Abstract
Background
Functional dyspepsia (FD) is a functional upper gastrointestinal disorder. The etiology and pathogenesis of FD remain unclear, with genetic factors playing an important role. Previous studies investigated the association of C825T in GNβ3 with FD, with conflicting results reported.
Aims
The aim of this meta-analysis is to assess the association of genetic variants in GNβ3 with FD.
Methods
We performed a systematic literature search in PubMed, Cochrane Library, Google Scholar and Web of Knowledge, and conducted a meta-analysis to assess the association of C825T in GNβ3 with FD. For sensitivity analysis, we analyzed the association between C825T and subtypes of FD. We also performed meta-analysis separately for individual ethnic groups/countries of origin.
Results
A total of 8 studies met the eligibility criteria and were included in our analyses. Our meta-analysis finds no association between 825CC and FD (OR=1.19, 95% CI: 0.84–1.67, p=0.328). The association, however, is significant under an additive model (OR=0.59, 95% CI: 0.38–0.92, p=0.018). Sensitivity analysis indicated a significant association of C825T with FD in participants from Korea but not in those from Japan, Europe, or the United States. We also detected a significant association of this SNP with dysmotility.
Conclusions
The genetic variant C825T in GNβ3 is significantly associated with FD under an additive model and the association is race specific. Further studies with larger samples sizes are needed to validate our findings and explore the potential mechanism underlying the association.
Keywords: functional dyspepsia, meta-analysis, GNβ3, polymorphism
Introduction
Functional dyspepsia (FD) is a functional upper gastrointestinal disorder characterized by recurrent or chronic abdominal symptoms in the absence of anatomical or biochemical abnormality [1]. It has been reported that up to 25% of the population experiences symptoms of FD, including postprandial discomfort, early satiety, upper abdominal burning or discomfort [1]. FD is a chronic disease, with over 80% of affected patients showing persistent symptoms after long-term follow-up [2, 3]. FD has two subtypes under the Rome III classification: postprandial distress syndrome (PDS), which refers to meal-induced dyspeptic symptoms characterized by postprandial fullness and early satiation, and epigastric pain syndrome (EPS) characterized by epigastric pain and burning [4].
The etiology and pathophysiology of FD remain unclear. Studies have identified several pathophysiology mechanisms, including delayed gastric emptying [5], visceral hypersensitivity [6], and dysfunction of the autonomic nervous system [7] as possible etiological factors. Previous studies also indicate that age, gender, smoking, Helicobacter pylori (H. pylori) infection and psychological disturbances could be potential risk factors for FD [8–11]. Recently, familial aggregation of FD has been reported, suggesting that genetic factors may play a role in the pathophysiology of FD [12, 13]. Many studies have been conducted to search for susceptibility genes for FD, including guanine nucleotide binding protein beta polypeptide 3 (GNβ3) [14], the serotonin transporter promoter (SERT-P) [15], the cyclooxygenase-1 (COX-1) [16] and the Catechol-o-methyltransferase (COMT) [17].
Guanine nucleotide-binding proteins (G-proteins), membrane receptors and signal transduction molecules are involved in intracellular signal transduction pathways. The Gβ3 protein is encoded by the GNβ3 gene, in which there is a common polymorphism C825T (rs5443), located on chromosome 12, with an exchange from cytosine to thymidine, producing three possible genotypes (i.e., CC, TC, and TT). The 825T allele is associated with alternative splicing of the gene and its protein activity [18]. Previous studies investigated the association of C825T with FD, with conflicting results reported [14, 15, 19, 20]. Therefore, in this study we performed meta-analysis to assess the association of C825T with FD.
Methods
Search Strategy and Study Selection
We did an extensive literature search in PubMed, Cochrane Library, Google Scholar and Web of Knowledge in November, 2012 for studies on the association of genetic variants in GNβ3 with FD. Search terms can be found in the supplementary file. The following inclusion criteria were used in determining study eligibility: 1) studies on human subjects; 2) outcomes of interest include FD; and 3) report of genotype data of individual genetic variants in GNβ3 in participants with and without FD (or provided odds ratios and their variances). All potentially relevant publications were retrieved and evaluated for inclusion. We also hand-searched references of all relevant publications for additional studies missed by the database search. Only studies published in the English language were included in our analysis. Two authors (FD and YL) performed the search independently. Disagreement over eligibility of a study was resolved by discussion until a consensus was reached.
Data Extraction
Two reviewers (YL and SG) independently extracted the following data according to a pre-specified protocol: first author’s name, year of publication, characteristics of the study participants (sample size, mean age, percentage of male and race/country of participants), genotype data for subjects with and without FD (or odds ratio and the corresponding variances), and the genetic model used (additive, allelic, dominant or recessive). Discrepancies were resolved by discussion, and extracted data were entered into a computerized spreadsheet for analysis.
Statistical Analysis
We used odds ratio (OR) as a measure of the association between the genetic variants in GNβ3 and FD. We used a random-effects model to calculate OR and the corresponding 95% confidence interval (CI) if there was significant heterogeneity between the studies; otherwise, a fixed-effect model was used. For random-effects meta-analysis, the inverse of the variance of each study was used as the weight for the study. A forest plot was used to graphically represent the calculated pooled ORs and their 95% CIs. Each study was represented by a square in the plot, the area of which is proportional to the weight of the study. The overall effect from the meta-analysis is represented by a diamond, with its width representing the 95% CI for the estimate. Between-study heterogeneity was assessed using a Q-test, and publication bias was assessed using Egger’s regression test [21].
Sensitivity Analysis
We analyzed the association between C825T and subtypes of FD (PDS, EPS, ulcer, dysmotility and non-specific FD). Meta-analyses were conducted when there were multiple studies for the analysis of each subtype. We also performed meta-analysis separately for individual ethnic groups/countries of origin (Korea, Japan and Europe/United States).
Meta-analysis was performed using Stata 11.2 (StataCorp LP, College Station, TX). All other analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Literature Search and Eligible Studies
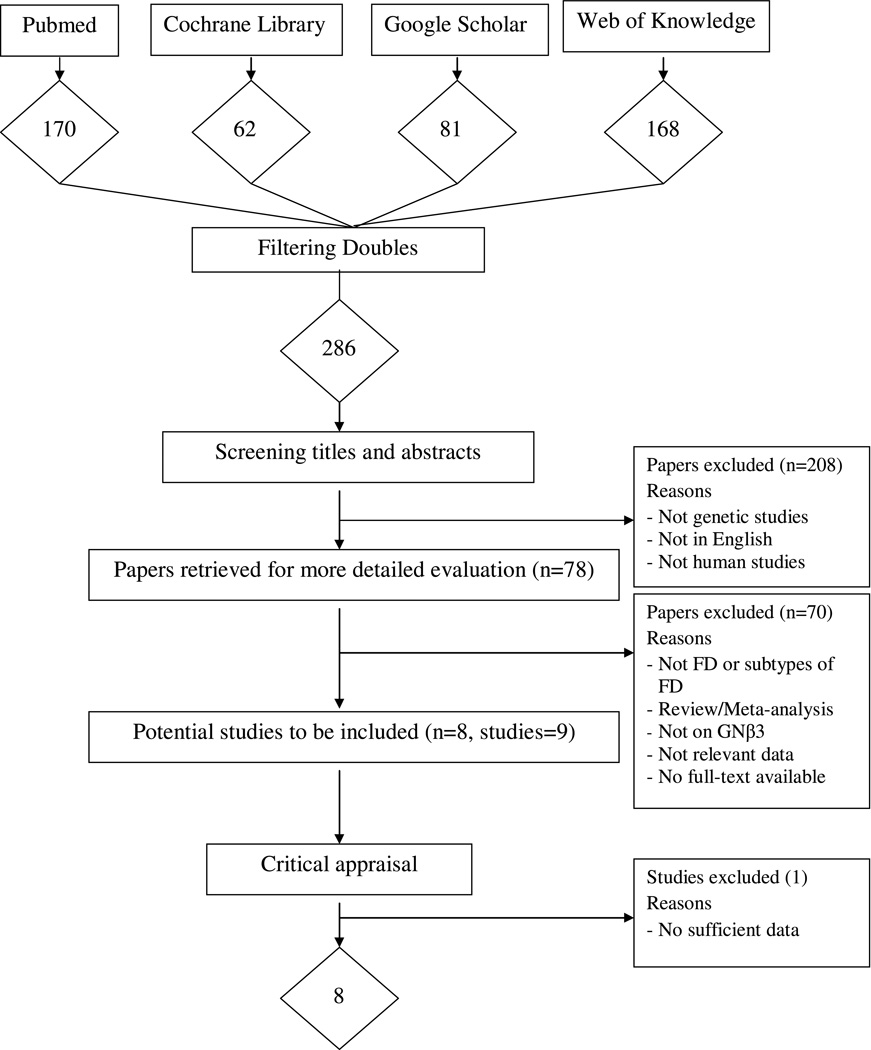
The flow diagram in Figure 1 adheres to the QUOROM statement and shows the selection of studies to be included in our analysis [22]. Using our pre-defined search strategy, we identified a total of 286 potential publications through our initial search. After screening the abstracts of these studies, 208 were excluded either because they were not genetic studies, not about human subjects, or not published in English. The remaining 78 studies were retrieved for more detailed evaluations, which excluded an additional 70 studies because they were not about FD or subtypes of FD, there were not sufficient data, they were meta-analyses, review studies or the full text was unavailable despite efforts to contact the authors. This left 8 potentially relevant publications (with 9 studies) to be included in our analysis. Further exploration of the data from these studies excluded one more study with insufficient data. A total of 8 studies met the eligibility criteria and were included in our analyses [14, 15, 19, 20, 23–26].
Figure 1.
Flow diagram of the selection process of the studies included in the meta-analyses
Note: Please see the Methods Section for additional details.
All qualified publications were published since 2004 and had sample sizes ranging from 89 to 829 (See Table 1). A total of 2,706 participants (718 with FD and 1,988 controls) were included in the meta-analysis. Prevalence of FD ranged from 8.2% to 56%. Of these 8 studies, three used Japanese participants, two used Korean participants, and three used participants from USA or Europe.
Table 1.
Basic characteristics of all studies.
| Study (author, year) |
Race/Country | Definition of FD |
FD | Control | ||||
|---|---|---|---|---|---|---|---|---|
| n | Age (Mean±SD) |
Male (%) |
n | Age (Mean±SD) |
Male (%) |
|||
| Park et al., 2012 | Koreas | Rome III | 102 | 11.2±3.6 | 31.3 | 148 | 10.8±3.9 | 54.7 |
| Kim et al., 2012 | Koreas | Rome III | 167 | 49±15 | 37.1 | 434 | 47±15 | 38.5 |
| Shimpuku et al., 2011 | Japanese | Rome III | 74 | 59.2±14.2 | 48.6 | 64 | 37.2±9.13 | 89.1 |
| Oshima et al., 2010 | Japanese | Rome III | 68 | 43(23–69)* | 36.8 | 761 | 45(19–83)* | 42.7 |
| Van-Lelyveld et al., 2008 | Netherlands/Caucasian | Rome II | 112 | 42.3±1 | 28 | 336 | 41.9±1 | 28 |
| Tahara et al., 2008 | Japanese | Rome II | 89 | 60.1±13.1 | 73 | 94 | 61.1±13.1 | 69 |
| Camilleri et al., 2006 | USA | Rome II | 50 | 55(34–79)* | 54 | 39 | 60(37–81)* | 44 |
| Holtmann et al., 2004 | Germany/Caucasian | Rome II | 56 | 46.4±1.9 | 35.7 | 112 | 44.4±1.3 | 35.7 |
FD: functional dyspepsia; SD: standard deviation.
Range of age.
Assessment of Publication Bias
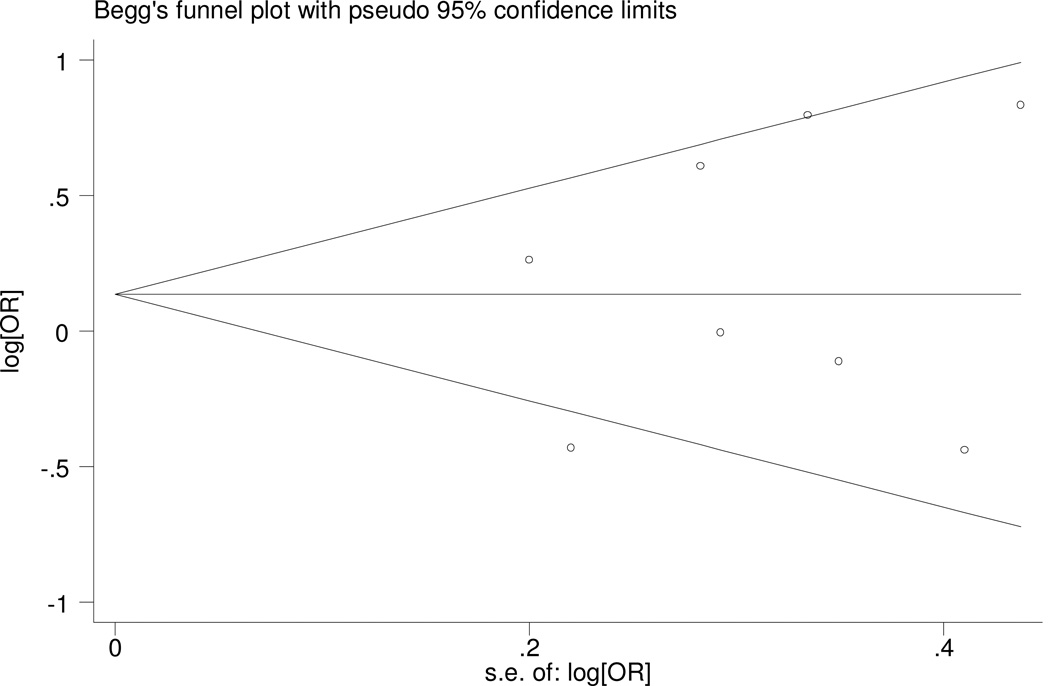
Examination of the funnel plot did not reveal severe deviance from symmetry (fig. 2). Egger’s test was also used to assess publication bias, and we found no publication bias for the meta-analysis (p=0.572). Assessment of publication bias for the meta-analysis of the association of C825T with subtypes of FD is not very meaningful due to limited number of studies included in the corresponding meta-analysis.
Figure 2.
Funnel plot for meta-analysis of C825T in GNB3.
The x-axis is the standard error of the log-transformed odds ratio (log[OR]), and the y-axis is log[OR]. The horizontal line in the figure represents the overall estimated log-transformed odds ratio. The two diagonal lines represent the pseudo 95% confidence limits of the effect estimate.
Association of C825T with FD
We calculated the association between C825T and FD assuming four different genetic models (additive, allelic, dominant and recessive). Due to space limits, we present the results for CC vs. TC and TT, which was used by many studies. Results obtained using other models can be found in the supplementary file.
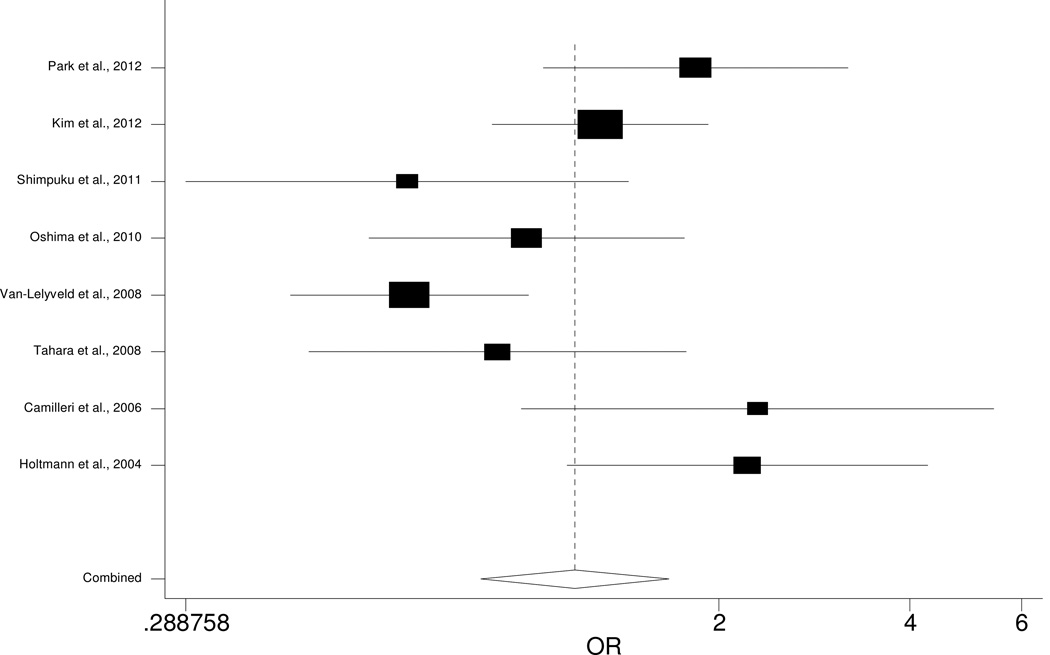
Of the eight studies included in our meta-analysis, only two show significant association between C825T and FD, with another two studies indicting marginal association (Table 2). Specifically, one study [14] indicated that CC carriers had increased risk of FD (OR=2.22, 95% CI: 1.15–2.47), as confirmed by a recent study (OR=1.84, 95% CI: 1.06–3.20) [26]. The other two studies reported marginal association of C825T with FD, but the direction of effect is inconsistent [15, 24]. Our meta-analysis indicates no significant association of C825T with FD (OR=1.19, 95% CI: 0.84–1.67, p=0.328; Fig. 3). The association, however, is significant under an additive model (reference genotype: CC; OR=0.59, 95% CI: 0.38–0.92, p=0.018; Supplementary Table 1).
Table 2.
Meta-analysis of the association of functional dyspepsia and C825T in GNB3a.
| Study | Case | Control | OR | P |
|---|---|---|---|---|
| Park et al., 2012 | 102 | 148 | 1.84 (1.06–3.20) | 0.031 |
| Kim et al., 2012 | 167 | 434 | 1.30 (0.88–1.92) | 0.187 |
| Shimpuku et al., 2011 | 74 | 64 | 0.65 (0.29–1.44) | 0.292 |
| Oshima et al., 2010 | 68 | 761 | 0.99 (0.56–1.76) | 0.973 |
| Van-Lelyveld et al., 2008 | 112 | 336 | 0.65 (0.42–1.00) | 0.052 |
| Tahara et al., 2008 | 89 | 94 | 0.89 (0.45–1.77) | 0.739 |
| Camilleri et al., 2006 | 50 | 39 | 2.30 (0.98–5.42) | 0.056 |
| Holtmann et al., 2004 | 56 | 112 | 2.22 (1.15–4.27) | 0.017 |
| Total | 718 | 1988 | 1.19 (0.84–1.67) | 0.328 |
CC vs. TC and TT.
Figure 3.
Forest plot for meta-analysis of C825T in GNB3.
Each study is represented by a square and a line. The mid-point of the square is the point estimate of that study, and the area of the square is proportional to the weight of the study. The width of the line represents the 95% confidence interval (CI) of the estimate of the study. The overall effect from meta-analysis is represented by a diamond whose width represents the 95% CI for the estimated odds ratio. The vertical dashed line is the line of no effect.
Sensitivity Analysis
Sensitivity analysis reveals significant association between C825T and dysmotility (p=0.001), but the association with other subtypes of FD is not significant, probably due to limited sample size in the calculation. We found significant association of C825T with FD in Korean participants (OR=1.46, 95% CI: 1.06–2.01, p=0.021) but not in Japanese (OR=0.87, 95% CI: 0.59–1.28, p=0.481) or Europe/United States participants (OR=1.43, 95% CI: 0.56–3.61, p=0.453).
Discussion
In this paper, we conducted a systematic literature search for publications on the association of genetic variants in GNβ3 with functional dyspepsia. Our results show no evidence for the association between 825CC genotype and functional FD. The association is significant, however, under the additive model. We also detected a significant association of this SNP with dysmotility. To the best of our knowledge, this is the first meta-analysis on the potential association of C825T with FD.
Studies on the association between C825T and FD have reported inconsistent results. The most significant results were reported in a study comprising 56 patients with FD and 112 controls (p=0.017) [14]. This study recruited Caucasian participants from across Germany. The positive findings in this study could not be replicated by most other subsequent studies except a recent one which reported a similar effect size in participants from Korea [26]. Inconsistencies of the results among these studies might also be due to the different genetic models used by individual studies. Although most studies compared the frequency of homozygous CC carriers vs. T-allele carriers, other studies also reported results using other genetic models [19, 20, 25]. Our meta-analysis found no significant association of 825CC with FD. However, C825T shows significant association with FD under an additive model (Supplementary Table 1). Another explanation for the inconsistencies might be the adjustment for different confounding factors: some studies did not control for any confounding factors, while others controlled for age and/or sex [14, 20]. Factors such as sample size, heterogeneity of the disease or sample selection, might also contribute to the conflicting results [27]. Specifically, one study recruited very young participants (aged 4–18 years old) [26] while other studies used data from older participants. Another important factor accounting for the conflicting results might be the difference in genetic composition of participants among different races/ethnic groups. The frequency of the C825TT allele is approximately 50% in Canadian Oi-Cree Indians [28], almost twice as high as in Germany [29]. Consequently, our sensitivity analysis found significant association of C825T with FD in Korean participants, but not in participants from Japan or Europe/United States. However, these results should be interpreted with caution because limited sample size may lead to insufficient power in detecting a significant association.
Although the two subtypes of FD (dysmolitity and PDS) employed different criteria in diagnosis, they share the same disturbed gastric motor function, including antral hypomotility, delay in gastric emptying and impaired gastric accommodation. The two-studies on dysmotility both reported significant and consistent association with C825T, and meta-analysis indicates that CC genotype carriers had 2-fold increased risk of having dysmotility (Table 3B) [14, 15]. Only one study examined the genetic association of C825T with PDS and found no significant association [19]. The study on PDS was limited to participants in Japan, while the studies on dysmotility were done in the US, and therefore might represent different genetic architectures across populations. More studies are certainly needed to quantify the association of C825T with PDS.
Table 3.
|
A Association of C825T with subtypes of functional dyspepsia (Rome III)a | |||||
|---|---|---|---|---|---|
| Symptom | Study | Case | Control | OR (95% CI) | P |
| PDS | Oshima et al. | 40 | 761 | 0.99 (0.48–2.07) | 0.989 |
| EPS | Oshima et al. | 43 | 761 | 0.68 (0.31–1.50) | 0.340 |
|
B Association of C825T with subtypes of functional dyspepsia (Rome II)a | |||||
|---|---|---|---|---|---|
| Symptom | Study | Case | Control | OR (95% CI) | P |
| Ulcer | Holtmann et al. | 16 | 112 | 1.84 (0.64–5.31) | 0.256 |
| Camilleri et al. | 3 | 39 | 9.00 (0.44–185.96) | 0.155 | |
| Total | 19 | 151 | 2.19 (0.81–5.94) | 0.123 | |
| Dysmotility | Holtmann et al. | 40 | 112 | 2.66 (1.26–5.65) | 0.011 |
| Camilleri et al. | 21 | 39 | 4.14 (1.26–3.57) | 0.019 | |
| Total | 61 | 151 | 3.02 (1.60–5.70) | 0.001 | |
| Non-specific | Camilleri et al. | 26 | 39 | 1.29 (0.48–3.50) | 0.612 |
CC vs. TC and TT.
Few other genetic variants in GNβ3 have ever been studied. Our extensive literature search found only one study that reported the genotype data of an additional SNP A814G in GNβ3 with FD [15]. There was no significant association of A814G with FD (OR 1.42, 95% CI: 0.39–5.26, p=0.596). The finding is not conclusive because of limited sample size (n=89). Knowledge about this genetic variant is scarce.
Heterotrimeric G-proteins, composed of α, β and γ subunits, are essential for stimulus-response coupling of a majority of known membrane receptors that are linked to intracellular effector systems [30–33]. Many hormones, neurotransmitters and sensory stimuli, which have been implicated in the generation of dyspeptic symptoms, exert their effects on cells through binding to G-protein coupled receptors (GPCRs) [24]. Changes in G-proteins could lead to disease by blocking or enhancing intracellular signal transduction [34]. The common polymorphism C825T in GNβ3 has been reported to be associated with a number of disorders, including obesity [35, 36], hypertension [29, 37, 38], coronary heart disease [39], stroke [40], insulin resistance [41] and depression [42].
The 825T allele is associated with enhanced G-protein activation and thereby altered signal transduction response [29]. It is associated with alternative splicing of the gene, resulting in a truncated deletion—but functionally active splicing variant—of 41 amino acids [29]. This can result in motor or sensory abnormalities of the gastrointestinal tract, which might be the pathophysiological mechanism underlying FD [43]. It was also reported that the T allele in C825T was associated with lower fasting gastric volume [44], which was found to contribute to symptoms in patients with functional dyspepsia [45]. The effect of C825T on gastrointestinal motor and sensory functions warrants further research to uncover the physiologic mechanism underlying the association.
Our study has some limitations. Although we conducted an extensive literature search, the number of participants included in our meta-analysis is limited, especially in the sensitivity meta-analysis to assess the association with FD subtypes. We would like to emphasize that the findings from these small number of studies are not conclusive and warrant further validation. Specifically, although we detected significant association of C825T with dysmotility, the sample size (total participants: 212) is too limited to reach a conclusive finding. Further studies are warranted to validate our findings and explore the potential mechanisms underlying the association, particularly studies with larger sample sizes that re-sequence more intensively genetic variants in GNβ3 to rigorously evaluate their cumulative contribution to disease pathogenesis. The definition of FD is different across studies, with earlier publications adopting Rome II criteria and later ones adopting Rome III criteria (Table 1). We cannot test the publication bias for sensitivity analyses due to limited number of studies. This might lead to bias in the data and possibly influence the results of our analysis. The appropriate model for testing the genetic association of GNβ3 with FD is not clear. Further biological studies are needed to elucidate the mechanism linking GNβ3 to FD in order to provide more evidence on the correct functional genetic model.
In summary, we did a systematic literature search and performed the first meta-analysis on the association of C825T in GNβ3 with FD. Under a dominant genetic model, we found no evidence of association between C825T and FD, but the association is significant if an additive model is used. We found that this genetic variant is significantly associated with dysmotility-like symptoms and symptoms in PDS. Further studies, particularly studies with larger samples sizes, are needed to validate our findings and to explore the potential mechanisms underlying the association.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Yang’s research was supported by R01AG036042 and the Illinois Department of Public Health.
Footnotes
Conflict of interest: no conflicting relationship exists for any author.
REFERENCES
- 1.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 2.Heikkinen M, Farkkila M. What is the long-term outcome of the different subgroups of functional dyspepsia? Alimentary pharmacology & therapeutics. 2003;18:223–229. doi: 10.1046/j.1365-2036.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- 3.Agreus L, Svardsudd K, Talley NJ, Jones MP, Tibblin G. Natural history of gastroesophageal reflux disease and functional abdominal disorders: a population-based study. The American journal of gastroenterology. 2001;96:2905–2914. doi: 10.1111/j.1572-0241.2001.04680.x. [DOI] [PubMed] [Google Scholar]
- 4.Geeraerts B, Tack J. Functional dyspepsia: past, present, and future. Journal of gastroenterology. 2008;43:251–255. doi: 10.1007/s00535-008-2167-8. [DOI] [PubMed] [Google Scholar]
- 5.Tack J, Masclee A, Heading R, et al. A dose-ranging, placebo-controlled, pilot trial of Acotiamide in patients with functional dyspepsia. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21:272–280. doi: 10.1111/j.1365-2982.2009.01261.x. [DOI] [PubMed] [Google Scholar]
- 6.Miwa H. Why dyspepsia can occur without organic disease: pathogenesis and management of functional dyspepsia. Journal of gastroenterology. 2012;47:862–871. doi: 10.1007/s00535-012-0625-9. [DOI] [PubMed] [Google Scholar]
- 7.Greydanus MP, Vassallo M, Camilleri M, Nelson DK, Hanson RB, Thomforde GM. Neurohormonal factors in functional dyspepsia: insights on pathophysiological mechanisms. Gastroenterology. 1991;100:1311–1318. [PubMed] [Google Scholar]
- 8.Ford AC. Eradicating Helicobacter pylori in functional dyspepsia. Gastroenterology. 2012;142:1613–1614. doi: 10.1053/j.gastro.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. The American journal of gastroenterology. 2004;99:2210–2216. doi: 10.1111/j.1572-0241.2004.40052.x. [DOI] [PubMed] [Google Scholar]
- 10.Mahadeva S, Goh KL. Anxiety, depression and quality of life differences between functional and organic dyspepsia. Journal of gastroenterology and hepatology. 2011;26(Suppl 3):49–52. doi: 10.1111/j.1440-1746.2011.06656.x. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Nie Y, Sha W, Su H. The link between psychosocial factors and functional dyspepsia: an epidemiological study. Chinese medical journal. 2002;115:1082–1084. [PubMed] [Google Scholar]
- 12.Locke GR, 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ., 3rd Familial association in adults with functional gastrointestinal disorders. Mayo Clinic proceedings. Mayo Clinic. 2000;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 13.Morris-Yates A, Talley NJ, Boyce PM, Nandurkar S, Andrews G. Evidence of a genetic contribution to functional bowel disorder. The American journal of gastroenterology. 1998;93:1311–1317. doi: 10.1111/j.1572-0241.1998.440_j.x. [DOI] [PubMed] [Google Scholar]
- 14.Holtmann G, Siffert W, Haag S, et al. G-protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126:971–979. doi: 10.1053/j.gastro.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Camilleri CE, Carlson PJ, Camilleri M, et al. A study of candidate genotypes associated with dyspepsia in a U.S. community. The American journal of gastroenterology. 2006;101:581–592. doi: 10.1111/j.1572-0241.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 16.Arisawa T, Tahara T, Shibata T, et al. Genetic polymorphisms of cyclooxygenase-1 (COX-1) are associated with functional dyspepsia in Japanese women. Journal of women's health. 2008;17:1039–1043. doi: 10.1089/jwh.2007.0720. [DOI] [PubMed] [Google Scholar]
- 17.Tahara T, Arisawa T, Shibata T, Nakamura M, Wang F, Hirata I. COMT gene val158met polymorphism in patients with dyspeptic symptoms. Hepatogastroenterology. 2008;55:979–982. [PubMed] [Google Scholar]
- 18.Baumgart D, Naber C, Haude M, et al. G protein beta3 subunit 825T allele and enhanced coronary vasoconstriction on alpha(2)-adrenoceptor activation. Circulation research. 1999;85:965–969. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 19.Oshima T, Nakajima S, Yokoyama T, et al. The G-protein beta3 subunit 825 TT genotype is associated with epigastric pain syndrome-like dyspepsia. BMC medical genetics. 2010;11:13. doi: 10.1186/1471-2350-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HG, Lee KJ, Lim SG, Jung JY, Cho SW. G-protein beta3 subunit C825T polymorphism in patients with overlap syndrome of functional dyspepsia and irritable bowel syndrome. Journal of neurogastroenterology and motility. 2012;18:205–210. doi: 10.5056/jnm.2012.18.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 23.Shimpuku M, Futagami S, Kawagoe T, et al. G-protein beta3 subunit 825CC genotype is associated with postprandial distress syndrome with impaired gastric emptying and with the feeling of hunger in Japanese. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23:1073–1080. doi: 10.1111/j.1365-2982.2011.01781.x. [DOI] [PubMed] [Google Scholar]
- 24.van Lelyveld N, Linde JT, Schipper M, Samsom M. Candidate genotypes associated with functional dyspepsia. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2008;20:767–773. doi: 10.1111/j.1365-2982.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 25.Tahara T, Arisawa T, Shibata T, et al. Homozygous 825T allele of the GNB3 protein influences the susceptibility of Japanese to dyspepsia. Digestive diseases and sciences. 2008;53:642–646. doi: 10.1007/s10620-007-9923-0. [DOI] [PubMed] [Google Scholar]
- 26.Park CS, Uhm JH. Polymorphisms of the Serotonin Transporter Gene and G-Protein beta3 Subunit Gene in Korean Children with Irritable Bowel Syndrome and Functional Dyspepsia. Gut Liver. 2012;6:223–228. doi: 10.5009/gnl.2012.6.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park MI. Is there enough evidence for the association of gNbeta3 C825T polymorphism with functional dyspepsia and irritable bowel syndrome? Journal of neurogastroenterology and motility. 2012;18:348–349. doi: 10.5056/jnm.2012.18.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegele RA, Harris SB, Hanley AJ, Cao H, Zinman B. G protein beta3 subunit gene variant and blood pressure variation in Canadian Oji-Cree. Hypertension. 1998;32:688–692. doi: 10.1161/01.hyp.32.4.688. [DOI] [PubMed] [Google Scholar]
- 29.Siffert W, Rosskopf D, Siffert G, et al. Association of a human G-protein beta3 subunit variant with hypertension. Nature genetics. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 30.Gollasch M, Kleuss C, Hescheler J, Wittig B, Schultz G. Gi2 and protein kinase C are required for thyrotropin-releasing hormone-induced stimulation of voltage-dependent Ca2+ channels in rat pituitary GH3 cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6265–6269. doi: 10.1073/pnas.90.13.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleuss C, Hescheler J, Ewel C, Rosenthal W, Schultz G, Wittig B. Assignment of Gprotein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–48. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 32.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- 33.Kleuss C, Scherubl H, Hescheler J, Schultz G, Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259:832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 34.Andresen V, Camilleri M, Kim HJ, et al. Is there an association between GNbeta3-C825T genotype and lower functional gastrointestinal disorders? Gastroenterology. 2006;130:1985–1994. doi: 10.1053/j.gastro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Siffert W, Forster P, Jockel KH, et al. Worldwide ethnic distribution of the G protein beta3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. Journal of the American Society of Nephrology : JASN. 1999;10:1921–1930. doi: 10.1681/ASN.V1091921. [DOI] [PubMed] [Google Scholar]
- 36.Hegele RA, Anderson C, Young TK, Connelly PW. G-protein beta3 subunit gene splice variant and body fat distribution in Nunavut Inuit. Genome research. 1999;9:972–977. doi: 10.1101/gr.9.10.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong Y, Zhu H, Sagnella GA, Carter ND, Cook DG, Cappuccio FP. Association between the C825T polymorphism of the G protein beta3-subunit gene and hypertension in blacks. Hypertension. 1999;34:1193–1196. doi: 10.1161/01.hyp.34.6.1193. [DOI] [PubMed] [Google Scholar]
- 38.Sartori M, Semplicini A, Siffert W, et al. G-protein beta3-subunit gene 825T allele and hypertension: a longitudinal study in young grade I hypertensives. Hypertension. 2003;42:909–914. doi: 10.1161/01.HYP.0000097600.58083.EE. [DOI] [PubMed] [Google Scholar]
- 39.Klintschar M, Stiller D, Schwaiger P, Kleiber M. DNA polymorphisms in the tyrosine hydroxylase and GNB3 genes: association with unexpected death from acute myocardial infarction and increased heart weight. Forensic science international. 2005;153:142–146. doi: 10.1016/j.forsciint.2004.09.103. [DOI] [PubMed] [Google Scholar]
- 40.Morrison AC, Doris PA, Folsom AR, Nieto FJ, Boerwinkle E. G-protein beta3 subunit and alpha-adducin polymorphisms and risk of subclinical and clinical stroke. Stroke; a journal of cerebral circulation. 2001;32:822–829. doi: 10.1161/01.str.32.4.822. [DOI] [PubMed] [Google Scholar]
- 41.Wascher TC, Paulweber B, Malaimare L, et al. Associations of a human G protein beta3 subunit dimorphism with insulin resistance and carotid atherosclerosis. Stroke; a journal of cerebral circulation. 2003;34:605–609. doi: 10.1161/01.STR.0000058159.63950.EA. [DOI] [PubMed] [Google Scholar]
- 42.Zill P, Baghai TC, Zwanzger P, et al. Evidence for an association between a G-protein beta3-gene variant with depression and response to antidepressant treatment. Neuroreport. 2000;11:1893–1897. doi: 10.1097/00001756-200006260-00018. [DOI] [PubMed] [Google Scholar]
- 43.Holtmann G, Gschossmann J, Neufang-Huber J, Gerken G, Talley NJ. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut. 2000;47:332–336. doi: 10.1136/gut.47.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camilleri M, Busciglio I, Carlson P, et al. Candidate genes and sensory functions in health and irritable bowel syndrome. American journal of physiology. Gastrointestinal and liver physiology. 2008;295:G219–G225. doi: 10.1152/ajpgi.90202.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Aros S, Camilleri M, Cremonini F, Ferber I, Stephens D, Burton DD. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–1694. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.