Abstract
A rate-limiting factor in the ontogeny of auditory eyeblink conditioning (EBC) is the development of sensory inputs to the pontine nucleus. One possible way to facilitate the emergence of EBC would be to use a conditioned stimulus (CS) that activates an earlier-developing sensory system. The goal of the current study was to investigate whether using a vibration CS would facilitate the ontogeny of delay EBC relative to an auditory CS. Rat pups received six sessions of delay EBC or unpaired training using either a tone or vibration CS on postnatal day (P)14-15, 17-18, 21-22, or 24-25. Conditioning with a vibration CS resulted in rapid learning as early as P17-18, whereas conditioning with a tone CS did not result in rapid conditioning until after P17-18. Control experiments verified that the differences in EBC were due to CS-specific sensory properties. The results suggest that the ontogeny of EBC depends on sensory system development.
Keywords: development, rat pup, associative learning, eyelid conditioning, cerebellum
INTRODUCTION
Eyeblink conditioning (EBC), a type of Pavlovian conditioning, is a particularly useful paradigm for studying the development of learning and memory. Because the unconditioned response (UR) is relatively simple (i.e., eyelid closure), even very young animals are able to perform the response as well as adults. This limits the influence of response system development and allows for easier across-age comparisons. Furthermore, the vast amount of research concerning both the behavior and neural circuitry involved in adult EBC makes it an ideal paradigm for studying the ontogeny of EBC in young animals (Stanton, Freeman, & Skelton, 1992).
The majority of previous research using EBC in adult animals has used a tone or light as the conditioned stimulus (CS). Consequently, developmental work concerning EBC has also used auditory and visual CSs. Developmental studies using an auditory or visual CS suggest that delay eyeblink conditioning develops between P17 and P24 (Stanton et al., 1992; Paczkowski, Ivkovich, & Stanton, 1999). Pups trained on P17 have low levels of conditioned responding to auditory or visual CSs. However, by P24-26 pups demonstrated high levels of conditioned responding to both auditory and visual CSs.
The developmental trajectory of EBC is related to sensory neural pathway development and the development of learning-related plasticity in the cerebellum (Freeman, 2010). At very young ages, sensory pathway development is a potential contributor to the ontogeny of learning (Alberts, 1984; Gottlieb, 1971). Although infant rats are able to respond to some auditory stimuli by P9, the ear canal does not fully open until approximately P13, and adult levels of auditory discrimination are not reached until approximately P16 (Crowley & Hepp-Raymond, 1966). Likewise, the rat visual system is considered late-developing. Pups do not open their eyes until approximately P14 (Gramsbergen, Schwartze, & Prechtl, 1970). Because auditory and visual sensory system development is necessary to perceive a tone or light CS, the earliest conceivable age at which EBC could be observed is P13 and P14 for auditory and visual stimuli, respectively. However, auditory and visual conditioning emerge nearly a week after ear canal and eye opening occurs. Literature concerning the development of another Pavlovian paradigm, fear conditioning, demonstrates that the development of learning in a given modality follows the developmental emergence of sensory input from that modality. Specifically, although pups may be able to respond to a given sensory stimulus, conditioning with that same stimulus does not emerge until nearly five days later (Rudy & Hyson, 1984; Moye & Rudy, 1985; Rudy, 1993).
Previous work from our laboratory concerning the development of CS inputs to the cerebellum supports the hypothesis that sensory pathway development is a primary rate-limiting factor in the ontogeny of EBC (Freeman, 2010). The most proximal part of the CS pathway in EBC is the pontine nuclei and their mossy fiber projections to the cerebellum (Steinmetz et al., 1987; Halverson & Freeman, 2010). Neuronal activity in the pontine nuclei during auditory conditioning shows developmental changes in the amplitude of responding and learning-related activity between the ages of P17-18 and P24-25 (Freeman & Muckler, 2003). These data suggest that pontine activity may play a role in the development of EBC by affecting CS input to the cerebellum. If pontine input to the cerebellum in younger animals is weak, cerebellar neurons will undergo less learning-related plasticity (Freeman, 2010). Conversely, if pontine input to the cerebellum is increased, younger animals should have facilitated acquisition of EBC. By using a pontine stimulation CS, as opposed to a tone CS, we were able to bypass late-developing sensory systems and thereby facilitate acquisition of EBC (Freeman et al., 2005; Campolattaro & Freeman, 2008). In fact, pups trained at P12-13, which do not normally acquire EBC, showed high levels of conditioned responding when trained with pontine stimulation as a CS. Increased pontine input to the cerebellum, therefore, reversed developmental deficits in EBC. Because these data suggest that sensory system development is in fact largely responsible for the delayed development of eyeblink conditioning with tone and light CSs, employing a sensory modality that emerges earlier in ontogeny could increase pontine neuronal activity, and thus increase pontine input to the cerebellum and facilitate learning in younger animals.
In contrast to the auditory and visual systems, the rat somatosensory system develops prenatally (Narayanan, Fox, & Hamburger, 1971; Smotherman & Robinson, 1988). In fact, research using the somatosensory system in other learning paradigms has demonstrated that animals are able to learn at a much younger age than observed with auditory and visual stimuli. For example, in the case of Pavlovian fear conditioning, Caldwell and Werboff (1962) found that one-day-old infant rats were able to form an association between a vibrotactile stimulus applied to the chest region and a foot shock.
The goal of Experiment 1 was to investigate the development of EBC in rat pups using a vibration or tone CS. Pups were given paired or unpaired training on P14-15, 17-18, 21-22, 24-25. If pups are able to learn EBC with a vibration CS earlier ontogenetically than with a tone CS, it will further strengthen the hypothesis that the ontogeny of EBC is mediated by the development of sensory inputs to the pontine nuclei. Experiment 2 examined whether or not the sound of the vibration CS contributed to CRs during EBC. Experiment 3 examined extinction of EBC with a vibration CS.
GENERAL METHODS
Subjects
The subjects were Long-Evans rat pups born and reared in the colony in Spence Laboratories of Psychology at the University of Iowa. The colony was maintained on a 12/12-hr light/dark cycle, with light onset at 7 am. Male and female breeders were pair housed in polycarbonate cages with wire lids. Each day cages were checked for births, with the day of birth being designated P0. On P2 litters were culled to eight pups. Subjects remained in their home cage until P19, at which time they were transferred to separate cages with same-sex littermates. Experimental groups included no more than two animals from the same litter (one male and one female). All training occurred from 7 am to 7 pm. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Surgery
Surgery was performed two days prior to training, on P12, P15, P19, or P22 to allow one day for the pup to recover. Isoflurane (1.5-3%) was used for anesthesia. Prior to surgery the rat’s head was fixed in a mouse stereotaxic head holder and aligned. During surgery rats were fitted with differential EMG electrodes to record blink activity and a bipolar stimulating electrode for US delivery. Two EMG electrodes were threaded through the left upper eyelid and a ground wire was attached to the skull with a small stainless steel skull screw. All three wires (two recording and one ground) terminated in small gold pins that were secured in a plastic connector. The plastic connector was cemented to the skull using bone cement (Zimmer), leaving only the gold pins exposed. The bipolar stimulating electrode for US delivery was placed subdermally immediately caudal to the left eye. The bipolar electrode terminated in a small plastic connector which was cemented to the skull using bone cement.
Conditioning Apparatus
Rat pups were trained in a conditioning chamber that was contained within a sound-attenuation chamber. One side of the conditioning chamber was fitted with two small speakers, which delivered the tone CS (2 kHz, 82 dB). The floor of the chamber, which delivered the vibration CS, consisted of a custom-built vibrating grid floor placed on top of a piece of bench paper that protected a thin foam pad. The vibrating grid floor was made of square wire that was attached to a vibration motor (model number C1030B028F; JinLong Machinery, Zhejiang, China). When completely assembled and placed on the bench paper and foam pad in the chamber, the vibration CS vibrated at 144 Hz, with an acceleration of 2.4 m/s2. The foam pad placed under the grid floor ensured that the vibrating grid floor produced a minimum amount of sound. This method of stimulus delivery has been used in previous studies of learning in rat pups (Spear & Smith, 1978; Markiewicz et al., 1986). Lightweight cables with connectors for both the recording EMG and the bipolar stimulating electrode were attached to a commutator above the conditioning chamber and threaded through a hole in the ceiling of the chamber. Computer software controlled the delivery of both CS and US while simultaneously recording differential eyelid EMG activity (sampling rate = 250 Hz). All EMG activity was amplified (× 2000), filtered (500-5000 Hz), and integrated (20 ms time constant).
Conditioning Procedure
Conditioning occurred over the course of two days. All pups received 6 sessions (3 per d) of either paired or unpaired delay EBC training with either a tone or vibration CS. Pups in the paired group received 100 trials per session of delay EBC with a 400 ms vibration or tone CS and a 25 ms periorbital stimulation US. Each session was divided into 10 equal blocks of 10 trials, each with an intertrial interval of approximately 30s. The first 9 trials of each block were paired CS-US presentations and the 10th trial of each block was a CS-alone probe trial. The probe trials were used to evaluate conditioned responding (CR) in the absence of the UR (Gormezano et al. 1983). Pups in the unpaired group received 200 trials per session, 100 CS-alone trials and 100 US-alone trials. Each trial consisted of an unpaired presentation of either the CS or the stimulation US. Unpaired trials were separated by intertrial intervals of approximately 15 s.
Data Analysis
Behavioral data were examined offline. CRs were defined as any blink response during the CS that crossed a .4 unit threshold above the pre-CS baseline EMG activity. Any responses that occurred within 80 ms of CS onset were considered startle responses. Trials with EMG activity that crossed the threshold prior to the CS onset were omitted from the analysis. A repeated measures ANOVA was performed on session data related to CR percentage, amplitude, onset latency, and peak latency. CR amplitude, onset latency, and peak latency measures were examined on CS-alone trials in which a CR occurred. Because group sizes were approximately 7-8 pups, pup sex was not analyzed. Significant group effects were further analyzed with the Bonferroni Test. An alpha level of 0.05 was used for all statistical tests.
EXPERIMENT 1
The purpose of Experiment 1 was to determine if sensory system development plays a role in the ontogeny of EBC. If sensory system development is a rate-limiting factor in the ontogeny of EBC, then training with an earlier-developing sensory system, the somatosensory system, should result in an earlier onset of conditioning. Pups were trained with either a vibration or tone CS on P14-15, 17-18, 21-22, 24-25.
Methods
The subjects were 108 Long-Evans rats pups derived from 58 different litters. The experimental design included 2 conditions (paired or unpaired), 2 CS modalities (tone or vibration), and 3 age groups (P17-18, P21-22, or P24-25). For vibration training only, there was an additional group trained on P14-15.
Results
Regardless of CS type, pups that were trained in the paired condition showed significantly more CRs that those trained in the unpaired condition (Fig. 1). Furthermore, during paired training age-matched pups trained with the vibration CS showed far greater levels of conditioned responding than those trained with the tone CS (Fig. 1). When trained with the vibration CS, significant levels of conditioned responding were observed as early at P14-15. Importantly, these increased levels of responding were not due to non-associative factors; levels of unpaired responding did not significantly differ across CS type or age.
Figure 1.
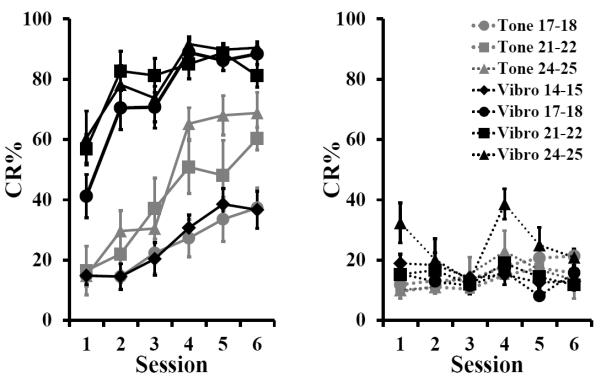
Mean (± SEM) eyeblink conditioned response (CR) percentage for rat pups given paired (left, solid lines) or unpaired (right, dashed lines) training with a tone (grey) or vibration (black) conditioned stimulus (CS). Pups were trained with the tone on postnatal days (P)17-18 (n = 8 paired, 8 unpaired), 21-22 (n = 7 paired, 9 unpaired), or P24-25 (n = 8 paired, 6 unpaired. Pups were trained with the vibration CS on P14-15 (n = 7 paired, 7 unpaired), P17-18 (n = 8 paired, 8 unpaired), P21-22 (n = 8 paired, 8 unpaired), or P24-25 (n = 8 paired, 8 unpaired).
A repeated measures ANOVA on the CR percentage data from all but the P14-15 age group confirmed these observations with an Age X CS-type X Condition (paired or unpaired) X Session interaction F(7.08, 290.36) = 2.592, p = 0.013 on CR percentage (Greenhouse-Geisser correction for sphericity). A repeated-measures ANOVA on the CR percentage for the vibration-trained pups only showed main effects of age F(10.53, 189.53) = 2.883, p < 0.001 and condition F(3.51, 189.53) = 27.595, p < 0.001 (Greenhouse-Geisser correction for sphericity).When data of paired, vibration-trained pups were further analyzed, post hoc tests indicated that the oldest three age groups trained with a vibration CS had a similar CR percentage (p > 0.05), but differed significantly from the youngest (P14-15) age group (p < 0.0001). Further comparisons confirmed that these differences were observed during all six training sessions (p < 0.01). When the data of paired tone-trained pups were further analyzed, post hoc tests examining the above-mentioned interaction indicated that the P24-25 and P17-18 groups had significantly different CR percentages on sessions 4-6 (p < 0.05). However, the P21-22 group performed intermediately and was not significantly different from either group.
In order to determine whether the youngest age group (P14-15) was able to learn the association between the vibration CS and the periorbital stimulation US, post hoc tests were performed on CR percentage between paired and unpaired groups across sessions. The paired vs. unpaired groups differed only on sessions 4-6 (p < 0.05.
The amplitude of the CR was also influenced by age, session, and condition (paired vs. unpaired)(Fig. 2). However, CS type did not influence response amplitude. There was an age- and session-related increase in CR amplitude during paired, but not unpaired training. A repeated measures ANOVA on all but the P14-15 age group (with Greenhouse-Geisser correction for sphericity) indicated a Session X Age X Condition (paired or unpaired) interaction F(6.86, 219.63)=2.09, p = 0.047. Post hoc tests on paired data indicate that there was an age-related increase in CR amplitude across sessions with both the P21-22 and P24-25 age groups having higher amplitude CRs than the P17-18 age group (p < 0.05). A repeated measures ANOVA on the vibration-trained pups indicated a Session X Age X Condition (paired or unpaired) interaction F(9.85,144.49) = 2.49, p = 0.009. Post hoc tests on the paired data from the vibration groups confirmed that there was an age-related increase in CR amplitude. The P14-15 group was significantly lower than the P21-22 and P24-25 groups and the P17-18 group was significantly lower than the P24-25 group.
Figure 2.
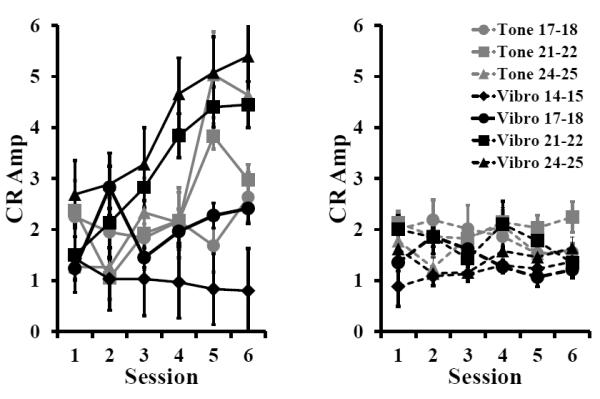
Mean (± SEM) eyeblink conditioned response (CR) amplitude for rat pups given paired (left, solid lines) or unpaired (right, dashed lines) training with a tone (grey) or vibration (black) conditioned stimulus (CS).
The UR amplitude, CR onset, and peak CR latencies did not differ between CS modalities or across age groups. Mean (+/- SD) session 1 UR amplitudes are as follows for vibration- and tone-trained pups, respectively: P14-15: 1.59(.78), P17-18: 1.94(.45), 2.6(1.08), P21-22: 3.34(1.02), 3.21(1.44), P24-25: 3.21(1.43), 2.87(1.07).
EXPERIMENT 2
In order to determine whether or not the sound of the vibration CS motor influenced CR production, we performed a control experiment. The goal of Experiment 2 was to determine whether the sound of the vibration CS had an impact on the CR. If the sound of the vibration CS contributed to the previously-observed level of conditioned responding, its presentation alone should also elicit a CR.
Methods
Subjects were 7 pups from 4 different litters trained on P17-18. Because Experiment 1 found robust and nearly identical learning at all but the youngest age group trained with a vibration CS, this experiment only utilized the P17-18 age group. The conditioning apparatus and methodology were identical to that described in Experiment 1 for CS-US acquisition sessions 1-5. However, during session 6 the vibration motor was suspended from the ceiling of the chamber by a lightweight cable where it could not vibrate the cage.
Results
As observed in Experiment 1, pups in the P17-18 age group readily showed CRs when trained with a vibration CS. Furthermore, their levels of CRs did not appear to be influenced by the sound of the vibration motor (Fig. 3). During session 6, when the sound of the CS was presented alone, pups showed an immediate drop in CRs (block 1 mean = 38.89 and blocks1-10 mean = 26.11). A repeated measures ANOVA across all six sessions confirmed these results F(1.87, 11.22)=29.72, p < 0.0001 (Greenhouse-Geisser correction for sphericity). Post hoc tests showed that there was a significant difference between the level of CRs on sessions 5 and 6 (p = 0.001), but not between the level of CRs on sessions 1 and 6 (p > 0.05).
Figure 3.
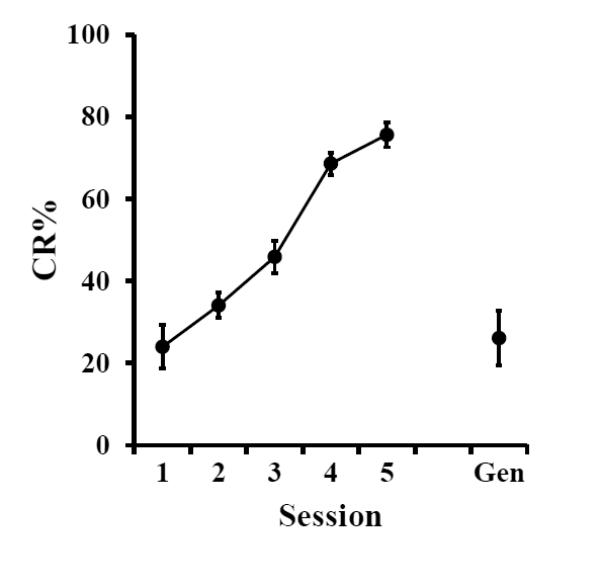
Mean (± SEM) eyeblink conditioned response (CR) percentage for rat pups given paired training with a vibration conditioned stimulus (CS) on P17-18 (sessions 1-5) followed by a CS-alone generalization test (Gen) for the sound of the vibration device.
EXPERIMENT 3
The goal of Experiment 3 was to investigate whether pups trained on P17-18 with a vibration CS were able to show extinction. Previous research in auditory EBC with this age group has yielded low levels of conditioned responding (Stanton, Freeman, & Skelton, 1992). Therefore, to date, there have not been any studies examining extinction in this age group. Assessing extinction with the vibration CS further assesses whether conditioning with a somatosensory CS has similar properties to conditioning with a visual or auditory CS.
Methods
Subjects were 8 pups from 5 different litters. Pups received 6 CS-US acquisition training sessions on P17-18, as in Experiment 1. The 2 CS-alone extinction sessions took place the following day, on P19.
Results
As in Experiments 1 and 2 P17-18 pups showed rapid acquisition of EBC using a vibration CS. However, this level of responding dropped off substantially during extinction training session 7 (Fig. 4). Overall, both extinction sessions showed similar levels of CRs. However, when examining block data within the two sessions, it became evident that session 7 and 8 had very different session profiles. Responding during session 7 started out high but dropped rapidly until halfway through the session, at which point responding leveled out. The profile of session 8 was substantially flatter, dropping only slightly across blocks. These observations were confirmed with a repeated-measures ANOVA across all 8 sessions F(7, 49) = 13.22, p < 0.0001 and post hoc tests indicated that there was a significant difference between the final session of acquisition training and the first session of extinction training (p = 0.003). A repeated-measures ANOVA across the block data of session 7, the first extinction session, showed a significant effect of training block F(9, 63) = 3.46, p = 0.002. Post hoc tests examining block data changes across session 7 showed that blocks 4-7 and 9-10 were significantly different from block 1.
Figure 4.
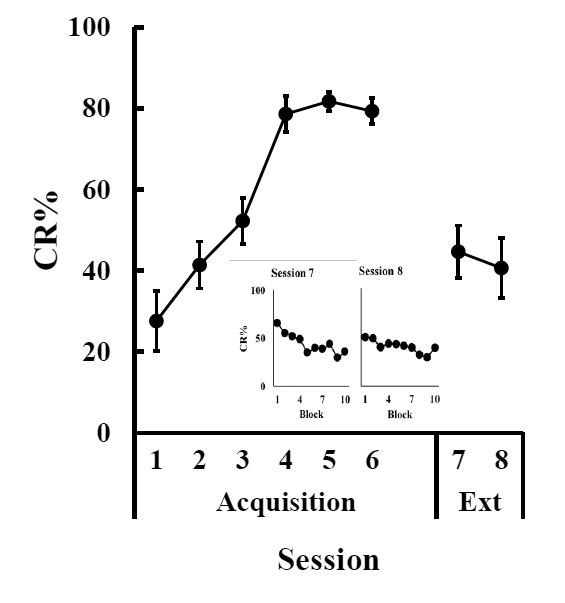
Mean (± SEM) eyeblink conditioned response (CR) percentage for rat pups given paired training with a vibration conditioned stimulus (CS) on P17-18 (Acquisition) followed by 2 CS-alone extinction sessions (Ext). Graph insert shows block data for sessions 7 and 8, the extinction sessions.
DISCUSSION
Eyeblink conditioning was faster and ontogenetically earlier with a vibration CS when compared to EBC with a tone CS. When trained with a tone CS, pups showed only modest levels of learning when trained on P17-18, a finding consistent with previous studies of the developmental trajectories of auditory and visual EBC (Stanton, Freeman, & Skelton, 1992; Paczkowski, Ivkovich, & Stanton, 1999). In contrast, training with a vibration CS resulted in robust learning by P17-18. In fact, pups as young as P14-15 showed low levels of EBC when trained with a vibration CS. This developmental increase in CRs does not appear to be the result of increased non-associative responding to the CS. Data from similar studies investigating the development of delay EBC with auditory and visual CSs show nearly identical levels of non-associative responding to those observed during unpaired presentation of the vibration CS (Ivkovich, Paczkowski, & Stanton, 2000). Our interpretation of the relatively early development of EBC with the vibration CS is that vibration is more salient than tones, in terms of the strength of neural inputs, to the cerebellum in younger pups. This increased neural salience for the vibration CS is hypothesized to be due to early development of the subcortical somatosensory system projections to the cerebellum.
The auditory CS pathway includes projections from the cochlear nucleus, superior olive, nucleus of the lateral lemniscus, and inferior colliculus to the medial auditory thalamus, directly and in series (Freeman and Steinmetz, 2011). Medial auditory thalamic neurons project to the lateral pontine nuclei which then project to the cerebellar cortex and deep nuclei (Campolattaro et al., 2007; Halverson et al., 2008; Halverson et al., 2010; Halverson & Freeman 2010). Development of auditory inputs to the lateral pontine nucleus plays a major role in the ontogeny of auditory eyeblink conditioning. Electrical stimulation of the pontine nuclei as a CS results in much faster conditioning in P17 pups, almost as fast as pups trained on P24 with a stimulation or tone CS (Freeman et al., 2005). This finding has been extended to pups trained with pontine stimulation as the CS on P12, also resulting in robust associative learning (Campolattaro & Freeman, 2008). These pontine stimulation studies demonstrate that the cerebellum is capable of eyeblink conditioning as early as P12 if it receives sufficient sensory input (Freeman, 2010). However, recordings of pontine neuronal activity in rat pups show a substantial developmental increase in sensory responses to a tone CS between P17 and P24 suggesting that auditory inputs to the pontine nuclei are continuing to develop past P17 (Freeman & Muckler, 2003). Furthermore, stimulation of the cochlear nucleus or medial auditory thalamus as the CS does not result in earlier learning in rat pups, which indicates that there are developmental changes in the auditory pathway projecting to the lateral pontine nuclei (Freeman & Campolattaro, 2008; Freeman & Duffel, 2008). Neuronal recordings from the medial auditory thalamus indicate that the thalamus, like the pontine nuclei, show substantial developmental changes in responsiveness to a tone CS and less learning-related activity (Ng & Freeman, 2012). In sum, these findings concerning the auditory pathway indicate that there are developmental changes in the strength of sensory information upstream of the pontine nuclei.
The neural pathway for a floor vibration CS has not been identified but probably includes tactile, vestibular, and proprioceptive afferent projections to the cerebellum. Vibration stimulation in the body activates spinal afferents that project to the dorsal column nuclei. The dorsal column nuclei then project to the medial pontine nucleus (Kosinski et al. 1986a; Kosinski et al. 1986b). Previous studies in rabbits and ferrets have shown that eyeblink conditioning using stimulation of the body with vibration or weak electrical shocks depends on the middle cerebellar peduncle (Lewis et al. 1987; Hesslow et al. 1999). Thus, although there are direct projections from the dorsal column nuclei to the cerebellum through the inferior cerebellar peduncle (Bengtsson and Jorntell 2009), the most likely CS pathway for a vibration CS is the dorsal column nuclear projection to the pontine nuclei. In contrast to the auditory and visual CS pathways (Halverson & Freeman, 2010a; 2010b), there do not appear to be direct projections from somatosensory thalamus to the pontine nuclei. In fact, the steep acquisition curve for somatosensory EBC in P17-18, P21-22, and P24-25 pups strongly resembles acquisition curves seen in pups that receive pontine stimulation as a CS (Freeman, Rabinak, & Campolattaro, 2005; Campolattaro & Freeman, 2008). Thus, the dorsal column projections to the cerebellum via the pontine nuclei appear to be mature by P17, whereas the auditory projections to the pontine nuclei are not mature until P24 or so.
Previous studies demonstrated associative learning with a vibration CS and an electrical stimulation US in neonatal rat pups (Caldwell & Werboff, 1962; Bachevalier & Blozovski, 1980). Conditioned limb flexion was measured in these studies of neonatal conditioning, which like EBC, depends on the cerebellum (Voneida, 2000; Mojtahedian et al., 2007). The findings of these limb flexion studies are consistent with the early EBC in the current study with a vibration CS. Neonatal rats showed only modest levels of conditioning (15-32%) in the previous studies relative to adult rats, comparable to the P14-15 group in the current study. A similar pattern of results was found by Schreurs et al. (2013) using a shock-shock (US-US) conditioning paradigm in rat pups. Somatosensory inputs to the cerebellum from the dorsal column nuclei may therefore continue to develop postnatally until P17, even though rat pups are clearly responsive to tactile stimuli prior to birth (Smotherman & Robinson, 1988). It is also possible that US pathway development (Nicholson & Freeman, 2003) plays a significant role in the early development of cerebellar learning, i.e., before P17.
In conclusion, this study demonstrated that using a vibration CS results in facilitated acquisition of delay EBC in rat pups relative to EBC with an auditory or visual CS. Pups are able to learn faster and at earlier ages than observed previously with other peripheral CSs. Furthermore, non-associative responding (as seen during unpaired training) does not differ from previously reported data using other CS modalities. The findings of the current study support the hypothesis that the ontogeny of cerebellar learning depends on the development of sensory systems and their inputs to the pontine nuclei.
Acknowledgments
This work was supported by National Institutes of Health grant NS038890 to J.H.F.
REFERENCES
- Alberts JR. Sensory-perceptual development in the Norway rat: a view toward comparative studies. In: Kail RE, Spear NE, editors. Comparative perspectives on memory development. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1984. pp. 65–101. [Google Scholar]
- Bachevalier J, Blozovski D. Acquisition and retention of classical conditioning in the newborn rat. Developmental Psychobiology. 1980;13:519–526. doi: 10.1002/dev.420130511. [DOI] [PubMed] [Google Scholar]
- Bengtsson F, Jorntell H. Sensory transmission in cerebellar granule cells relies on similarly coded mossy fiber inputs. Proceedings of the National Academy of Sciences (USA) 2009;106:2389–2394. doi: 10.1073/pnas.0808428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell DF, Werboff J. Classical conditioning in newborn rats. Science. 1962;136:1118–1119. doi: 10.1126/science.136.3522.1118. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Eyeblink conditioning in 12-day-old rats using pontine stimulation as the conditioned stimulus. Proceedings of the National Academy of Sciences (USA) 2008;105:8120–8123. doi: 10.1073/pnas.0712006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolattaro MM, Halverson HE, Freeman JH. Medial auditory thalamic stimulation as a conditioned stimulus for eyeblink conditioning in rats. Learning & Memory. 2007;14:152–159. doi: 10.1101/lm.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley DE, Hepp-Reymond MC. Development of cochlear function in the ear of the infant rat. Journal of Comparative and Physiological Psychology. 1966;62:427–432. [Google Scholar]
- Freeman JH, Muckler AS. Developmental changes in eyeblink conditioning and neuronal activity in the pontine nuclei. Learning and Memory. 2003;10:337–345. doi: 10.1101/lm.63703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Campolattaro MM. Ontogenetic change in the auditory conditioned stimulus pathway for eyeblink conditioning. Learning & Memory. 2008;15:823–828. doi: 10.1101/lm.1131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Duffel JW. Eyeblink conditioning using cochlear nucleus stimulation as a conditioned stimulus in developing rats. Developmental Psychobiology. 2008;50:640–646. doi: 10.1002/dev.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learning & Memory. 2011;18:666–677. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learning & Memory. 2005;12:255–259. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH. Developmental neurobiology of cerebellar learning. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. Oxford University Press; New York, NY: 2010. pp. 546–572. [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning with the rabbit. Progress in Psychobiology and Physiological Psychology. 1983;10:197–275. [Google Scholar]
- Gottlieb G. Ontogenesis of sensory function in birds and mammals. In: Toback E, Aronson LR, Shaw E, editors. The biopsychology of development. Academic Press; New York: 1971. pp. 67–128. [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HF. The postnatal development of behavioral states in the rat. Developmental Psychobiology. 1970;3:267–80. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic input to the lateral pontine nuclei is necessary for auditory eyeblink conditioning. Neurobiology of Learning and Memory. 2010;93:92–98. doi: 10.1016/j.nlm.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Ventral lateral geniculate input to the medial pons is necessary for visual eyeblink conditioning in rats. Learning & Memory. 2010;17:80–85. doi: 10.1101/lm.1572710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Lee I, Freeman JH. Associative plasticity in the medial auditory thalamus and cerebellar interpositus nucleus during eyeblink conditioning. The Journal of Neuroscience. 2010;30:8787–8796. doi: 10.1523/JNEUROSCI.0208-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Poremba A, Freeman JH. Medial auditory thalamus inactivation prevents acquisition and retention of eyeblink conditioning. Learning & Memory. 2008;15:532–538. doi: 10.1101/lm.1002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiology of Learning and Memory. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- Ivkovich D, Paczkowski CM, Stanton ME. Ontogeny of delay versus trace eyeblink conditioning in the rat. Developmental Psychobiology. 2000;36:148–160. doi: 10.1002/(sici)1098-2302(200003)36:2<148::aid-dev6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kosinski RJ, Azizi SA, Border BG, Mihailoff GA. Origin and ultrastructural identification of dorsal column nuclear synaptic terminals in the basilar pontine gray of rats. Journal of Comparative Neurology. 1986a;253:92–104. doi: 10.1002/cne.902530108. [DOI] [PubMed] [Google Scholar]
- Kosinski RJ, Neafsey EJ, Castro AJ. A comparative topographical analysis of dorsal column nuclear and cerebral cortical projections to the basilar pontine gray in rats. Journal of Comparative Neurology. 1986b;244:163–173. doi: 10.1002/cne.902440204. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Lo Turco JJ, Solomon PR. Lesions of the middle cerebellar peduncle disrupt acquisition and retention of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1987;101:151–157. doi: 10.1037//0735-7044.101.2.151. [DOI] [PubMed] [Google Scholar]
- Markiewicz B, Kucharski D, Spear NE. Ontogenetic comparison of memory for Pavlovian conditioned aversions to temperature, vibration, odor, or brightness. Developmental Psychobiology. 1986;19:139–54. doi: 10.1002/dev.420190206. [DOI] [PubMed] [Google Scholar]
- Mojtahedian S, Kogan DR, Kanzawa SA, Thompson RF, Lavond DG. Dissociaton of conditioned eye and limb responses in the cerebellar interpositus. Physiology & Behavior. 2007;91:9–14. doi: 10.1016/j.physbeh.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of learning: VI. learned and unlearned responses to visual stimulation in the infant hooded rat. Developmental Psychobiology. 1985;18:395–409. doi: 10.1002/dev.420180505. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Fox MW, Hamburger V. Prenatal development of spontaneous and evoked activity in the rat (rattus norvegicus albinus) Behaviour. 1971;40:100–134. doi: 10.1163/156853971x00357. [DOI] [PubMed] [Google Scholar]
- Ng KH, Freeman JH. Developmental changes in medial auditory thalamic contributions to associative motor learning. The Journal of Neuroscience. 2012;32:6841–6850. doi: 10.1523/JNEUROSCI.0284-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr. Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nature Neuroscience. 2003;6:532–537. doi: 10.1038/nn1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowski C, Ivkovich D, Stanton ME. Ontogeny of eyeblink conditioning using a visual conditional stimulus. Developmental Psychobiology. 1999;35:253–263. doi: 10.1002/(sici)1098-2302(199912)35:4<253::aid-dev1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Contextual conditioning and auditory cue conditioning dissociate during development. Behavioral Neuroscience. 1993;107:887–891. doi: 10.1037//0735-7044.107.5.887. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Hyson RL. Ontogenesis of learning: III. Variation in the rat's differential reflexive and learned responses to sound frequencies. Developmental Psychobiology. 1984;17:285–300. doi: 10.1002/dev.420170308. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behavioral Neuroscience. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Spear NE, Smith GJ. Alleviation of forgetting in preweanling rats. Developmental Psychobiolgy. 1978;11:513–29. doi: 10.1002/dev.420110602. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Voneida TJ. The effect of brachium conjunctivum transection on a conditioned limb response in the cat. Behavioural Brain Research. 2000;109:167–175. doi: 10.1016/s0166-4328(99)00169-2. [DOI] [PubMed] [Google Scholar]


