Abstract
Resistance to endocrine therapy is a major impediment to successful treatment of breast cancer. Preclinical and clinical evidence links resistance to anti-estrogen drugs in breast cancer cells with the overexpression and/or activation of various pro-oncogenic tyrosine kinases. Disruption of circadian rhythms by night shift work or disturbed sleep-wake cycles may lead to an increased risk of breast cancer and other diseases. Moreover, light exposure at night (LEN) suppresses the nocturnal production of melatonin that inhibits breast cancer growth. In this study, we used a rat model of ERα+ MCF-7 tumor xenografts to demonstrate how altering light/dark cycles with dim LEN (dLEN) speeds the development of breast tumors, increasing their metabolism and growth and conferring an intrinsic resistance to tamoxifen therapy. These characters were not produced in animals where circadian rhythms were not disrupted, or in animals subjected to dLEN if they received nocturnal melatonin replacement. Strikingly, our results also showed that melatonin acted both as a tumor metabolic inhibitor and a circadian-regulated kinase inhibitor to re-establish the sensitivity of breast tumors to tamoxifen and tumor regression. Together, our findings show how dLEN-mediated disturbances in nocturnal melatonin production can render tumors insensitive to tamoxifen.
Keywords: Melatonin, Tamoxifen, Circadian, Breast, Warburg
Introduction
Approximately 60-75% of breast cancers express estrogen receptor alpha (ERα) and/or progesterone receptor (PR), which are markers and determinants for the use of endocrine therapies including selective ERα modulators such as tamoxifen (TAM) (1, 2), pure anti-estrogens, and aromatase inhibitors (3-5). However, the development of resistance to TAM and other endocrine therapies has become a major impediment in endocrine therapy such that anywhere from 30% to 50% of patients with ERα-positive breast tumors display intrinsic resistance while most patients are initially responsive but will eventually develop acquired resistance to TAM (6).
Compelling data has emerged from breast tumor biopsies and in vitro studies indicating that elevated expression and signaling of receptor tyrosine kinases, including members of the epidermal growth factor receptor (EGFR) family and downstream mitogen-activated protein kinase/extracellular signal-related kinase (MAPK/ERK) and phosphatidylinositol 3-kinase/protein B (PI3K/AKT), can drive TAM-resistance through phosphorylation of either ERα at Ser167 or Ser118 to increase DNA-binding or coregulator binding (7-12) to regulate cell proliferation and apoptosis. Additionally, other signaling pathways are elevated or activated in TAM-resistant breast tumors including SRC, focal adhesion kinase (FAK), signal transducer and activator of transcription 3 (STAT3), and nuclear factor-kappa B (13, 14).
Numerous, studies have shown that the circadian melatonin signal regulates signaling and metabolic activities to inhibit breast cancer initiation, promotion, and progression (15-17). Based on early studies showing the anti-cancer actions of melatonin in breast cancer Stevens (18) hypothesized that suppression of nighttime melatonin production by the pineal gland by light at night might explain the rise in breast cancer rates that have accompanied industrialization and electrification in the United States and other westernized countries. Light exposure at night (LEN) is a well-recognized environmental disruptor of the central circadian timing system located in the suprachiasmatic nucleus (SCN) of the brain (23). Nighttime production of melatonin by the pineal gland represents a highly reliable circadian output signal of the circadian clock whose suppression by LEN is intensity-, duration-, and wavelength-dependent (21, 22). These and other data led the World Health Organization to designate night shift work involving LEN-induced circadian/melatonin disruption as a probable carcinogen (class 2a) and risk factor for the development of breast cancer (23). Furthermore, employing our novel tissue-isolated MCF-7 human breast cancer xenograft model in circadian/melatonin intact female nude rats, we also report increases in tumor growth rates and enhanced ERK, AKT, and AKT stimulatory 3-phosphoinositide-dependent kinase-1 (PDK1) activity (24-27), and repression of GSK3β activity in breast tumor xenografts in response to light exposure during the night or day and that these changes are blocked by melatonin (28).
The above studies, in addition to those demonstrating that physiological nocturnal concentrations of melatonin significantly increase the sensitivity of ERα+ MCF-7 human breast cancer cells to TAM in vitro (29), prompted us to postulate that dim light exposure at night (dLEN), by virtue of its ability to suppress nocturnal melatonin production, will promote partial or complete resistance to TAM therapy in vivo. In the present study, we demonstrate for the first time that exposure to dLEN, via suppression of the nighttime melatonin signal, induces tumor progression and intrinsic resistance to TAM in human breast tumor xenografts. Furthermore, the maintenance of the endogenous melatonin signal or its replacement with exogenous melatonin under dLEN conditions preserves TAM-sensitivity and drives tumor regression.
Materials and methods
Chemicals and reagents
All chemicals and tissue culture reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell culture medium, RPMI 1640 and fetal bovine serum (FBS) were purchased from Invitrogen Corporation (Carlsbad, CA). High-performance liquid chromatography (HPLC)-grade reagents were purchased from Fisher Chemical (Pittsburgh, PA). Free fatty acid, cholesterol ester, triglyceride, phospholipid, rapeseed oil methyl ester standards, as well as boron trifluoride-methanol, potassium chloride, sodium chloride, perchloric and trichloroacetic acids were purchased from Sigma-Aldrich (St. Louis, MO). The HPLC standards, (+/−) 5-HETE and 13(S)-HODE) were purchased from Cayman Chemical Co. (Ann Arbor, MI).
Cell line and cell culture
The ERα−/PR-positive, TAM-sensitive MCF-7 human breast cancer cell line (passage numbers 18-20) used in these studies was obtained from American Tissue Culture Collection (Manassas, VA). These cells were tested and authenticated by ATCC and immediately expanded, and frozen-down as stock for future studies. Cells from low passage frozen stocks (passage numbers 18-20) were used in these studies. Briefly, cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 50 mM minimum essential medium non-essential amino acids, 1 mM sodium pyruvate, 2 mM glutamine, 10 mM basal medium eagle, 100 mg/mL streptomycin, and 100 U/mL penicillin and maintained at 37° C in a humidified atmosphere of 5% CO2 and 95% air.
Animals, housing conditions, and diet
Female athymic, inbred nude rats (Hsd:RH-Foxn1rnu), 1-2 weeks of age, used in this study were purchased from Harlan Laboratories (Indianapolis, IN). Upon arrival, animals were maintained in environmentally controlled rooms (25° C; 50-55% humidity) with controlled diurnal lighting schedule of 12 h light:12 h dark at subjective night (LD 12:12) [304 lux; 123 μW/cm2; lights on, 0600 hr and off at 1800 hr). Animal rooms were completely devoid of light contamination during the dark phase (30). One week prior to tumor implantation two-thirds of the animals were switched to a 12 h light:12 h dim light at night, subjective night (dLEN) cycle [0.2 lux, with lights on at 0600 hr and off at 1800 hr, and dLEN on at 1600 hr and off at 0600 hr) in an AAALAC-accredited facility and in accordance with The Guide. Animals were given free access to food (Purina 5053 Irradiated Laboratory Rodent Diet, Richmond, IN), and acidified water as previously described (30-32). All procedures employed for animal studies were approved by the Tulane University Institutional Animal Care and Use Committee.
Arterial blood collection
Diurnal plasma melatonin levels (pg/mL; mean ± 1 SD) of naïve, female nude rats (n = 12) maintained initially in the control LD 12:12 cycle (304 lux; 123 μW/cm2; lights on, 0600 hr) or in the dLEN cycle were measured as previously described (47, 48). In experimental animals during the course of these studies blood was collected over time at six circadian time points (0400-, 0800-, 1200-, 1600-, 2000, and 2400-hr) for the measurement of levels of melatonin, total fatty acids, glucose, and lactic acid (1, 24, 26, 32).
MCF-7 tumor xenografts development in nude mice
Ovariectomized athymic nude mice (4–5 week old females) were obtained from Charles River (Indianapolis, IN) and maintained in pathogen-free aseptic conditions with phytoestrogen-free food and water ad libitum. All mice were supplemented with estrogen pellets (0.72 mg of 17β-estradiol 60-day release from Innovative Research of America (Sarasota, FL) and estradiol-dependent MCF-7 xenografts were propagated. Exponentially growing MCF-7 cells (Passage numbers 18-20) were harvested and approximately 5×106 MCF-7 cells in 150 μl of PBS-matrigel mixture were orthotopically and bilaterally implanted into the mammary fat pads of female nude mice, as previously described (33).
Tumor transplantation into athymic nude rats
After one week of photoperiod acclamation (LD 12:12 or dLEN for Study I: dLEN or dLEN + nighttime melatonin supplementation for Study II), nude rats were implanted in a tissue-isolated fashion with (ER+) MCF-7 human tumor xenografts obtained from the tumor xenografts initially developed in mice, as described previously (24, 26, 32). Once implanted tissue-isolated tumor xenografts reached a palpable size (approximately pea size) in the nude rats, tumors were measured every day for estimated tumor weights, as described previously (24, 27, 32).
Tumor growth studies
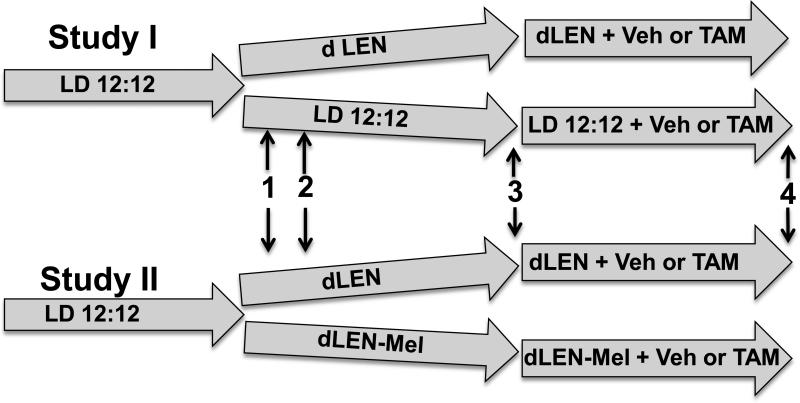
Two separate approaches to evaluate the effects of dLEN and melatonin on the responsiveness of breast tumor xenografts to 4OH-TAM were employed as shown in figure 1. In Study I, tissue-isolated (ERα+) MCF-7 human breast tumor xenografts were implanted into nude rats maintained in a control LD 12:12 (elevated endogenous nocturnal melatonin) lighting schedule or in a dLEN (repressed nocturnal melatonin) schedule. In Study II, all nude rats were maintained in a dLEN schedule with or without melatonin supplementation at night. In each study once tumors reached an estimated weight of 2.5 g, rats were treated with 4OH-TAM (80ug/kg/day) or vehicle until tumors regressed to approximately 1.5 g, until they grew to an estimated weight of 8 g, or until 40 days post implantation. At the appropriate times tumors were freeze clamped with liquid nitrogen and stored at -80° C until they were used for analyses.
Figure 1. Experimental design.
In Study I (upper track), upon arrival rats were acclimated in a standard LD 12:12 lighting schedule for one week after which half were switched to either dLEN or kept on LD 12:12 lighting cycles. (1) After one week of acclamation to appropriate lighting cycles arterial blood was collected at circadian time points and measured for melatonin. (2) Tissue-isolated breast tumor xenografts were implanted into rats housed in LD 12:12 or dLEN lighting schedules. (3) Administration of vehicle or 4OH-TAM was initiated as tumors in each lighting schedule reached an estimated weight of 2.5 g. (4) As tumors reached 8 g (estimated weight), regressed to half their weight (approximately 1.5 g), or reached 40 days post tumor implantation, arterial and venous blood were collected for tumor metabolic analysis and tumors were harvested and snap frozen in liquid nitrogen for protein analysis. In Study II (lower track) rats were housed in LD12:12 and (1) half were transferred to dLEN lighting cycle and the other half to a dLEN lighting cycle but supplemented with nighttime melatonin and the study conducted as described above for Study I.
Study I
When tumor weights reached approximately 2.5 g estimated weight, one-half the animals (n = 3/group) maintained in either the dLEN lighting or LD 12:12 lighting environments were treated daily at 1600 hr with 0.1 ml of 4OH-TAM (Sigma-Aldrich, St. Louis, MO.) solution (80 μg/Kg/day) via i.p. Injection. The second group of animals were maintained in these lighting environments (n = 3/group) and injected with vehicle. Thus, there were four experimental groups in this study: Group 1 (dLEN Controls); Group II (dLEN treated with TAM); Group III (LD 12:12, controls); and Group IV (LD 12:12 treated with TAM).
Study II
All animals were maintained in the dLEN lighting schedule and were randomized so that half received a vehicle at nighttime and the other half received a supplement of melatonin in their nighttime drinking water (0.1 μg/ml) such that they received approximately 2.5 μg melatonin daily intake, based on a daily water intake of about 25 ml, which simulates the high normal nighttime physiologic levels of melatonin. When tumor weights reached approximately 2.5 g estimated weight, one half the animals (n = 3/group) maintained in either the dLEN lighting or dLEN supplemented with melatonin were treated daily at 1600 hr with either 0.1 ml of 4OH-TAM (80 μg/Kg/day) or diluent by i.p. injection. Thus this study consisted of 4 groups: Group I (dLEN treated with vehicle); Group II (dLEN treated with TAM); Group III (dLEN supplemented with melatonin and treated with vehicle); and Group IV (dLEN supplemented with melatonin and treated with TAM).
A-V tumor measurements
When estimated tumor weights reached an estimated weight of 8 g, (Groups I, II, III), were out 40 days past implantation, or regressed to 1.6 g (Group IV) following TAM-administration, tumors were prepared for in situ tumor vein cannulation (32). Experiments were conducted between 2400 and 0400 hr following a normal nocturnal feeding period. Animal preparation, including anesthesia administration and blood sample collection was described previously (24, 27, 32). Analysis of arterial glucose, lactate, acid/gas, fatty acids, and melatonin were conducted as previously described (30, 31).
Tumor lysate extraction and Western blot analysis
Frozen tumors were pulverized and homogenized in RIPA buffer (1 × PBS, 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1% SDS) containing a protease inhibitor cocktail (Sigma Scientific) and phosphatase inhibitor cocktails. Total tumor protein was isolated from tumor lysates as previously described (28) and aliquots stored at −80° C. One hundred twenty micrograms of protein from each sample was separated on a criterionTM precast gel (Bio-Rad, Richmond, CA) and transferred onto nitrocellulose membranes (Bio-Rad). After incubation with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween 20, immunoblots were probed with various antibodies including cleaved caspase 3, phospho- (p)-ERK1/2 Thr202/Tyr204, total (t)-ERK1/2, p-AKT Ser473, t-AKT, p-NF-kB Ser536, t-NF-KB, p-SRC Tyr416, t-SRC, p-FAK Tyr576/577, t-FAK, p-CREB Ser133, t-CREB, p-STAT3 Tyr707 and t-STAT3, and LC3BI and LC3BII from Cell Signaling (Danvers, MA). For analysis of p-ERα, antibodies for p-ERα Ser118 and Ser167 (Bethyl Laboratories Inc., Montgomery, TX), and l- α (Novocastra Laboratories, Newcastle, UK) were employed. The blots were stripped and re-probed with anti-β-actin antibody (Sigma, St. Louis, MO) to evaluate loading. Quantitation of Western blots and differences in expression of total and phosphorylated proteins were determined by digital quantitation of phosphorylated and total protein levels and normalizing phosphorylated levels to the levels of the total protein of interest and comparing the levels of TAM treated, LD 12:12, dLEN + melatonin in the presence or absence of TAM, to the dLEN control to determine the percent or fold change.
Statistical analysis
Data are represented as the mean ± standard error of the mean. Statistical differences between mean values in the LEN-exposed group versus the control group at circadian time points were assessed by the Student's t test. Statistical differences among group means in tumor perfusion studies were determined by a one-way ANOVA followed by Bonferroni's multiple comparison test. Differences in the tumor growth rates among groups were determined by regression analyses and tests for parallelism (Student's t test). Differences were considered to be statistically significant at p < 0.05.
Results
Study I
Plasma melatonin levels
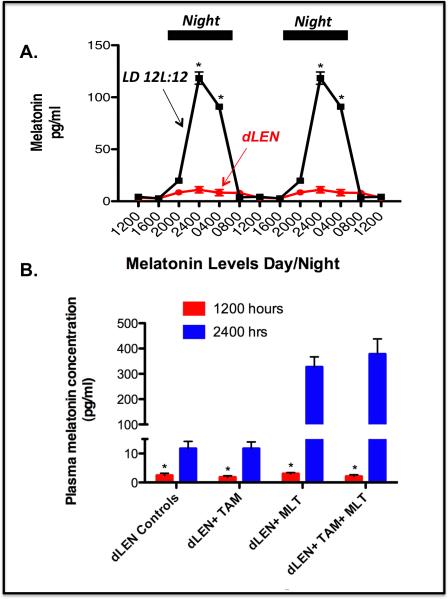
Figure 2A shows that under LD 12:12 conditions, prior to TAM treatment, plasma melatonin levels increased and remained high during the dark phase reaching a peak at 2400 hr that was more than 70-fold higher than during the light phase. In rats under dLEN lighting schedule, melatonin levels remained consistently low to undetectable throughout the 24 h period (Fig. 2B).
Figure 2.
Effect of dLEN versus LD 12:12 lighting schedules or administration of exogenous melatonin in the dLEN lighting schedule on the serum melatonin profile in female nude rats. Female nude rats with (ERα+) tissue-isolated breast tumor xenografts were housed under control (LD 12:12) or experimental, dLEN (with light at 0.2 lux) lighting schedules, or dLEN and supplemented with nighttime melatonin (MLT), and treated with diluent or 4OH-tamoxifen (TAM). (A) Diurnal plasma melatonin levels (pg/ml; mean ± 1 SD) of female nude rats maintained in a controlled LD 12:12 or experimental (dLEN) lighting cycle (n=12/group) were measured as described in “Materials and Methods”. Data are double-plotted to better visualize rhythmicity (n = 12/group). Significant differences (p < 0.05) in serum melatonin levels in rats under the different lighting schedules are denoted by an asterisk (*). (B) Plasma melatonin levels from Study II at 1200 hr (red bars) and 2400 hh (blue bars) from animals maintained in dLEN and treated with vehicle (dLEN Controls) or TAM (dLEN + TAM), or from animals on dLEN but supplemented with melatonin (in nighttime drinking water) and treated with vehicle (dLEN+TAM) or TAM (dLEN+TAM+MLT), as described in “Materials and Methods”. Significant differences (n=3/group, p < 0.05) in serum melatonin levels in rats under the different lighting schedules are denoted by an asterisk (*).
dLEN promotes tumor growth and intrinsic resistance to 4OH-Tamoxifen in (ER+) MCF-7 tissue-isolated breast tumor xenografts
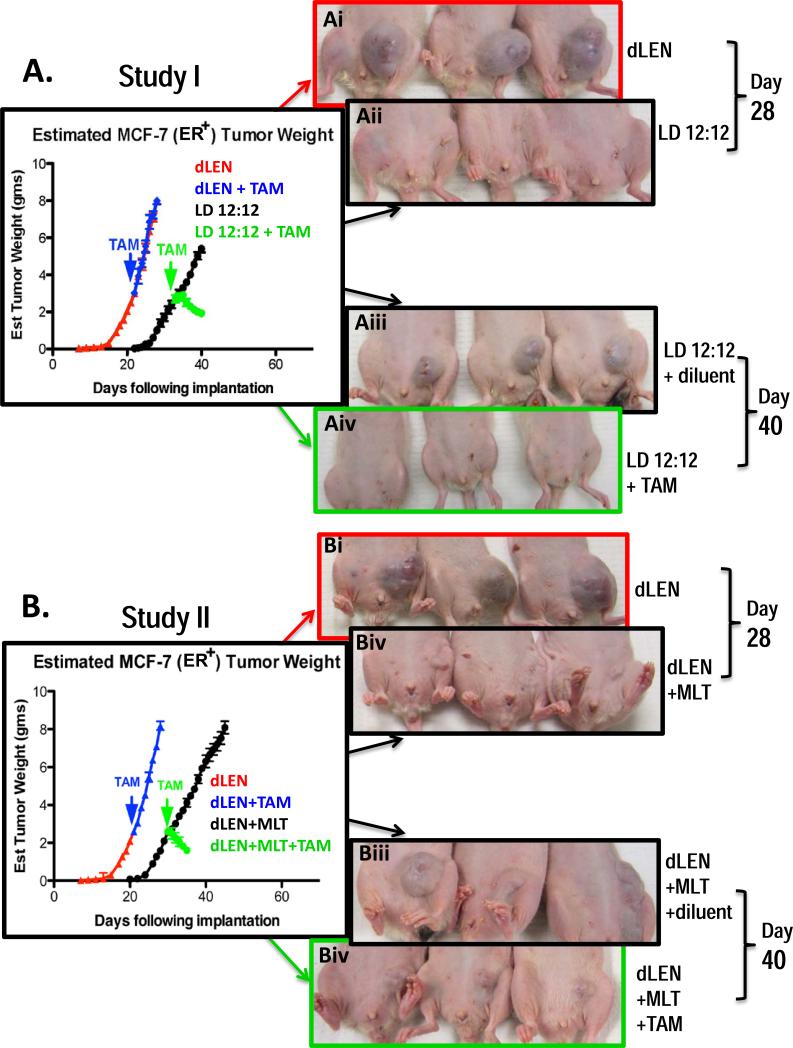
Figure 3A shows that breast tumor xenografts from rats housed in dLEN had a significantly reduced (p < 0.001) latency-to-onset and a significantly faster (p < 0.001) growth rate (2.6-fold, 0.73 g/day) compared to tumor xenografts grown under the LD 12:12 lighting schedule (0.28 g/day). Tumors from rats in dLEN showed complete intrinsic resistance to TAM growing at the same rate (0.69 g/day) as vehicle treated xenografts in dLEN. However, late afternoon (1600 hr/4 p.m.) administration of TAM significantly inhibited (p < 0.001) tumor growth in rats housed in LD 12:12 as tumors regressed at a rate of −0.14 g/day. Figure 3A also shows the visual dramatic difference in tumor growth and regression between xenografts from dLEN with our without melatonin supplementation and LD 12:12 groups treated with TAM.
Figure 3.
Differential effects of 4OH-TAM of on the growth and regression of (ERα+) MCF-7 tissue-isolated breast tumor xenografts in female nude rats housed in standard lighting schedule (LD 12:12) or in dLEN lighting schedules with or without melatonin supplementation. (A) Study I - Estimated tumor weight [g/day] of MCF-7 (ERα+) human breast tumor xenografts from nude rats exposed to a dLEN lighting schedule and treated diluent (red triangles) with 4OH-TAM (blue triangles) [80ug/kg/day] or a LD 12:12 lighting schedule and treated with diluent (black circles) or 4OH-TAM (green circles). (B) Study II − Estimated tumor weight [g/day] of MCF-7 (ERα+) human breast tumor xenografts from nude rats exposed to a dLEN lighting schedule and treated with vehicle (red triangles) or 4OH-TAM (blue triangles) [80ug/kg/day] or in a dLEN lighting schedule supplemented with exogenous melatonin at night and treated vehicle (black circles) or 4OH-TAM (green circles). Tumor weights were estimated daily in as described in “Materials and Methods.” Images of tumor-bearing nude rats in Studies I (Ai, ii, iii, and iv) and II (Bi, ii, iii, and iv) maintained on either experimental (dLEN) or control (LD12:12) lighting schedules, as described in “Materials and Methods.” Panel A represents xengografts from experimental animals 28 days after tumor implant in dLEN (Ai upper) and LD 12:12 (Aii panel). Panel Aiii and iv are xenografts from experimental animals 40 days after tumor implant in LD 12:12 and diluent treatment (Aiii panel) and LD 12:12 after 28 days of treatment with TAM (Aiv panel). Photographs in Panel B are xenografts from experimental animals 28 days after tumor implant in control dLEN lighting schedule (Bi panel) and dLEN supplemented with exogenous nighttime melatonin (Bii panel). Panel Biii is xenografts from experimental animals 40 days after tumor implant in dLEN supplemented with exogenous nighttime melatonin and administered the vehicle for TAM. Panel Biv is xenografts from experimental animals 40 days after tumor implant in dLEN but supplemented with exogenous nighttime melatonin and after treatment with TAM.
Tumor cAMP levels
Tumor cAMP levels were 14- and 20-fold higher at the mid-dark phase in rats in vehicle- and TAM-treated dLEN rats, respectively, as compared with the same treatment groups on LD 12:12 (Table 1A). Administration of 4OH-TAM to rats in dLEN did not affect tumor cAMP levels, however, it further diminished tumor cAMP levels by 40% as compared to vehicle treated animals on LD 12:12.
Table 1.
A. Tumor cAMP levels, LA uptake, 13-HODE formation, [3H]thymidine incorporation into DNA and DNA content during the mid-dark phase (2400 hrs) in tissue-isolated MCF-7 (ER+) human breast cancer xenografts in nude female rats (Study I) exposed to either LD 12:12 or dLEN and treated with either vehicle or tamoxifen (80 μd). Values are means + SD (n =3/group).
| Treatmenta | cAMP (nmol/g tissue) | LA uptake (ng/min/g) | 13-HODE (ng/min/g) Arterial Supply | 13-HODE (ng/min/g) Venous Output | 3H-Thymidine Incorporation (dpms/ug DNA) | DNA Content (mg/g) |
|---|---|---|---|---|---|---|
| dLEN Vehicle | 1.55 ± 0.28 | 3.86 ± 0.73 (34.8 ± 1.7%)ψ | 0 | 6.58 ± 0.71 | 66.5 ± 2.6 | 3.15 ± 0.16 |
| dLEN Tamoxifen | 1.38 ± 0.14 | 3.94 ± 0.49 (35.8 ± 2.7%)ψ | 0 | 6.04 ± 0.65 | 67.4 ± 1.9 | 3.20 ± 0.28 |
| LD 12:12 Vehicle | 0.11 ± 0.05* | 0 | 0 | 0 | 5.6 ± 1.0 * | 2.0 ± 0.1* |
| LD 12:12 Tamoxifen | 0.07 ± 0.02** | 0 | 0 | 0 | 6.5 ± 1.0** | 1.9 ± 0.2** |
| B Tumor cAMP levels, LA uptake, 13-HODE formation, [3H]thymidine incorporation into DNA and DNA content during the mid-dark phase (2400 hrs) in tissue-isolated MCF-7 (ER+) human breast cancer xenografts in nude female rats (Study II) exposed to dLEN and treated with either vehicle, tamoxifen (80 μg/kg/d), melatonin (2.5μg/d), or tamoxifen + melatonin. Values are means + SD (n =3/group). | ||||||
|---|---|---|---|---|---|---|
| Treatmenta | cAMP (nmol/g tissue) | LA uptake (ng/min/g) | 13-HODE (ng/min/g) Arterial Supply | 13-HODE (ng/min/g) Venous Output | 3H-Thymidine Incorporation(dpms/μg DNA) | DNA Content (mg/g) |
| dLEN Vehicle | 1.63 ± 0.20 | 2.5 ± 0.7 (35.3 ± 1.5%)ψ | 0 | 6.9 ± 0.7 | 67.4 ± 1.1 | 3.5 ± 0.2 |
| dLEN Tamoxifen | 1.62 ± 0.22 | 2.6 ± 0.5 (34.4 ± 2.8%)ψ | 0 | 6.8 ± 0.5 | 69.2 ± 3.3 | 3.4 ± 0.2 |
| dLEN Melatonin | 0.16 ± 0.06* | 0 | 0 | 0 | 5.8 ± 1.0* | 2.0 ± 0.1* |
| dLEN Tamoxifen + Melatonin | 0.06 ± 0.02*** | 0 | 0 | 0 | 5.8 ± 0.1 ** | 2.0 ± 0.1** |
Three animals (tumors)/group
(+ SD) tumor weights, MCF-7 (ER+) dLEN group, vehicle treatment = 7.26 ± 0.21 g; tamoxifen treatment = 7.08 ± 0.22 g; LD 12:12 group vehicle treatment = 5.53 ± 0.20 g; tamoxifen treatment = 1.95 ± 0.09 g, respectively. All tumors were harvested at 2400 hrs.
Values expressed as % of arterial LA supply.
p < 0.05 vs. dLEN vehicle
p < 0.05 vs dLEN + tamoxifen.
Three animals (tumors)/group
(± SD) tumor weights, in MCF-7(ER+) dLEN groups, vehicle treatment = 7.26 ± 0.21 g; tamoxifen treatment = 7.08 ± 0.22 g; dLEN melatonin treatment = 5.53 ± 0.20 g; dLEN tamoxifen + melatonin treatment = 1.95 ± 0.09 g respectively. All tumors were harvested at 2400 hrs.
Values expressed as % of arterial LA supply.
p< 0.05 vs. vehicle
p < 0.05 vs. tamoxifen
p < 0.05 vs. tamoxifen and melatonin.
Tumor linoleic acid (LA) uptake, 13-HODE production, and proliferative activity
Tumor LA uptake and 13-HODE formation were completely suppressed during the mid-dark phase in both vehicle- and TAM-treated rats on LD 12:12, while high levels of tumor LA metabolism was seen in both vehicle- and TAM-treated rats maintained on dLEN (Table 1A). Also tumor incorporation of [3H]-thymidine into DNA in vehicle-and TAM-treated dLEN rats was elevated by 10-fold at the mid-dark phase as compared with vehicle- and TAM-treated rats on LD 12:12, respectively. No difference was observed between vehicle- and TAM-treated groups in both photoperiods.
Warburg Effect - tumor glucose and O2 uptakes, and lactic acid and CO2 production
Tumor glucose and O2 uptake were increased by 2- and 1.5-fold, respectively, at 2400 hr (mid dark phase) in vehicle-treated dLEN rats as compared with vehicle-treated rats on the standard LD 12:12 photo-schedule. In dLEN rats tumor glucose and O2 uptake were also increased by 4- and 3.5-fold, respectively, following TAM-treatment versus LD 12:12 tumors following TAM-treatment (Table 2A). Tumor glucose and O2 uptake were significantly reduced by 77% and 57%, respectively, in TAM-treated and vehicle-treated rats on LD 12:12.
Table 2.
A. Tumor uptake of glucose and O2 and production of lactate and CO2 production in vivo (Warburg effect) during the mid- dark phase (2400 hrs) in tissue-isolated MCF-7 (ER+) human breast cancer xenografts in nude female rats (Study I) exposed to either LD 12:12 or dLEN and treated with either vehicle or tamoxifen (80 μg/kg/d). Values are means + SD (n =3/group).
| Treatmenta | Glucose uptake (μg/min/g) | Lactate release (nmol/min/g) | O2 uptake (% of supply) | CO2 production (% of original value) |
|---|---|---|---|---|
| dLEN Vehicle | 4.3 ± 0.1 (32.8 ± 5.7%)ψ | 28.9 ± 4.4 | 75.8 ± 1.1 | 136.2 ± 10.5 |
| dLEN Tamoxifen | 5.1 ± 1.0 (31.4 ± 3.5%)ψ | 30.2 ± 5.1 | 76.5 ± 0.9 | 133.0 ± 4.3 |
| LD 12:12 Vehicle | 2.3 ± 0.2* (12.9 ± 1.5%)ψ,* | 15.9 ± 0.8* | 50.5 ± 3.5* | 69.4 ± 3.1* |
| LD 12:12 Tamoxifen | 1.3 ± 0.4** (2.8 ± 1.5%)ψ,** | 2.3 ± 0.3** | 21.9 ± 3.3** | 49.2 ± 2.4** |
| B Tumor uptake of glucose and O2 and production of lactate and CO2 production in vivo (Warburg effect) during the mid- dark phase (2400 hrs) in tissue-isolated MCF-7 (ER+) human breast cancer xenografts in nude female rats (Study II) exposed to dLEN and treated with either vehicle, tamoxifen (80 μg/kg/d), melatonin (2.5|ig/d), or tamoxifen + melatonin. Values are means + SD (n =3/group). | ||||
|---|---|---|---|---|
| Treatmenta | Glucose uptake (μg/min/g) | Lactate release (nmol/min/g) | O2 uptake (% of supply) | CO2 production (% of original value) |
| dLEN Vehicle | 4.4 ± 0.5 (33.7 ± 2.8%)ψ | 27.3 ± 1.1 | 78.9 ± 0.8 | 133.6 ± 8.7 |
| dLEN Tamoxifen | 4.4 ± 0.5 (34.5 ± 1.7%)ψ | 25.8 ± 2.4 | 78.6 ± 12.5 | 136.2 ± 5.7 |
| dLEN Melatonin | 1.6 ± 0.1* (12.5 ± 0.5%)ψ,* | 10.4 ± 0.4* | 60.5 ± 3.7* | 72.9 ± 9.2* |
| dLEN Tamoxifen + Melatonin | 1.9 ± 0.6*** (3.6 ± 0.5%)*** | 2.0 ± 1.0** | 22.7 ± 1.8** | 46.8 ± 4.4** |
Three animals (tumors)/group
(+ SD) tumor weights, MCF-7 (ER+) dLEN group, vehicle treatment = 7.26 ± 0.21 g; tamoxifen treatment = 7.08 ± 0.22 g; LD 12:12 group vehicle treatment = 5.53 ± 0.20 g; tamoxifen treatment = 1.95 ± 0.09 g, respectively. All tumors were harvested at 2400 hrs.
Values expressed as % of arterial glucose supply.
p < 0.05 vs. dLEN vehicle
p < 0.05 vs. LD 12:12 + tamoxifen and dLEN + tamoxifen.
Three animals (tumors)/group
(± SD) tumor weights, in MCF-7(ER+) dLEN groups, vehicle treatment = 7.26 ± 0.21 g; tamoxifen treatment = 7.08 ± 0.22 g; dLEN melatonin treatment = 5.53 ± 0.20 g; tamoxifen + melatonin treatment = 1.95 ± 0.09 g respectively. All tumors were harvested at 2400 hrs.
Values expressed as % of arterial glucose supply.
p< 0.05 vs. vehicle
p < 0.05 vs. tamoxifen and melatonin
p < 0.05 vs. tamoxifen.
Tumor lactate and CO2 production were also increased by 1.8- and 2-fold, respectively, at the 2400 hr (mid dark phase) in vehicle-treated and 3.5- and 2.7-fold, respectively, in TAM-treated LD dLEN rats as compared with vehicle- and TAM-treated rats in LD 12:12, respectively (Table 2A). Tumor lactate and CO2 production were reduced by 29% in LD 12:12 rats, and by 93% in TAM-treated rats on LD 12:12.
Modulation of apoptosis in breast tumor xenografts exposed to dLEN or nighttime melatonin and treated with 4OH-TAM
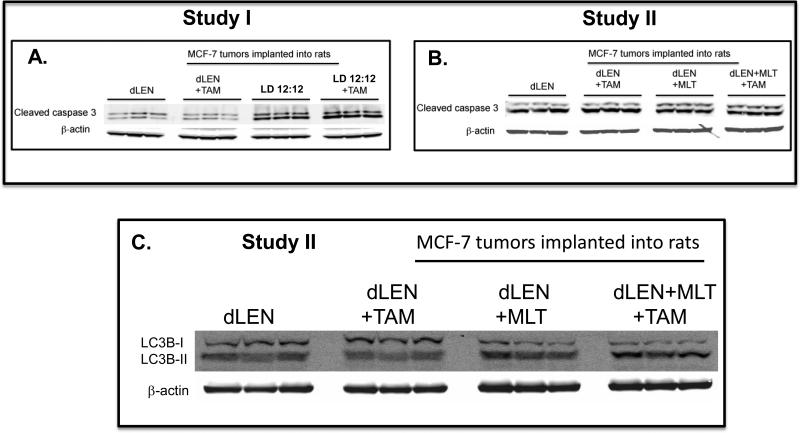
Caspase 3 has high homology to the CED-3 protease and is a key player in apoptosis. Caspase 3 is cleaved by caspases 8 and 9 into two bands of (17 and 19 kDa) that in turn induce apoptosis. The increased levels of cleaved caspase 3 are markers of apoptosis in Study I, (Fig. 4A) showed a significant 28% and 42% increase in LD 12:12 tumors with or without 4OH-TAM treatment, respectively, versus dLEN tumors.
Figure 4.
Differential effects of 4OH-TAM on apoptosis and autophagy of (ERα+) MCF-7 tissue-isolated breast tumor xenografts in female nude rats housed in LD 12:12 or dLEN lighting schedules with or without melatonin supplementation. (A) Study I - Expression of cleaved caspase 3 apoptotic protein (upper band) in breast tumor xenografts from nude rats in dLEN and treated with vehicle (dLEN) or 4OH-TAM (dLEN+TAM) or in LD 12:12 lighting schedule and treated with vehicle (12L:12D) or 4OH-TAM (12L:12D+TAM). (B) Study II - Expression of cleaved caspase 3 (upper band) apoptotic protein in breast tumor xenografts from rats exposed to a dLEN lighting schedule and treated with vehicle (dLEN) or 4OH-TAM (dLEN+TAM) or a dLEN lighting schedule but supplemented with exogenous nighttime melatonin and treated with vehicle (dLEN+MLT) or 4OH-TAM (dLEN+MLT+TAM). (C) Study II - Expression of the autophagy markers Light Chain 3B I (upper band) and LC3BII (lower band) in breast tumor xenografts from rats exposed to a dLEN lighting schedule and treated with vehicle (dLEN) or 4OH-TAM (dLEN+TAM) or in a dLEN lighting schedule but supplemented with exogenous nighttime melatonin and treated with vehicle (dLEN+MLT) or 4OH-TAM (dLEN+MLT+TAM). In both Studies I and II, tumors were harvested at 2400 hr (mid-dark phase) from 3 animals in each group. Total cell lysates (120 μg of protein/sample) from tumors were analyzed by Western blotting for expression of cleaved caspase 3 (A and B) or conversion of LC3BI to LC3BII by lipidation (C). β-actin was used as a control for equal loading.
Modulation of key proliferation and survival signaling molecules in breast tumor xenografts exposed to dLEN or nighttime melatonin treated with 4OH-TAM
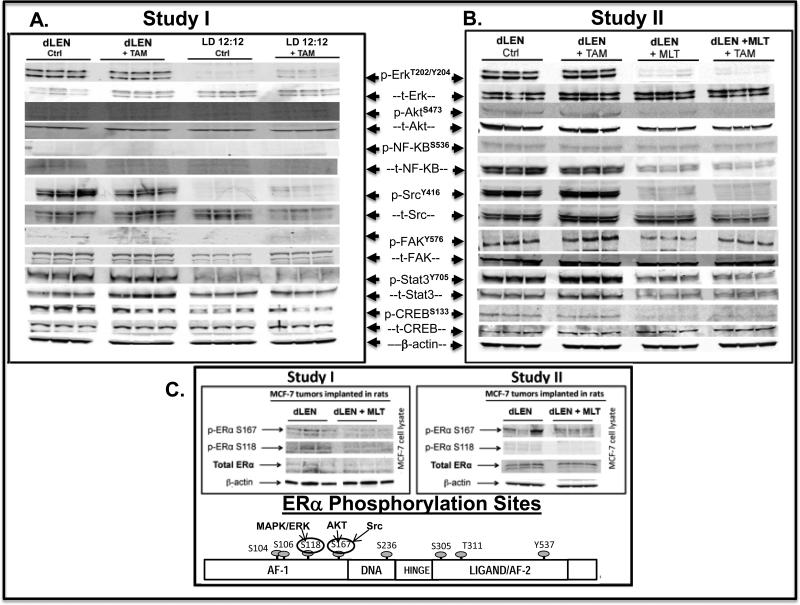
Tumors from animals in dLEN treated with vehicle or TAM, harvested at 2400 hr (mid-dark phase, subjective night) showed high levels of Erk1/2 phospho-activation, while tumors from animals in LD 12:12 with elevated nighttime melatonin showed almost complete suppression of ERK1/2 phospho-activation (Fig. 5A). No increase in AKT, either total or phosphorylated at ser473 (p-AKTS473) or NF-kB/p65, was observed in response to dLEN or LD 12:12 in either the presence or absence of TAM.
Figure 5.
Modulation of signaling kinases and transcription factors in tissue-isolated (ER+) MCF-7 human breast tumor xenografts from female nude rats exposed to control LD 12:12, dLEN or dLEN supplemented with nighttime melatonin and treated with 4OH-TAM. (A) Study I - Total and phospho- Western blot analysis of total tissue lysates from tissue-isolated MCF-7 breast tumor xenografts harvested rats in dLEN lighting schedule and treated with vehicle (dLEN) or 4OH-TAM (dLEN+TAM) or a LD 12:12 lighting schedule and treated with vehicle (12L:12D) or 4OH-TAM (12L:12D+TAM). (B) Study II- Total and phospho- Western blot analysis of total tissue lysates from breast tumor xenografts harvested rats in dLEN lighting schedule and treated with vehicle (dLEN) or 4OH-TAM (dLEN+TAM) or in dLEN but supplemented with nighttime melatonin and treated with vehicle (dLEN+MLT) or 4OH-TAM (dLEN+MLT+TAM). In both studies, tumors were harvested at 2400 hr (mid-dark phase) from 3 animals in each group. Total cell lysates (120 μg of protein per sample) from each tumor were analyzed by Western blot for expression of phosphorylated and total forms of ERK1/2, AKT, NF-kB, SRC, FAK, STAT3, and CREB. β-actin was used as a control for equal loading. (C) Study II - Total tissue lysates from tissue-isolated MCF-7 (ERα+) breast tumor xenografts harvested from nude rats housed under dLEN photoperiod (dLEN) or dLEN and supplemented with nighttime melatonin (dLEN+MLT). As above, tumors were harvested at 2400 h (mid-dark phase) from 3 animals in each group. Total cellular protein was analyzed by Western blotting for expression of total and phosphorylated ERα (p-ERα S118 and S167). β-actin was used as a control for equal loading.
Tumors from dLEN groups (vehicle or TAM-treated) also showed elevated levels of p-SRC and p-FAK at sites Y416 and Y576/577, respectively, but repression of the phospho-active forms was seen in LD 12:12 tumors (Fig. 5A). The levels of p-STAT3 (Y705) were also elevated in dLEN tumors treated with vehicle or TAM, but were almost completely inhibited in LD 12:12 tumors (vehicle- and TAM-treated). Figure 5A shows cAMP response element binding protein (CREB), a transcription factor phospho-activated by cAMP/PKA and up regulated in many breast tumors (34, 35), is phospho-activated in dLEN tumors treated with or without TAM, but inhibited in LD 12:12 tumors.
dLEN activation and melatonin repression of ERα phosphorylation and activation
Elevated phosphorylation of Ser118 and Ser167 of the ERα was observed in tumors from the dLEN group without significant change in total ERα protein levels (Fig. 5A) compared to those in the LD 12:12 groups.
Study II
Plasma melatonin levels in Study II
Melatonin levels remained low throughout the 24 h period in rats under the dLEN lighting schedule prior to melatonin and/or TAM treatment. As shown in Figure 2B, following administration of melatonin in the drinking water (2.5 μg/day) plasma melatonin levels at 2400 hr (mid dark phase/subjective night) were 70- to 90-fold greater than at 1200 hr in control (dLEN) rats, respectively.
dLEN promotes tumor growth and intrinsic resistance to 4OH-TAM in (ER+) MCF-7 tissue-isolated breast tumor xenografts
Figure 3B demonstrates that (ER+) MCF-7 breast tumor xenografts from rats housed in dLEN had a significantly shorter (p < 0.001) latency-to-onset and a significantly increased growth rate (2.2-fold) compared to tumors in rats in dLEN that were supplemented with exogenous melatonin at night. Tumor xenografts from rats in dLEN showed complete intrinsic resistance to 4OH-TAM, growing at the same rate as vehicle treated dLEN xenografts. Tumor xenografts from rats in dLEN supplemented with nighttime melatonin showed a highly significant response (p<0.0001) to 4OH-TAM administered at 1600 hr (4 p.m.), regressing at a rate of −0.17 g/day. Figure 3B shows the visual dramatic difference in tumor growth and regression between the various dLEN groups.
Tumor cAMP levels
Table 1B shows that tumor cAMP concentrations at 2400 hr in dLEN animals given either vehicle or TAM were 10- and 27-fold higher, respectively, than in the same treatment groups in dLEN rats supplemented with nighttime melatonin. In rats in dLEN supplemented with melatonin, TAM administration further decreased tumor cAMP levels by 63% compared to vehicle controls.
Tumor linoleic acid (LA) uptake, 13-HODE production, and proliferative activity
Table 1B shows elevated levels of tumor LA uptake and 13-HODE formation at 2400 hr (mid-dark phase) in both dLEN groups (vehicle- and TAM-treated) without melatonin supplementation. However, uptake of LA and its conversion to 13-HODE were completely suppressed at 2400 hr in dLEN groups supplemented with nighttime melatonin. Furthermore, incorporation of [3H]-thymidine into the DNA of dLEN tumors, both vehicle- and 4OH-TAM-treated, was elevated by 10-fold compared with the same treatment groups in dLEN tumors and supplemented with melatonin.
Warburg Effect - tumor glucose and O2 uptakes, and lactic acid and CO2 production
In Table 2B tumor glucose and O2 uptake increased by 2.8- and 1.3-fold and 2.3- and 3.4-fold, in vehicle- and TAM-treated dLEN rats, respectively as compared to the same treatment groups from dLEN melatonin-supplemented tumors. Also, tumor lactate and CO2 production increased by 2.7- and 1.8-fold and 1.3- and 2.9-fold in vehicle- and TAM-treated dLEN rats, respectively, versus vehicle- and TAM-treated dLEN melatonin-supplemented rats.
Modulation of apoptosis and autophagy in breast tumor xenografts exposed to dLEN or nighttime melatonin and treated with 4OH-TAM
Caspase 3 cleavage, a marker of apoptosis, was increased by 15% and 23% in dLEN melatonin-supplemented tumors treated with vehicle or 4OH-TAM, respectively, compared to the same treatment groups in dLEN tumors without melatonin-supplementation (Fig. 4B). Furthermore, an increase in the conversion of LC3BI (upper band) to LC3BII (lower band) is observed in dLEN melatonin-supplemented tumors treated with vehicle or 4OH-TAM versus xenografts compared to dLEN tumors treated with vehicle or 4OH-TAM (Fig. 4C).
Modulation of key proliferation and survival signaling molecules in breast tumor xenografts exposed to dLEN or nighttime melatonin treated with 4OH-TAM
In Study II dLEN tumors treated with diluent or 4OH-TAM showed high levels of phospho-active ERK1/2, SRC, FAK, STAT3, and CREB (Fig. 5B) and modest, but consistent, induction of phospho-active AKTS473. A large and consistent increase in t-NF-kB/p65 but not p-NF-kB/p65 was observed in dLEN tumors treated with vehicle or 4OH-TAM. Conversely, the corresponding treatment groups in dLEN melatonin-supplemented tumors showed almost complete suppression of p-ERK1/2, p-AKT, p-SRC, p-FAK, p-STAT3, and p-CREB and a significant reduction of t-NF-kB/p65 was also observed.
dLEN activation and melatonin repression of ERα phosphorylation and activation
Elevated phosphorylation of Ser118 and 167 was observed in tumors from rats house in dLEN, without significant change in total ERα protein levels (Fig. 5C). Supplementation with exogenous nighttime melatonin in dLEN tumors greatly suppressed ERα phosphorylation at Ser118 and 167 by 68% and 76%, respectively.
Discussion
Unlike exposure to bright light at night, which both disrupts the activity of the central circadian clock and suppresses pineal melatonin synthesis, dLEN suppresses only melatonin production while normal SCN-driven circadian feeding and drinking activity persists (24). In these studies dLEN was at a light intensity of 0.2 lux, which is equivalent to a crack of light under a door in a completely dark room, or the intensity of light from a television perceived through closed eyelids. As shown in Study I, female nude rats on LD 12:12 lighting schedule evinced a circadian rhythm of plasma melatonin, that closely mimics the normal melatonin circadian profile in adult human female subjects (26, 32) while dLEN only suppressed the nocturnal melatonin signal as we previously reported (24, 26). Study II, demonstrated that supplementation of the drinking water with melatonin is an effective replacement strategy for reconstituting nighttime levels of melatonin in dLEN rats.
This study clearly demonstrates that under dLEN conditions, latency-to-tumor onset was shortened while the growth of tumors and their development of intrinsic TAM-resistance were stimulated. Conversely, in the presence of the endogenous nocturnal melatonin signal or in response to exposure to melatonin at night under dLEN conditions, tumor latency-to-onset was prolonged while TAM-resistance was negated resulting in tumor regression. These in vivo studies demonstrate for the first time that the presence of the endogenous circadian melatonin signal inhibits intrinsic TAM-resistance and thus confers increased tumor sensitivity to TAM. These findings are supported by our earlier in vitro work (29) and demonstrate that the loss of the nocturnal circadian melatonin signal, in response to dLEN-induced circadian/melatonin disruption, is an underlying and novel mechanism for the development of intrinsic resistance to TAM therapy.
We anticipated that the tumor regression driven by the combination of melatonin and 4OHTAM would be associated with significant increases in apoptosis and autophagy. However, only moderate increases in apoptosis and autophagy were observed at the mid dark phase time point, which do not correlate with the rapid rates of tumor regression observed. Thus, it is likely that apoptosis and autophagy are increased at other circadian time points, and/or that the degree of tumor regression induced by the combination of TAM and melatonin is mediated by multiple mechanisms including apoptosis, autophagy, and diminished cell proliferative activity.
While the evolving list of mechanisms by which melatonin inhibits human breast cancer growth includes the inhibition of multiple signaling, transcriptional and metabolic pathways, the mechanism(s) by which this indoleamine increases breast cancer responsiveness to TAM is unknown. Elucidating this mechanism(s) is essential for understanding how dLEN-induced melatonin suppression promotes breast cancer growth progression and TAM resistance. With respect to breast cancer metabolism, melatonin suppresses the metabolism of glucose via the inhibition of aerobic glycolysis (Warburg effect) as well as the uptake of LA and its metabolism to its mitogenic end product 13-HODE. Both of these processes, which are linked to key signaling pathways for cell proliferation/survival, are important in supplying both the energetics and infrastructure involved in building tumor biomass. We recently reported (37) that in human breast cancer xenografts LA metabolism, the Warburg effect, their associated cell signaling pathways, and cell proliferative activity exhibit robust melatonin-driven circadian rhythms characterized by suppressed activity during the night and peak activity during the day in our breast cancer xenografts. Numerous studies have linked the activation of ERK/12, AKT, NF-kB, cAMP SRC, STAT-3, and interleukin-6 (IL-6) to the potentiation of the Warburg effect in various tumor types (38-40). Since some of these same pathways are phospho-activated in response to dLEN in the present studies, it is possible that dLEN may have amplified the Warburg effect through up-regulation of phospho-active ERK1/2, cAMP, SRC, and IL-6. Based on the fact that the Warburg effect can drive some tumors to chemo-resistance (41), it is conceivable that dLEN may drive breast cancer to intrinsic resistance to TAM via constitutive activation of the Warburg effect.
On the other hand, in animals exposed to LD 12:12, with an intact endogenous melatonin signal, or to dLEN and receiving melatonin-replacement therapy, it is also possible that the rapid tumor regression observed in response to 4OH-TAM therapy can be primarily ascribed to inhibition of key proliferative and survival signaling pathways. Our results clearly demonstrate that the nocturnal exposure to melatonin dramatically suppresses or ablates the expression of total or phospho- activated ERK1/2, AKT, cAMP, SRC, FAK, STAT3, CREB, and NF-kB. Numerous reports have described the importance of each of these pathways, either individually or collectively, in driving breast cancer toward proliferation, progression, and TAM resistance (42, 43). The potential importance of melatonin as a natural endogenous nighttime inhibitor of pERK1/2, p-SRC, p-FAK and p-STAT3 pathways is supported by the work of Hiscox et al. (44) showing that inhibition of c-SRC suppresses the expression of cyclin D1 and c-Myc, and thus inhibits the emergence of a TAM-resistant phenotype in breast cancer cell lines. Future studies over multiple circadian time points will be required to determine the precise temporal sequence of kinase and transcription factor phosphorylation, defining the efficacy of endocrine therapies depending on the timing of their administration.
It is important to note that STAT3 is regarded as a point of convergence for many oncogenic signals and its aberrant constitutive activity is crucial for the survival, proliferation, and metastatic activity of tumors of different origins (45). Moreover, STAT3 activity can be driven by activation of ERK1/2, SRC, and FAK, all of which are activated by dLEN. STAT3, in turn, induces the expression of various genes involved in tumor promotion and progression including Myc, and cyclin D1 (44). Furthermore, activation of STAT3 promotes a feed-forward loop involving continuous production of the pro-inflammatory cytokine interleukin 6 (IL-6) and activation of NF-kB (46). Supporting a report by Alvarez-Garcia et al. (47), analysis of tissue-isolated breast tumor xenografts from rats in a LD 12:12 lighting schedule showed a significant 80% reduction in IL-6 mRNA expression at night as compared to those in dLEN (data not shown). Thus, the ability of melatonin to inhibit p-STAT3 levels in these breast cancer xenografts could result from either suppression of c-SRC/FAK phospho-activation, IL-6 expression, or both, to inhibit cell survival, proliferation, metastasis, angiogenesis, and endocrine resistance.
Our studies also suggest that ERα phosphorylation and transactivation is circadian regulated by the nocturnal melatonin signal and that dLEN-induced melatonin suppression is associated with elevated phosphorylation of the ERα at S118 and S167 and possibly involved in TAM resistance. These data combined with our reports (48) and that of others (49) that melatonin inhibits ERα transcriptional activity, and our unpublished data in cell lines that EGF or IGF-1 stimulate phosphorylation of ERα at both Ser118 and Ser167and that melatonin administration can inhibit this phosphorylation suggest that melatonin regulates ERα transcriptional activity through a phosphorylation-mediated mechanism.
In conclusion, the present investigation highlights and validates the importance of an intact endogenous nocturnal circadian melatonin signal in sensitizing human breast tumor cells to TAM-therapy. Given that nighttime melatonin significantly suppresses tumor kinase signaling; one could consider melatonin a broadly based “circadian-regulated broad kinase inhibitor” (CRKI) that exhibits potent anti-metabolic, -proliferative, and –progressive/metastatic activity in breast cancer. Moreover, our work demonstrates that a comprehensive understanding and maintenance of host/cancer circadian biology and the circadian-regulated nature of cancer metabolism and signaling are essential to derive the maximal efficacy from TAM and possibly other endocrine therapies. In this regard, the maximal efficacy of such therapies would appear to be dependent on their optimal temporal administration in alignment with the circadian timing of patients or with exogenous melatonin in patients with LEN-induced circadian/melatonin disruption. It is plausible that many, if not all, breast cancer patients are likely to be subjected to various degrees of LEN and may be circadian/melatonin disrupted as a result of stress, lack of sleep, and/or continued late night shift work. Therefore, LEN may represent a unique and previously unappreciated risk factor that could account for some forms of intrinsic and possibly acquired TAM-resistance and may even lead to a shortened survival time and even a decreased survival rate.
Acknowledgments
Financial Support This work was supported by the following grants NIH Grant R21CA129875–04 (to DEB) and an American Association for Laboratory Animal Science GLAS grant (RTD and DEB). Part of the cost of this work was defrayed with the support of the Edmond and Lily Safra Endowed Chair for Breast Cancer Research (SMH recipient) at Tulane Cancer Center.
Footnotes
The Authors disclose no potential conflicts of interest.
References
- 1.Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;8:1284–91. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- 2.Lewis-Wambi JS, Jordan VC. Treatment of Postmenopausal Breast Cancer with Selective Estrogen Receptor Modulators (SERMs). Breast Dis. 2006;24:93–105. doi: 10.3233/bd-2006-24108. [DOI] [PubMed] [Google Scholar]
- 3.Lønning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer. 2013;20:R183–201. doi: 10.1530/ERC-13-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72:7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingle JN. Endocrine therapy trials of aromatase inhibitors for breast cancer in the adjuvant and prevention settings. Clin Cancer Res. 2005;11:900–05. [PubMed] [Google Scholar]
- 6.Sabnis G, Brodie A. Understanding resistance to endocrine agents: molecular mechanisms and potential for intervention. Clin Breast Cancer. 2010;18:E6. doi: 10.3816/CBC.2010.n.014. [DOI] [PubMed] [Google Scholar]
- 7.Werner H, Bruchi I. The insulin-like growth factor-1 receptor as an oncogene. Arch Physiol Biochem. 2009;115:58–71. doi: 10.1080/13813450902783106. [DOI] [PubMed] [Google Scholar]
- 8.Fagan DH, Yee D. Crosstalk between IGF1R and estrogen receptor signaling in breast cancer. J. Mammary Gland Biol Neoplasia. 2008;13:423–29, 2008. doi: 10.1007/s10911-008-9098-0. [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Cui YK, Huang WH, Man K, Zhang GJ. Phosphorylation of estrogen receptor α at serine 118 is correlated with breast cancer resistance to tamoxifen. Oncol Lett. 2013;1:118–24. doi: 10.3892/ol.2013.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29:1289–91. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.deGraffenried LA, Chandrasekar B, Friedrichs WE, Donzis E, Silva J, Hildalgo M, et al. NF-kappa B inhibition markedly enhances sensitivity of resistant breast tumor cells to tamoxifen. Ann Oncol. 2004;15:885–90. doi: 10.1093/annonc/mdh232. [DOI] [PubMed] [Google Scholar]
- 12.Shah YM, Rowan BG. The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cell through phosphorylation-dependent stabilization of estrogen receptor (alpha) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol. 2005;19:732–48. doi: 10.1210/me.2004-0298. [DOI] [PubMed] [Google Scholar]
- 13.Vallabhaneni S, Nair BC, Cortez V, Challa R, Chakravarty D, Tekma RR, et al. Significance of ER-Src axis in hormonal therapy resistance. Breast Cancer Res Treat. 2011;130:377–85. doi: 10.1007/s10549-010-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EH Y, Lee CS, Lee JK, Lee YJ, Shin MK, et al. STAT3-RANTES autocrine signaling is essential for tamoxifen resistance in human breast cancer cells. Mol Cancer Res. 2013;11:31–42. doi: 10.1158/1541-7786.MCR-12-0217. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 16.Madigan MP, Ziegler RG, Benichou J, Byrne C, Hoover RN. Proportion of breast cancer cases in the United States explained by well-established risk factors. J. Natl Cancer Inst. 1997;87:1681–85. doi: 10.1093/jnci/87.22.1681. [DOI] [PubMed] [Google Scholar]
- 17.Hahn RA, Moolgavkar SH. Nulliparity, decade of first birth, and breast cancer in Connecticut cohorts, 1855-1945: an ecological study. Am J Public Health. 1989;79:1503–07. doi: 10.2105/ajph.79.11.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens RG. Electric power used and breast cancer: a hypothesis. Am J Epidemiol. 1987;125:556–61. doi: 10.1093/oxfordjournals.aje.a114569. [DOI] [PubMed] [Google Scholar]
- 19.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris. 2011;105:170–82. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Stevens RG. Artificial lighting in the industrialized world: circadian disruption and breast cancer. Cancer Causes Control. 2006;17:501–507. doi: 10.1007/s10552-005-9001-x. [DOI] [PubMed] [Google Scholar]
- 21.Rüger M, St Hilaire MA, Brainard GC, Khalsa SB, Kronauer RE, Czeisler CA, Lockley SW. Human phase response curve to a single 6.5 h pulse of short-wavelength light. J Physiol. 2013;591:353–363. doi: 10.1113/jphysiol.2012.239046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revell VL, Skene DJ. Light-induced melatonin suppression in humans with polychromatic and monochromatic light. Chronobiol Int. 2007;24:1125–37. doi: 10.1080/07420520701800652. [DOI] [PubMed] [Google Scholar]
- 23.Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift work, painting, and fire fighting. Lancet Oncol. 2007;8:1065–66. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 24.Dauchy RT, Blask DE, Sauer LA, Brainard GC, Krause JA. Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett. 1999;144:131–36. doi: 10.1016/s0304-3835(99)00207-4. [DOI] [PubMed] [Google Scholar]
- 25.Blask DE, Hill SM, Dauchy RT, Xiang S, Yuan L, Duplesis T, et al. Circadian regulation of molecular, dietary, and metabolic signaling mechanisms of human breast cancer growth by the nocturnal melatonin signal and the consequences of its disruption by light at night. J Pineal Res. 2011;51:259–69. doi: 10.1111/j.1600-079X.2011.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC. Growth and fatty acid metabolism of human breast cancer (MCF-7) xenografts in nude rats: impact of constant light-induced nocturnal melatonin suppression. Breast Cancer Res Treat. 2003;279:313–20. doi: 10.1023/a:1024030518065. [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Dauchy RT, Tirrell PC, Wu SS, Lynch DT, Jitawatanarat P, Burrington CM, et al. Light at night activates IGF-1R/PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res. 2011;71:2622–31. doi: 10.1158/0008-5472.CAN-10-3837. [DOI] [PubMed] [Google Scholar]
- 28.Mao L, Dauchy RT, Blask DE, Slakey LM, Xiang S, Yuan L, et al. Circadian gating of epithelial-to-mesenchymal transition in breast cancer cells via melatonin regulation of GSK3β. Mol Endocrinol. 2012;26:1808–20. doi: 10.1210/me.2012-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson ST, Blask DE, Lemus-Wilson AM. Melatonin augments the sensitivity of MCF-7 human breast cancer cells to tamoxifen in vitro. J Clin Endocrinol Metab. 1992;75:669–70. doi: 10.1210/jcem.75.2.1639964. [DOI] [PubMed] [Google Scholar]
- 30.Dauchy RT, Dauchy EM, Tirrell RP, Hill CR, Davidson LK, Greene MW, et al. Dark-phase light contamination disrupts circadian rhythms in plasma measures of physiology and metabolism. Comp Med. 2010;60:348–56. [PMC free article] [PubMed] [Google Scholar]
- 31.Dauchy RT, Dauchy EM, Tirrell RP, Hill CR, Davidson LK, Greene MW, et al. Dark-phase light contamination disrupts circadian rhythms in plasma measures of physiology and metabolism. Comp Med. 2010;60:348–356. [PMC free article] [PubMed] [Google Scholar]
- 32.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005;65:11174–84. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 33.Anbalagan M, Carrier L, Glodowski S, Hangauer D, Shan B, Rowan BG. KX-01, a novel Src kinase inhibitor directed toward the peptide substrate site, synergizes with tamoxifen in estrogen receptor α positive breast cancer. Breast Cancer Res Treat. 2012;132:391–409. doi: 10.1007/s10549-011-1513-3. [DOI] [PubMed] [Google Scholar]
- 34.Lazennec G, Thomas JA, Katzenellenbogen BS. Involvement of the cAMP response element binding protein (CREB) and estrogen receptor phosphorylation in the synergistic action of the estrogen receptor by estradiol and protein kinase activators. J Steroid Biochem Mol Biol. 2001;77:193–203. doi: 10.1016/s0960-0760(01)00060-7. [DOI] [PubMed] [Google Scholar]
- 35.Cho YS, Park YG, Lee YN, Kim MK, Bates S, et al. Extracellular protein kinase A as a cancer biomarker: its expression by tumor cells and reversal by a myristate-lacking C alpha and RIIbeta subunit overexpression. Proc Natl Acad Sci USA. 2000;97:835–40. doi: 10.1073/pnas.97.2.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 37.Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM, Greene MW, et al. Light exposure at night disrupts host/cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling and tumor growth prevention. PLOS ONE. 2014 doi: 10.1371/journal.pone.0102776. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soga T. Cancer metabolism: key players in metabolic reprograming. Cancer Science. 2013;104:275–81. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaria M, Giorgi C, Lebiedzinska M, Esposito G, D'Angeli L, et al. A Stat3-mediated metabolic switch is involved in tumor transformation and Stat3 addiction. Aging. 2010;2:823–42. doi: 10.18632/aging.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demaria M, Poli V. PMK2, Stat3 and HIF-1α: The Warburg's vicious circle. JAKSTAT. 2012;1:194–96. doi: 10.4161/jkst.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez-Outschoorn UE, Lin Z, Ko YH, Goldberg AF, Flomenberg N, et al. Understanding the metabolic basis of drug resistance: therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle. 2011;10:2521–28. doi: 10.4161/cc.10.15.16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Browne BC, Hochgrafe F, Wu J, Millar EK, Barraclough J, Stone A, et al. Global characterization of signaling networks associated with tamoxifen resistance in breast cancer. FEBS J. 2013;280:5237–57. doi: 10.1111/febs.12441. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Becerra R, Santos N, Diaz L, Comacho J. Mechanisms of resistance to endocrine therapy in breast cancer: focus on signaling pathways, miRNAs and genetically based resistance. Int J Mol Sci. 2012;14:108–45. doi: 10.3390/ijms14010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–74. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 45.Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–24. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez-Garcia V, Gonzalez A, Alonso-Gonzales C, Martinez-Campla C, Cos S. Melatonin interferes in the desmoplastic reaction in breast cancer by regulating cytokine production. J Pineal Res. 2012;52:282–90. doi: 10.1111/j.1600-079X.2011.00940.x. [DOI] [PubMed] [Google Scholar]
- 48.Kiefer T, Ram PT, Yuan L, Hill SM. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res. 2002;71:37–48. doi: 10.1023/a:1013301408464. [DOI] [PubMed] [Google Scholar]
- 49.Del Rio B, Garcia Pedrero JM, Martinez-Campa C, Zuazua P, Lazo PS, Ramos S. Melatonin, an endogenous-specific inhibitor of estrogen receptor alpha via calmodulin. J Biol Chem. 2004;279:38294–302. doi: 10.1074/jbc.M403140200. [DOI] [PubMed] [Google Scholar]