molds are considered an important risk factor for asthma (3-5). Simultaneous exposure to several allergens is also common (6, 7). Indeed, exposure to multiple allergens and increased levels of total allergens in the home are significantly associated with developing asthma (8). In addition, certain allergens, such as Alternaria, the house dust mite (HDM), mouse, and cockroach, are detected together at high levels in home environment (8). Most patients with allergic asthma are sensitized to multiple allergens (9). Therefore, the conventional asthma models in mice that rely on OVA sensitization and challenge, or exposure to a single allergen, may not capture the impact and complexity of chronic exposure to multiple airborne allergens in humans.
Recent studies suggest that the biological properties of allergen molecules likely play important roles in developing type 2 immunity to inhaled allergens. For example, the response to HDM is mediated by recognition of the major HDM allergen Der p 2 and perhaps endotoxin contained in fecal pellets through TLR4 (10, 11). Alternatively, many Th2-inducing allergens have enzymatic activities, such as proteases from HDM and fungi (12-14) and phospholipases from bee venom (15), which may trigger protease-activated receptors or other as yet unidentified receptor(s). Allergen proteases may also increase the passage of allergens across the epithelial barrier (12, 13). Thus, several immune receptors and pathways are likely triggered by exposure to natural allergens. It would be critical to identify key fundamental factor(s) that play central roles in these complex immune responses to allergens.
Using a mouse model, we sought to investigate the mechanisms involved in pathological processes induced by chronic exposure to airborne allergens. To accomplish our goal of mimicking natural allergen exposure in humans, we simultaneously exposed animals for a prolonged period to several allergens that are relevant to human asthma. Previously, combined sensitization of mice to HDM, ragweed, and Aspergillus broke tolerance and established the chronic features of asthma (16). In this study, we used HDM and Alternaria allergens because they are often found together in U.S. homes and increase the risk for asthma (8). We also included Aspergillus because it is implicated in severe and treatment-resistant asthma in humans (5, 14). Chronic intranasal exposure of naïve animals to a combination of these allergens triggered robust type 2 immune responses, which involve both innate and adaptive immunity; the allergens worked synergistically to induce a strong response. Importantly, among various immunological factors, interleukin (IL)-33 appeared to be particularly important in mediating the process because it is significantly increased during both the early and chronic phases of exposure and because blockade of the pathway attenuated airway pathology. Thymic stromal lymphopoietin (TSLP) also contributed significantly, whereas IL-25 and IL-1 appeared to play minimal roles.
MATERIALS AND METHODS
Reagents
Extracts of natural allergens, including Alternaria alternata, Aspergillus fumigatus, and HDM (D. pteronyssinus), were purchased from Greer Laboratories (Lenoir, NC). These extracts contained undetectable levels of endotoxin (<10 ng/mg extract, <0.50 ng/dose). Endotoxin-free OVA was prepared in our laboratory as previously described (17).
Mice
Balb/c, C57BL/6, Rag1−/− and Il1r1−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). ST2−/− (Il1rl1−/−) (18), Il17rb−/− (19), Tslpr−/− (20), and Il13+/eGFP mice (19) were generated on the Balb/c background and have been bred in the animal facility at the Mayo Clinic (Rochester, MN). Il5+/venus mice (21) were a gift from Dr. Kiyoshi Takatsu (University of Toyama, Toyama, Japan). Six- to 12-week-old female mice were used in this study. All the experiments in this study were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Chronic allergen exposure model
Mice were exposed intranasally (i.n.) to a mixture of allergen extracts [10 μg each (dry weight) of OVA, Alternaria, Aspergillus, and HDM in 50 μl PBS], 3 times a week, for up to 8 weeks under isoflurane inhalation anesthesia. The levels of protein in the 10 μg doses of Alternaria, Aspergillus and HDM extracts were 2.0, 3.5 and 3.2 μg, respectively. Each allergen extract plus OVA or a mixture of allergen extracts without OVA were also used for certain experiments. Alternatively, each allergen alone (10 μg/dose or 30 μg/dose in 50 μl PBS) was used for some experiments. Twenty-four hours after the final exposure, airway hyperresponsiveness (AHR) to inhaled methacholine was analyzed by whole body plethysmography as previously described (Buxco Electronics Ltd., Sharon, CT) (22). Mice were nebulized with increasing doses of methacholine (1.6–50 mg/ml), and AHR was represented as a percentage of baseline enhanced pause (Penh). AHR in anesthetized mice was also analyzed in certain experiments by FlexiVent® forced oscillation technique (Scireq, Montreal, Canada) (23). Thereafter, 100 μl of blood was collected by retroorbital bleeding under isoflurane anesthesia. Mice were then killed, and bronchoalveolar lavage (BAL) was performed by intratracheal instillation of 1.0 ml of HBSS. In some experiments, the lungs were also collected, homogenized in 0.5 ml PBS, and centrifuged at 10,000 ×g at 4°C for 15 min; the protein concentrations in the supernatant were quantified with the Pierce™ BCA Protein Assay kit (Thermo Fisher, Rockford, IL). Total leukocyte counts in BAL fluids were determined using a hemocytometer after staining with Randolph’s stain. For cell differentiation, cytospin preparations from BAL fluids were stained with Wright-Giemsa. Cytokine levels in the supernatants of lung homogenates were measured using ELISA. We also collected the spleen for analyses of cytokine production in vitro.
Acute allergen exposure model
Naïve mice were exposed once i.n. to a mixture of allergen extracts (10 μg each of OVA, Alternaria, Aspergillus, and HDM in 50 μl PBS). One, 3, 6, 12, or 24 hours after the exposure, mice were killed, and BAL samples and the lungs were collected. Cytokine levels in the supernatants of BAL fluids and lung homogenates were measured using ELISA.
ELISA
The levels of IL-1α, IL-1β, IL-4, IL-5, IL-13, IL-17E (IL-25), IL-33, IFN-γ, and TSLP in the supernatants of lung homogenates and BAL fluids were measured using ELISA kits (R&D systems, Minneapolis, MN) following the procedure recommended by the manufacturer. The levels of OVA-specific IgE or IgG1 in the plasma were measured using sandwich ELISA as previously described (17). To measure the levels of allergen-specific IgG1 antibodies in the plasma, ELISA plates were coated with recombinant Alt a 1, natural Asp r 1 (Asp f 1 homologue) or recombinant Der p 1 (all from Indoor Biotechnologies, Charlottesville, VA) overnight at 4 °C (15 μg/ml in 0.1 M carbonate buffer, pH 9.5). The plates were blocked with PBS containing 1% BSA for 2 hours. Plasma samples, which were diluted 1:20 in PBS containing 1% BSA and 0.05% Tween 20, were added to the plates and incubated for 4 hours. Thereafter, the plates were incubated with HRP-conjugated rat anti-mouse IgG1 (BD Bioscience, San Jose, CA) for 1 hour. After washing, TMB substrate (Thermo Scientific, Rockford, IL) was added, and the reaction was stopped with 2 M H2SO4. The absorbance at 450 nm was read in a microplate autoreader (Thermomax; Molecular Devices).
Splenocyte cytokine production in vitro
Splenocytes were suspended in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured at 4×105 cells/well in 96-well round bottom tissue culture plates in the presence or absence of 100 μg/ml OVA antigen. Five days later, the concentration of IL-13 in the cell-free supernatants was measured using ELISA.
Lung immunohistochemistry
To identify lymphocytes in mouse lungs, lung tissue was embedded in optimal cutting temperature (OCT) compound, frozen, and sectioned. Sections were fixed in ice-cold acetone and incubated with a dual endogenous enzyme block (Dako, Carpinteria, CA) and a blocking reagent (Background Sniper for CD3 and B220, Rodent Block M for CD4 and CD8; Biocare Medical, Concord, CA). The sections were then stained with rat anti-mouse CD3 (145-2C11, BioLegend, San Diego, CA), CD4 (GK1.5, eBioscience, Inc., San Diego, CA), CD8a (YTS 168AG, Abcam, Cambridge, MA), B220 (RA3-6B2, eBioscience), or the appropriate isotype-matched rat IgG control (eBioscience). Staining was visualized using a rat-on-mouse HRP-polymer kit and a DAB detection system (Biocare Medical). Sections were counterstained with hematoxylin QS (Vector Laboratories, Inc., Burlingame, CA).
Flow cytometry
Lungs were minced using a gentleMACS Dissociator (Miltenyi Biotec, Auburn, CA), and digested with LiberaseTM Research Grade (Roche, Mannheim, Germany) in RPMI 1640 medium in the presence of DNase I solution (STEMCELL Technologies, Vancouver, Canada) for 1 hour at 37 °C. After digestion, single lung cells were hemolyzed with ammonium-chloride-potassium (ACK) buffer, and washed with PBS containing 0.1% sodium azide and 1% bovine serum albumin (PAB). To quantitate the numbers of lymphocytes, single lung cells were stained with FITC-CD3e (145-2C11), phycoerythrin (PE)-B220 (RA3-6B2), peridinin chlorophyll protein complex (PerCP)-CD4 (RM4-5), and allophycocyanin (APC)-CD8 (53-6.7, BD Bioscience, San Jose, CA) for 30 min at 4°C. After washing in PAB buffer, the expression levels of CD3, CD4, CD8, and B220 were detected by FACS (BD FACSCalibur, BD Bioscience). To examine the expression levels of cytokines by type 2 innate lymphoid cells (ILC2s) or CD4+ T cells, single lung cells from Il13+/eGFP mice or Il5+/venus mice were stained with a PE-conjugated lineage cocktail [CD3 (145-2C11), CD14 (rmC5-3), CD16/32 (2.4G2), B220 (RA3-6B2)], APC-CD25 (PC61), PerCP-Cy5.5-CD44 (IM7, BD Biosciences) or PE-CD4, and PerCP-CD3. Lung ILC2s and CD4+ T cells were identified as Lin−CD25+CD44hi cells and CD3+CD4+ cells, respectively, as previously described (24).
Quantitation of airway remodeling
For histological analyses, lung tissues were fixed in 10% formalin and embedded in paraffin. Six-millimeter sections were stained with periodic acid-Schiff (PAS) or Masson’s Trichrome. Computer analysis was performed to evaluate objectively the area stained with PAS or Masson’s Trichrome. Briefly, using image-analysis software (KS400 Image Analysis System, Carl Zeiss Microscopy, Thornwood, NY), the threshold for the PAS- or Masson’s Trichrome-negative areas was calibrated to a baseline value that did not have any positive pixels. This background threshold was used to analyze the stained area. The threshold for the total tissue was also calibrated to detect the lumen. The airway basement membrane (B.M.) of the picture was traced, and the length of B.M. was calculated. The pixels of the PAS- or Masson Trichrome-positive area in the epithelium were recorded in randomly selected airway sections (>10) per tissue section and normalized as unit per μm B.M.
Statistical Analysis
Data are expressed as the mean±SEM for the mice indicated. The statistical significance of the differences between the various treatment groups was assessed with Student’s t test by using PRISM software and InStat software (both from GraphPad Software, La Jolla, CA); p<0.05 was considered significant.
RESULTS
Chronic exposure to natural airborne allergens induces robust type 2 immune responses followed by airway remodeling
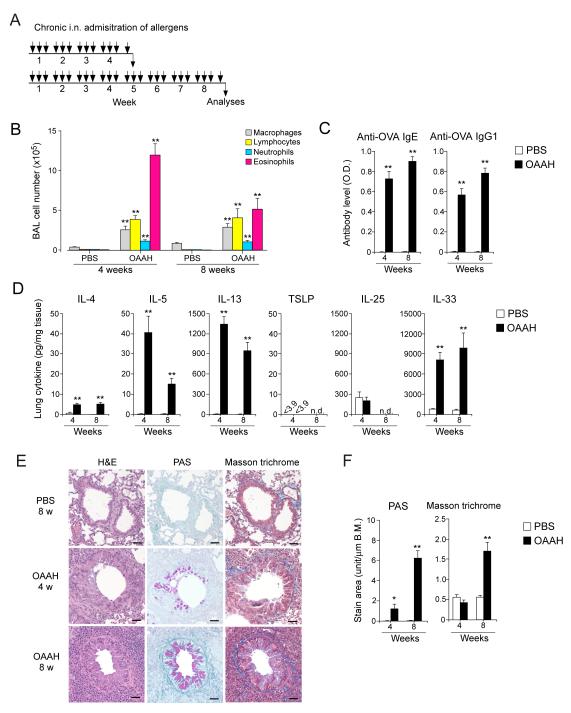
To investigate the immunological mechanisms involved in airway pathology induced by chronic exposure to multiple airborne allergens, we intranasally administered a combination of allergen extracts three times a week for up to 8 weeks to naïve Balb/c mice (Figure 1A); BAL and lung specimens were analyzed 24 hours after the last exposure. No systemic immunization, such as intraperitoneal or subcutaneous injection of allergens, was used throughout the procedure. For allergens, we used HDM, Alternaria, and Aspergillus. HDM and Alternaria are commonly found in homes and exposure to them increases the risk of developing asthma (3, 5, 8). Aspergillus is implicated in severe treatment-resistant asthma (5, 14). While the cockroach is also implicated in human asthma (4), we did not include it in this study because cockroach extracts are highly contaminated with endotoxin (i.e. 7,000 EU/mg). A cocktail of HDM, Alternaria, and Aspergillus extracts (10 μg/dose each) was spiked with a small amount of endotoxin-free OVA (10 μg/dose), which allows us to monitor the development of antigen-specific adaptive immunity; thus, the allergen mix was named OAAH (i.e. short for OVA, Alternaria, Aspergillus, and HDM).
Figure 1.
Chronic airway exposure of naïve mice to natural airborne allergens induces type 2 immune responses, eosinophilic inflammation, and airway remodeling. (A) Schematic representation of the exposure protocol. Naïve Balb/c mice were exposed i.n.. 3 times/week to a combination of ovalbumin (OVA) and extracts of Alternaria, Aspergillus, and house dust mite (HDM) (named OAAH) for up to 8 weeks. (B) BAL fluids were analyzed for the number of inflammatory cells. (C) Plasma specimens were analyzed for the levels of anti-OVA antibodies. (D) Lung homogenates were analyzed for cytokine levels. (Panels B to D) Results are the mean ± SEM (n=5-6 in each group). *: p<0.05, **: p<0.01, compared to mice exposed to PBS. n.d.: not determined. (E) Representative photomicrographs of lung specimens. Scale bar: 100 μm. (F) The magnitude of airway remodeling was analyzed by computer-assisted image analysis. Results are the mean ± SEM (n=10 in each group). *: p<0.05, **: p<0.01, compared to mice exposed to PBS.
Chronic and multiple exposures to OAAH induced a robust increase in BAL eosinophils in 4 weeks; eosinophils made up approximately 70% of total BAL cells (Figure 1B). Plasma concentrations of OVA-specific IgE and IgG1 antibodies continued to rise for at least 8 weeks (Figure 1C). Increased lung levels of type 2 cytokines, including IL-4, IL-5, and IL-13, were also observed in 4 weeks (Figure 1D). The IL-5 and IL-13 levels appeared to decline in 8 weeks. Notably, a marked increase in the lung levels of IL-33 was observed (please note the y-axis scale in Figure 1D) in 4 weeks, and the levels remain elevated for at least 8 weeks. In contrast, TSLP was undetectable, and the IL-25 levels were not affected by allergen exposure. Lung pathology specimens showed apparent evidence of structural changes in 8 weeks, including increased PAS-positive airway epithelial cells and connective tissues stained with Masson’s trichrome; these changes were minimal or not apparent in 4 weeks (Figures 1E and 1F). Together, these findings suggest that chronic exposure to multiple allergens induces robust eosinophilic inflammation and IL-5 and IL-13 production, followed by airway remodeling. Continued increases in specific IgE and IgG1 antibodies in peripheral blood and IL-33 in the lungs for 8 weeks were also noted.
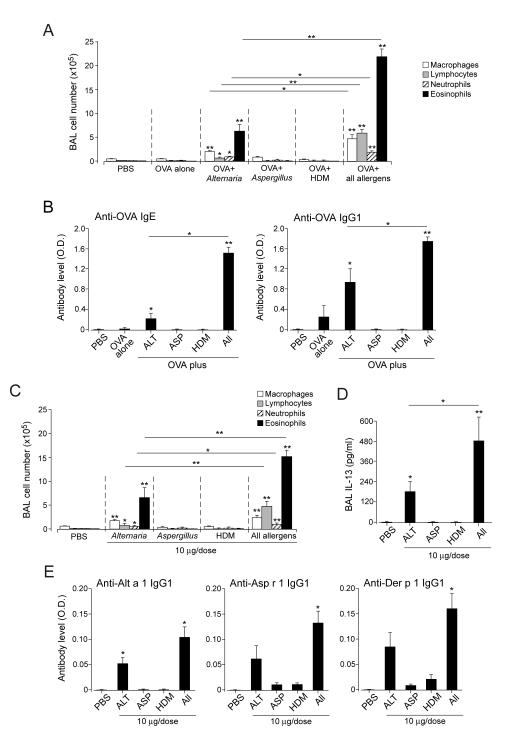
Allergens act synergistically to induce robust responses
We performed subsequent mechanistic studies by focusing on the 4-week time point, which represents the peak for airway eosinophilia and cytokine levels (Figures 1B and 1D). First, we examined the contribution of each allergen at the dose used in this study (i.e. 10 μg/dose). Exposure to OVA alone, OVA plus Aspergillus, or OVA plus HDM produced minimal airway inflammation (Figure 2A) whereas exposure to OVA plus Alternaria induced modest airway eosinophilia. Importantly, the three allergens when combined induced marked increases in BAL lymphocytes and eosinophils, approximately 10-fold and 3-fold higher, respectively, compared to OVA plus Alternaria. The production of specific antibodies, in particular of the IgE isotype, was also enhanced dramatically by exposure to a combination of three allergens (Figure 2B).
Figure 2.
Allergens work synergistically to induce robust airway inflammation and antibody production. (A) Naïve mice were exposed i.n. to PBS, OVA alone, OVA plus each allergen (10 μg/dose), or OVA plus a mixture of all allergens (Alternaria, Aspergillus, HDM; 10 μg/dose each) as indicated, 3 times/week, for 4 weeks. BAL fluids were analyzed for the number of inflammatory cells. (B) Plasma was analyzed for anti-OVA antibody levels; ALT, Alternaria; ASP, Aspergillus; HDM, house dust mite. (C) Naïve mice were exposed to each allergen (10 μg/dose) or a combination of all allergens (Alternaria, Aspergillus, HDM; 10 μg/dose each) for 4 weeks. BAL fluids were analyzed for the number of inflammatory cells. (D) The levels of IL-13 in BAL fluids were analyzed. (E) The levels of IgG1 antibodies to Alt a 1, Asp r 1 (Asp f 1 homologue) and Der p 1 in the plasma were analyzed. Results are the mean ± SEM (n=3 in panels A and B and n=5 in panels C through E). *: p<0.05, **: p<0.01, compared to mice exposed to PBS, or between groups, as indicated by the horizontal lines.
Second, to examine whether the OVA that was used to monitor adaptive immunity was necessary to induce type 2 responses to allergens, we compared the effects of the OAAH allergen mix and one without OVA (i.e. AAH). Removal of OVA from OAAH did not affect the magnitude and characteristics of airway inflammation (Supplemental Figure 1A). The plasma levels of total IgE and BAL levels of IL-13 were not affected by omitting OVA from OAAH, and OVA-specific IgE was undetectable in AAH-exposed animals (Supplemental Figure 1B).
Third, to examine the effects of allergens alone without OVA, mice were exposed to each allergen (10 μg/dose) or a mixture of all three allergens (10 μg/dose each) for 4 weeks. Exposure to Aspergillus or HDM produced minimal airway inflammation (Figure 2C) whereas exposure to Alternaria induced modest airway eosinophilia. A mixture of the three allergens induced marked increases in BAL lymphocytes and eosinophils and BAL levels of IL-13 (Figures 2D and 2E). Mice also developed IgG1 antibodies to Alt a 1, Asp r 1 and Der p 1 in plasma when they were exposed to a mixture of the three allergens (Figure 2E); exposure to Alternaria alone induced a significant increase in anti-Alt a 1 antibody.
Finally, the effects of each allergen were examined at a higher dose (i.e. 30 μg/dose). At this dose, not only Alternaria but also HDM induced airway eosinophilia and specific IgG1 antibodies (Supplemental Figure 2). Altogether, these findings suggest allergens act synergistically during chronic exposure even if the effects of each allergen may not be apparent in a relatively low dose.
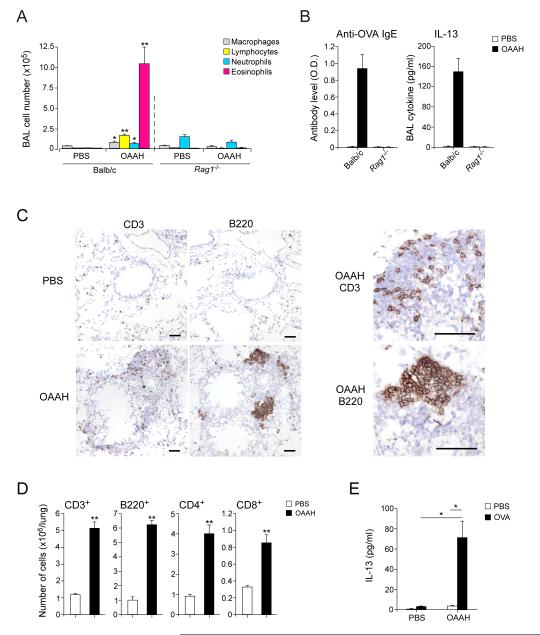
Adaptive immunity is necessary to drive chronic type 2 immunity to allergens
Recent studies suggest that acute airway exposure to certain natural or model allergens, such as Alternaria (24, 25) and the cysteine protease papain (26), activate type 2 innate immunity, and type 2 ILC2s are sufficient to mediate airway inflammation even in the absence of adaptive immunity. Therefore, to examine the role of adaptive immunity in the chronic allergen exposure model, we used Balb/c Rag1−/− mice, which are deficient in mature T cells and B cells. Whereas wild-type mice showed increases in BAL eosinophils, lymphocytes, and IL-13, as well as in plasma anti-OVA IgE, these responses were abolished in Rag1−/− mice (Figure 3A and 3B).
Figure 3.
Adaptive immunity is necessary for chronic type 2 immune responses induced by airborne allergens. (A) Naïve wild-type or Rag1−/− Balb/c mice were exposed to PBS or allergens (OAAH) for 4 weeks. BAL fluids were analyzed for the number of inflammatory cells. (B) The levels of plasma IgE antibody and BAL IL-13 were analyzed. (C) Lung specimens from wild-type mice exposed to PBS or allergens were stained with anti-CD3 and anti-B220 antibodies. Scale bars: 100 μm. (D) Number of lymphocyte subpopulations in the lungs was quantitated by flow cytometry. (E) Splenocytes were cultured in vitro with PBS or OVA antigen for 4 days, and the concentration of IL-13 in cell-free supernatants was determined. Results are the mean ± SEM (n=5 in each group). *: p<0.05, **: p<0.01, compared to mice exposed to PBS.
Immunohistochemical analyses revealed occasional CD3+ T cells and B220+ B cells scattered in the lung parenchyma in wild-type mice exposed to PBS (Figure 3C). When wild-type mice were exposed to the allergens for 4 weeks, CD3+ cells and B220+ cells increased in number and accumulated in the perivascular and peribronchial regions. B220+ cells formed distinct clusters while CD3+ cells were distributed rather diffusely. As estimated by flow cytometry, the total numbers of CD3+ cells and B220+ cells in the lungs increased by 5~6-fold in OAAH-exposed animals as compared to PBS-exposed animals (Figure 3D); increases in both the CD4+ and CD8+ subsets of T cells were observed.
To verify the involvement of adaptive immunity, we cultured splenocytes from PBS- or OAAH-exposed animals with OVA antigen in vitro. Splenocytes from OAAH-exposed animals, but not those from PBS-exposed animals, produced IL-13 when stimulated with OVA in vitro (Figure 3E). Altogether, unlike acute models, intact adaptive immunity is necessary for airway inflammation in the chronic-exposure model.
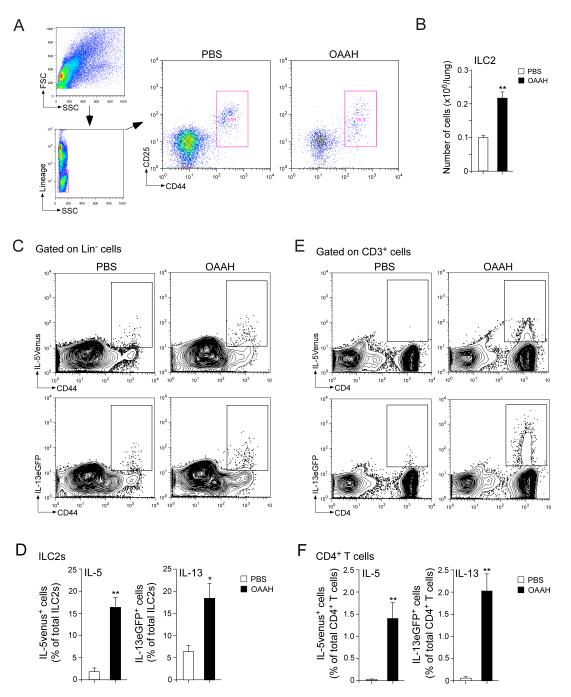
Both CD4+ T cells and ILC2s are sources of type 2 cytokines
We then investigated whether innate immunity is involved. Because ILC2s are recognized as an important source of type 2 cytokines during the acute and innate phase of the immune response (27, 28), we quantitated ILC2s in the lungs of this chronic-exposure model. Lung ILC2s were identified as lineage-negative (Lin−) and CD25- and CD44-double-positive cells (Figure 4A), as described previously (24). ILC2s were present in the lungs of naïve (data not shown) as well as PBS-exposed animals, and the number increased by >2-fold when animals were exposed to OAAH for 4 weeks (Figure 4B).
Figure 4.
ILC2s and CD4+ T cells are likely the source of IL-5 and IL-13. (A) Naïve wild-type mice were exposed i.n. to PBS or allergens (OAAH) for 4 weeks. Lung single-cell suspensions were gated for the lineage-negative (Lin−) cell population, and ILC2s were identified as CD25+CD44hi cells. (B) The number of ILC2s in the lung specimens was quantitated. (C) IL-5+/venus mice (upper panels) or IL-13+/eGFP mice (lower panels) were exposed to PBS or allergens (OAAH) for 4 weeks. Lung single-cell suspensions were gated for the Lin− cell population, and the expression levels of IL-5venus or IL-13eGFP in the CD44hi population were analyzed by flow cytometry. (D) The proportions of CD44hiIL-5venus+ cells or CD44hiIL-13eGFP+ cells among the ILC2 population were quantitated. (E) Lung single-cell suspensions were gated for the CD3+ population, and the expression levels of IL-5venus or IL-13eGFP in the CD4+ cell population were analyzed by flow cytometry. (F) The proportions of CD44hiIL-5venus+ cells or CD44hiIL-13eGFP+ cells among the CD4+ T cell population were quantitated. Results are the mean ± SEM (n=4 in panel B, n=3 in panels D and F) and are representative of two independent experiments. *: p<0.05, **: p<0.01, compared to mice exposed to PBS.
To examine the functions of these ILC2s during chronic inflammation, we used cytokine reporter mice, including the IL-5 reporter IL-5+/venus mice and IL-13 reporter IL-13+/eGFP mice. In PBS-exposed mice, a small fraction of CD44hi cells within the Lin-population expressed IL-5venus or IL-13eGFP (Figure 4C); no other cell populations within the Lin− population expressed these cytokines. Because the Lin−CD44hi cell population in the lungs consists exclusively of ILC2s (24) (Figure 4A), these findings suggest that a small fraction of ILC2s express IL-5 or IL-13 when mice are exposed to PBS. Importantly, when mice were exposed to OAAH, the proportion of IL-5- or IL-13-producing ILC2s increased by approximately 3~7-fold, and approximately 15~20% of total ILC2s showed ongoing production of these cytokines (Figures 4C and 4D).
To examine the functions of T cells, similar analyses were performed by gating on CD3+ T cells. Unlike ILC2s, IL-5venus or IL-13eGFP signals were undetectable or minimal within the CD3+ cell population in PBS-exposed animals. When mice were exposed to OAAH, the prevalence of IL-5- or IL-13-positive cells increased dramatically (Figures 4E). These cytokine-positive CD3+ T cells expressed CD4 (Figure 4E), and no CD4− cells were positive for IL-5 or IL-13. Approximately 1.4~2.0% of total CD4+ T cells expressed cytokines when mice were exposed to OAAH (Figure 4F). On average, 12.3×103 and 7.7×103 ILC2s/lung were positive for IL-5venus and IL-13eGFP, respectively, and 55.5×103 and 58.3×103 CD4+ T cells/lung were positive for IL-5venus and IL-13eGFP, respectively, after OAAH exposure (n=3). Altogether, both ILC2s and CD4+ T cells likely contribute to the increased IL-5 and IL-13 production in mice after chronic airway exposure to airborne allergens; CD4+ T cells appear to outnumber ILC2s.
Innate cytokines, including IL-33, increase rapidly after allergen exposure in naïve mice
The molecular and cellular mechanisms that initiate and maintain type 2 immunity remain topics of active investigation. Evidence suggests that epithelial cells make important contributions by producing several cytokines that facilitate the development of type 2 immunity, including IL-25, IL-33, and TSLP (29-31). IL-1-family cytokines, such as IL-1α and IL-β, have been considered prototypic innate cytokines with diverse immunological activities (32), and IL-1α has been shown to induce IL-33 release from airway epithelial cells (33). Therefore, to further investigate the immunological mechanisms of chronic type 2 immune responses to airborne allergens, we focused on the roles for these cytokines.
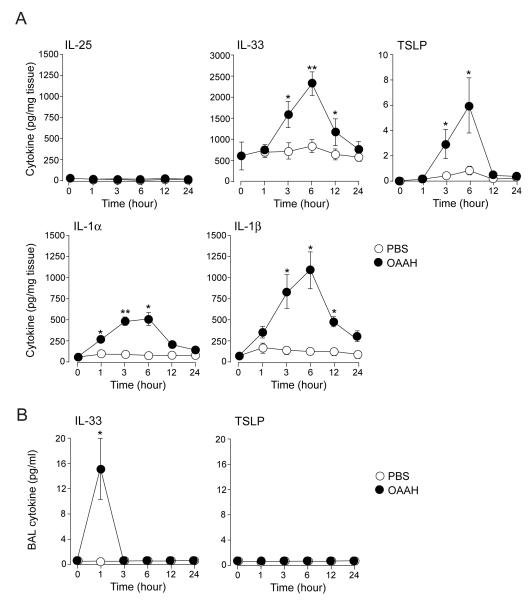
We investigated whether these innate cytokines are produced when mice are exposed acutely to the combination allergen OAAH. Naïve Balb/c mice were exposed to PBS or OAAH only once, and the kinetic changes of these cytokines in the lungs were analyzed. Substantial amounts of IL-33 (600 pg/mg tissue) were detected in the lungs of naïve non-treated mice, suggesting that this cytokine is constitutively produced (Figure 5A). When mice were exposed to OAAH, the lung levels of IL-33, IL-1α, and IL-1β increased quickly within one to several hours and peaked at 6 hours, and the levels declined to the baseline values by 12 or 24 hours. The increase in TSLP was also observed while the levels were considerably lower than IL-33, IL-1α, or IL-1β. The kinetic changes in these four cytokines were similar. In contrast, IL-25 levels did not change significantly for up to 24 hours.
Figure 5.
The lung levels of IL-33, TSLP, and IL-1 increase quickly following exposure to allergens. Naïve Balb/c mice were exposed i.n. to PBS or allergens (OAAH) only once. (A) The kinetics changes of cytokines in lung homogenates were analyzed. (B) Changes in the kinetics of cytokine production in BAL fluid supernatants were analyzed. Results are the mean ± SEM (n=3 in each group). *: p<0.05, **: p<0.01, compared to mice exposed to PBS.
When supernatants of BAL fluids were analyzed, IL-33 was detectable within 1 hour after exposure to OAAH. The IL-33 levels declined quickly, and IL-33 became undetectable 3 hours after the exposure. No TSLP was detectable in BALF supernatants at any time points. These findings suggest that expression of IL-1-family cytokines and TSLP increase transiently when naive mice are exposed to allergens.
IL-33 and to a lesser degree TSLP play pivotal roles in chronic inflammation
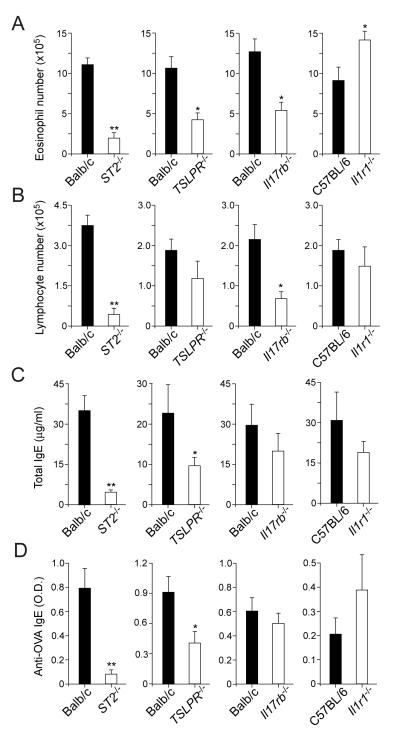
To examine the roles of these innate cytokines, we investigated mice that are deficient in their receptors, including ST2−/− (i.e. IL-33 receptor deficient), TSLPR−/−(TSLP receptor deficient), Il17rb−/− (i.e. IL-25 receptor deficient), and Il1r1−/− (i.e. deficient in the receptors for IL-1α and IL-1β) mice. Wild-type mice or receptor knockout mice were exposed to PBS or OAAH for 4 weeks. PBS-exposed wild-type mice or knockout mice did not show any apparent signs of airway inflammation: BAL eosinophils, <0.1×105 cells; BAL lymphocytes, <0.1×105 cells; and IL-4, IL-5 and IL-13 levels in lung homogenate, <5.0 pg/mg tissue. When exposed to OAAH, ST2−/− mice showed 70~80% reduction in BAL eosinophil and lymphocyte numbers (Figures 6A and 6B) and in plasma levels of total IgE and OVA-specific IgE (Figures 6C and 6D), compared to wild-type mice exposed to OAAH. TSLPR−/− mice also showed ~50% reduction in BAL eosinophils as wells as in plasma IgE antibody levels. While Il17rb−/− mice showed partial reduction in BAL eosinophil and lymphocyte numbers, IgE antibodies were not affected. In contrast, Il1r1−/− mice did not show significant changes in any of these immunological parameters; the number of BAL eosinophils rather increased in these Il1r1−/− mice (p<0.05).
Figure 6.
IL-33 and TSLP play critical roles in airway inflammation and IgE antibody production in response to chronic allergen exposure. Naïve wild-type mice (Balb/c or C57/BL6) or mice deficient in cytokine receptors were exposed i.n. to allergens (OAAH) for 4 weeks. The number of eosinophils (panel A) and lymphocytes (panel B) in BAL fluids, or plasma levels of total IgE (panel C) or anti-OVA IgE (panel D) were analyzed. Results are the mean ± SEM (n=5 in each group) and are representative of two independent experiments. *: p<0.05, **: p<0.01, compared to wild-type mice.
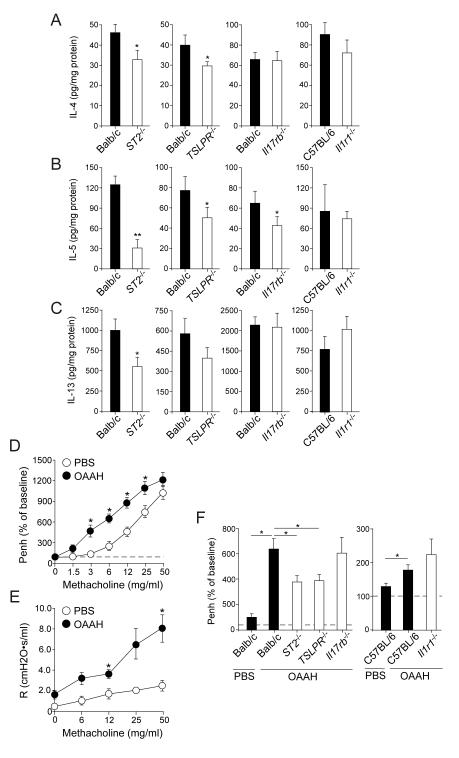
Figures 7A though 7C show the levels of lung cytokines. The levels of IL-4, IL-5, and IL-13 were significantly decreased in ST2−/− mice; IL-5 levels appeared to be affected the most. Partial reduction in IL-5 was also observed in TSLPR−/− and Il17rb−/− mice, and no apparent changes were observed in Il1r1−/− mice. We finally examined the roles of these cytokines in airway reactivity to inhaled methacholine. When naïve wild-type mice were exposed to OAAH for 4 weeks, they developed enhanced reactivity to inhaled methacholine as compared to PBS-exposed wild-type mice (Figure 7D). Analysis of airway reactivity with a forced oscillation technique also verified this observation (Figure 7E). Although we evaluated a full-range dose-response curve for inhaled methacholine (Supplemental Figure 3), the data at 6 mg/ml methacholine are summarized in Figure 7F for clarity. Wild-type Balb/c mice or C57BL/6 mice exposed to OAAH showed significant increases in airway reactivity to methacholine, compared to those exposed to PBS. The increase in reactivity was significantly attenuated in ST2−/− and TSLPR−/− mice (p<0.05) but not in Il17rb−/− or Il1r1−/− mice. Together, these findings suggest that IL-33, and to a lesser degree TSLP, play pivotal roles in airway inflammation, IgE antibody production, and AHR when mice are exposed to multiple airborne allergens for a prolonged period. The role of IL-25 is likely minimal, and IL-1α/β may play no role or rather act as an inhibitor of airway eosinophilia in this model.
Figure 7.
IL-33 and TSLP play significant roles in type 2 cytokine production and the development of airway hyperreactivity in response to chronic allergen exposure. (A, B, and C) Naïve wild-type mice or mice deficient in cytokine receptors were exposed to allergens as in Figure 6. The lung levels of IL-4 (A), IL-5 (B) or IL-13 (C) were analyzed. Results are the mean ± SEM (n=5 in each group) and are representative of two independent experiments. *: p<0.05, **: p<0.01, compared to wild-type mice. (D through F) Naïve wild-type mice or mice deficient in cytokine receptors were exposed i.n. to PBS or allergens (OAAH) for 4 weeks. Airway reactivity to inhaled methacholine was analyzed as described in the Materials and Methods. (D) The dose response to methacholine in wild-type Balb/c mice as examined by whole body plethysmography is presented. Results are the mean ± SEM (n=20 in each group, a pool of 4 experiments). *: p<0.05, compared to mice exposed to PBS. (E) The dose response to methacholine in wild-type Balb/c mice as examined by a forced oscillation technique is presented. Results are the mean ± SEM (n=5 in each group). *: p<0.05, compared to mice exposed to PBS. (F) Airway reactivity to 6 mg/ml methacholine is presented. Results are the mean ± SEM (n=5 in each group). *: p<0.05, between the groups indicated by horizontal lines.
DISCUSSION
Animal models of disease are critical in research to investigate mechanisms and to identify and validate targets for therapeutic interventions. While mouse models have been invaluable in asthma research, their limitations have also been identified and discussed (34, 35). In this study, by attempting to mimic natural environmental exposure, three different airborne allergens that are relevant to human asthma were combined and administered to the airways of naive mice for a prolonged period, up to 8 weeks. While each allergen itself provoked minimal responses at the dose used in this study (i.e. 10 μg/dose), they synergistically induced robust eosinophilic airway inflammation and IgE antibody production in 4 weeks. Airway remodeling continued to occur at least for 8 weeks. The results from this new model extend previous observations and suggest that IL-33 is involved in mediating chronic type 2 immune responses to natural airborne allergens, including airway inflammation, production of IgE antibody, and AHR. In contrast, although IL-1α and IL-1β showed apparent increases during the acute phase (Figure 5), mice deficient in IL-1 receptor were not protected from developing chronic airway inflammation. A previous report also suggested that IL-1α is necessary for the induction of IL-33 release from airway epithelial cells when mice are exposed to HDM extract (33). However, the results from this study indicate that IL-33 can be involved in type 2 immunity independent of the IL-1 pathway. Another new series of studies focusing on the 8-week time point or even longer is necessary to investigate the roles of IL-33 and potentially other cytokines in airway remodeling induced by chronic allergen exposure.
The role of TSLP in regulating type 2 immunity in the lungs has remained a topic of some controversy. Previously, when mice were sensitized by intraperitoneal injection of the OVA antigen, TSLP was found to play a key role in the lung Th2 response (36, 37). Furthermore, transgenic overexpression of TSLP in the airway also induced asthma-like pathological changes (36). However, more recently, by exposing naïve mice intranasally to HDM extract for 10 days, Chu et al demonstrated that Tslpr−/− mice showed comparable levels of IgE antibody and airway eosinophilia as did wild-type mice (38). The results from our study suggest that TSLP is involved in airway eosinophilia and IgE antibody production when animals are exposed to natural allergens for a prolonged period, although the contribution of TSLP may not be as robust as that of IL-33 (Figures 6 and 7). The mechanism and ability of TSLP to induce type 2 immunity is likely different from that of IL-33. For example, overexpression of TSLP alone in the airways caused a relatively weak innate immune response (39). Both overexpression of TSLP and airway administration of the OVA antigen were required to induce a disease phenotype (39),suggesting that TSLP conditions the lung milieu toward the optimal development of adaptive immunity against innocuous antigens. In contrast, IL-33 appears to be involved in both the innate and adaptive phases of type 2 immunity by acting on a variety of cell types including but not limited to ILC2s, dendritic cells (DCs), CD4+ T cells, mast cells, and eosinophils (40). Therefore, potential differences in the observations regarding the role of TSLP in this study and those by Chu et al. (38) can be explained by the variance in the models, in particular the length of allergen exposure (e.g. 4 weeks vs. 10 days) and possibly the types of allergens used.
Although the expression profile of IL-33 has been well characterized in normal tissues (41, 42), relatively little is known regarding its expression during inflammation. One of the major findings in this study is the dynamic change in lung IL-33 levels in response to chronic allergen exposure. IL-33 was the most abundant cytokine examined in lung tissues from naïve non-treated animals (Figure 5). IL-33 levels then increased quickly, similarly to other IL-1 family cytokines, after acute allergen exposure. In contrast, IL-33 secretion into the airway lumen was detectable only at the earliest time point of examination (i.e. 1 hour), suggesting increased total lung levels of IL-33 do not necessarily predict secretion of the protein to the extracellular space. Interestingly, while IL-33 levels in the lungs decreased temporarily to baseline in 24 hours, they were augmented dramatically by >20-fold during the chronic phase of allergen exposure, and remained elevated for at least 8 weeks (Figure 1D). IL-33 can be produced by airway tissue cells, such as epithelial cells, fibroblasts, vascular endothelial cells and smooth muscle cells, as wells as by immune cells such as DCs and macrophages (43-45). Studies using genetic tools, such as IL-33-reporter mice (46, 47), combined with immunological techniques, will be necessary to map the source(s) of IL-33 precisely during each of the acute and chronic phases of allergen exposure. Questions also remain as to the mechanism of enhanced IL-33 production and release. The direct effects of allergens on airway epithelial cells (48, 49), the influence of the cytokine milieu (50), and potential autocrine mechanisms of the IL-33/ST2 pathway (46) may need to be considered.
Unlike conventional OVA-mediated “asthma” models in mice (35), type 2 airway inflammation induced by chronic exposures to natural airborne allergens is likely mediated by complex and perhaps intertwining networks of innate and adaptive immunity. ILC2s have been known to provide a critical early source of type 2 cytokines, such as IL-5 and IL-13, when animals are exposed acutely to allergens (24-26). On the other hand, the roles of ILC2s in chronic inflammation have not been well understood. In this study, when exposed to allergens for a prolonged period (i.e. 4 weeks), both CD4+ T cells and ILC2s were identified as the potential source of IL-5 and IL-13 (Figure 4). The results are consistent with previous analyses of the source of IL-13 in OVA-induced type 2 responses, indicating that CD4+ T cells and ILC2s are the sources of IL-13 (51). Nonetheless, T cells were essential for robust eosinophilic inflammation and type 2 cytokine production (Figures 3A and 3B), consistent with previous findings (52). Although further studies will be necessary to identify key element(s) that regulate the network of innate and adaptive immunity, potential crosstalk between CD4+ T cells and ILC2s may also need to be considered. For example, T cell-derived cytokines, such as IL-2, sustain the numbers and activities of ILC2s (24). ILC2s may also regulate the differentiation and longevity of CD4+ T cells. For example, IL-13 derived from lung ILC2s likely enhances development of Th2-type antigen-specific CD4+ T cells by optimizing antigen presentation by dendritic cells (53). A new mouse model to remove ILC2s specifically and to leave the other immune cell compartments intact, such as those using the Cre-Lox system (54), will likely dissect the importance of ILC2s in the chronic phase of type 2 airway inflammation in vivo.
A potential weakness and at the same time a strength of this study is the complexity of its model. Each allergen itself, such as HDM and Alternaria, is reported to be sufficient to trigger several immune pathways when administered to the airways. For example, cysteine proteases, Der p 2, LPS, and chitin in the HDM extract could activate protease-activated receptors, TLR4, and dectin, as well as other as yet unidentified receptors; proteases also increase epithelium tight junction permeability (12). Protease-like activities in Alternaria extract can trigger ATP release, resulting in the activation of purinergic receptors on airway epithelia cells (48). A more recent study suggests that, among various natural allergens, serine protease activity from Alternaria uniquely induces IL-33 secretion in mice (55). Conceivably, a combination of these allergens can trigger multiple receptors that may work synergistically or even in some instances antagonistically to regulate airway immunity. Therefore, our model is unlikely to be optimal to dissect immune mechanisms in detail, such as the identification of specific receptor(s) to recognize allergens. Nonetheless, we could also consider this model relevant to a natural condition where human airways are exposed constantly to a number of environmental factors, including multiple allergens as well as other environmental stresses. Considering its complexity, the evidence obtained in this study in support of the central importance of IL-33 and perhaps TSLP may be highly valuable. Further studies on the molecular and cellular mechanisms that are involved in IL-33 and TSLP production, in particular those in chronically inflamed airway tissues, will help us better understand the immunological mechanisms of asthma and develop novel therapeutic strategies for the disease.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Kiyoshi Takatsu for providing IL-5+/venus mice, Mr. James E. Tarara for technical assistance, and Ms. LuRaye Eischens for secretarial help.
This work was supported by the grants from the National Institute of Health, R01AI34486, R01AI49235 and R01 HL117823, and by Mayo Foundation.
Abbreviations
- ACK
ammonium-chloride-potassium
- AHR
airway hyperresponsiveness
- APC
allophycocyanin
- BAL
bronchoalveolar lavage
- B.M.
basement membrane
- DCs
dendritic cells
- HDM
house dust mite
- ILCs
innate lymphoid cells
- ILC2s
type 2 ILCs
- i.n.
intranasal
- Lin−
lineage-negative
- OCT
optimal cutting temperature
- PAS
periodic acid-Schiff
- PerCP
peridinin chlorophyll protein complex
- TSLP
thymic stromal lymphopoietin
REFERENCES
- 1.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J. Allergy Clin. Immunol. 1997;100:S2–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 2.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J. Allergy Clin. Immunol. 2003;112:362–368. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 3.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N. Engl. J. Med. 1990;323:502–507. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 4.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N. Engl. J. Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 5.Knutsen AP, Bush RK, Demain JG, Denning DW, Dixit A, Fairs A, Greenberger PA, Kariuki B, Kita H, Kurup VP, Moss RB, Niven RM, Pashley CH, Slavin RG, Vijay HM, Wardlaw AJ. Fungi and allergic lower respiratory tract diseases. J. Allergy Clin. Immunol. 2012;129:280–291. doi: 10.1016/j.jaci.2011.12.970. [DOI] [PubMed] [Google Scholar]
- 6.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, Jankun T, Ren P, Je J. E. McSharry, Platts-Mills TA, Chapman MD, Bracken MB. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ. Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, 3rd, Stout J, Malindzak G, Smartt E, Plaut M, Walter M, Vaughn B, Mitchell H. Results of a home-based environmental intervention among urban children with asthma. N. Engl. J. Med. 2004;351:1068–1080. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 8.Salo PM, Arbes SJ, Jr., Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J. Allergy Clin. Immunol. 2008;121:678–684. e672. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson A, Tan VY, Winn J, Svensen M, Bishop CM, Heckerman DE, Buchan I, Custovic A. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am. J. Respir. Crit. Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 10.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat. Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory LG, Lloyd CM. Orchestrating house dust mite-associated allergy in the lung. Trends Immunol. 2011;32:402–411. doi: 10.1016/j.it.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakib F, Ghaemmaghami AM, Sewell HF. The molecular basis of allergenicity. Trends Immunol. 2008;29:633–642. doi: 10.1016/j.it.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Porter PC, Ongeri V, Luong A, Kheradmand F, Corry DB. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol. 2011;32:43–49. doi: 10.1016/j.it.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependnet on the receptor ST2 and conferes protective immunoty. Immunity. 2013 doi: 10.1016/j.immuni.2013.10.006. doi:10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goplen N, Karim MZ, Liang Q, Gorska MM, Rozario S, Guo L, Alam R. Combined sensitization of mice to extracts of dust mite, ragweed, and Aspergillus species breaks through tolerance and establishes chronic features of asthma. J. Allergy Clin. Immunol. 2009;123:925–932. doi: 10.1016/j.jaci.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi T, Iijima K, Radhakrishnan S, Mehta V, Vassallo R, Lawrence CB, Cyong JC, Pease LR, Oguchi K, Kita H. Asthma-related environmental fungus, Alternaria, activates dendritic cells and produces potent Th2 adjuvant activity. J. Immunol. 2009;182:2502–2510. doi: 10.4049/jimmunol.0802773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 2000;191:1069–1076. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpino N, Thierfelder WE, Chang MS, Saris C, Turner SJ, Ziegler SF, Ihle JN. Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Mol. Cell Biol. 2004;24:2584–2592. doi: 10.1128/MCB.24.6.2584-2592.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J. Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T, Iijima K, Kita H. Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice. J. Immunol. 2003;170:5756–63. doi: 10.4049/jimmunol.170.11.5756. [DOI] [PubMed] [Google Scholar]
- 23.Wagner EM, Jenkins J. Effects of airway distension on leukocyte recruitment in the mouse tracheal microvasculature. J. Appl. Physiol. 2007;102:1528–1534. doi: 10.1152/japplphysiol.01054.2006. [DOI] [PubMed] [Google Scholar]
- 24.Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. Immunol. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doherty TA, Khorram N, Sugimoto K, Sheppard D, Rosenthal P, Cho JY, Pham A, Miller M, Croft M, Broide DH. Alternaria induces STAT6-dependent acute airway eosinophilia and epithelial FIZZ1 expression that promotes airway fibrosis and epithelial thickness. J. Immunol. 2012;188:2622–2629. doi: 10.4049/jimmunol.1101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 27.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 28.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat. Rev. Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 29.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat. Rev. Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat. Rev. Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 33.Willart MA, Deswarte K, Pouliot P, Braun H, Beyaert R, Lambrecht BN, Hammad H. Interleukin-1alpha controls allergic sensitization to inhaled house dust mite via the epithelial release of GM-CSF and IL-33. J. Exp. Med. 2012;209:1505–1517. doi: 10.1084/jem.20112691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmes AM, Solari R, Holgate ST. Animal models of asthma: value, limitations and opportunities for alternative approaches. Drug Discov. Today. 2011;16:659–670. doi: 10.1016/j.drudis.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Mullane K, Williams M. Animal models of asthma: Reprise or reboot? Biochem. Pharmacol. 2013 doi: 10.1016/j.bcp.2013.06.026. doi:10.1016/j.bcp.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 36.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 38.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, In T. Seunghyun, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J. Allergy Clin. Immunol. 2013;131:187–200. doi: 10.1016/j.jaci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J. Immunol. 2009;182:1641–1647. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartemes KR, Kita H. Dynamic role of epithelium-derived cytokines in asthma. Clin. Immunol. 2012;143:222–235. doi: 10.1016/j.clim.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PloS one. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuchler AM, Pollheimer J, Balogh J, Sponheim J, Manley L, Sorensen DR, De Angelis PM, Scott H, Haraldsen G. Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am. J. Pathol. 2008;173:1229–1242. doi: 10.2353/ajpath.2008.080014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pichery M, Mirey E, Mercier P, Lefrancais E, Dujardin A, Ortega N, Girard JP. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. Immunol. 2012;188:3488–3495. doi: 10.4049/jimmunol.1101977. [DOI] [PubMed] [Google Scholar]
- 44.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J. Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 45.Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, Lewis L, Finkelman FD, Smith DE, Bryce PJ, Kurt-Jones EA, Wang TC, Sivaprasad U, Hershey GK, Herbert DR. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J. Exp. Med. 2012;209:607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hardman CS, Panova V, McKenzie AN. IL-33 citrine reporter mice reveal the temporal and spatial expression of IL-33 during allergic lung inflammation. Eur. J. Immunol. 2013;43:488–498. doi: 10.1002/eji.201242863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, McKenzie AN. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J. Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Polumuri SK, Jayakar GG, Shirey KA, Roberts ZJ, Perkins DJ, Pitha PM, Vogel SN. Transcriptional regulation of murine IL-33 by TLR and non-TLR agonists. J. Immunol. 2012;189:50–60. doi: 10.4049/jimmunol.1003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meephansan J, Komine M, Tsuda H, Karakawa M, Tominaga S, Ohtsuki M. Expression of IL-33 in the epidermis: The mechanism of induction by IL-17. J. Dermatol. Sci. 2013;71:107–114. doi: 10.1016/j.jdermsci.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 52.Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol. Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 53.Halim T,Y, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014 doi: 10.1016/j.immuni.2014.01.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. U S A. 1988;85:5166–5170. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33 mediated asthma exacerbation. J. Allergy Clin. Immunol. 2014 doi: 10.1016/j.jaci.2014.02.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.