Abstract
Background
Little is known about how colorectal cancer screening test preferences operate together with test access and navigation to influence screening adherence in primary care.
Methods
We analyzed data from a randomized trial of 945 primary care patients to assess the independent effects of screening test preference for fecal immunochemical test (FIT) or colonoscopy (CX), mailed access to FIT and CX, and telephone navigation for FIT and CX, on screening.
Results
Preference was not associated with overall screening, but individuals who preferred FIT were more likely to complete FIT screening (p = 0.005), while those who preferred CX were more likely to perform CX screening (p = 0.032). Mailed access to FIT and CX was associated with increased overall screening (OR = 2.6, p = 0.001), due to a 29-fold increase in FIT use. Telephone navigation was also associated with increased overall screening (OR = 2.1, p = 0.005), mainly due to a 3-fold increase in CX performance. We estimated that providing access and navigation for both screening tests may substantially increase screening compared to a preference-tailored approach, mainly due to increased performance of non-preferred tests.
Conclusions
Preference influences the type of screening tests completed. Test access increases FIT and navigation mainly increases CX. Screening strategies providing access and navigation to both tests may be more effective than preference-tailored approaches.
Impact
Preference tailoring in colorectal cancer screening strategies should be avoided if the objective is to maximize screening rates, although other factors (e.g., costs, necessary follow-up) should also be considered.
Keywords: colorectal cancer, screening, access, navigation
INTRODUCTION
In the United States, colorectal cancer (CRC) screening among adults aged 50 and older is a little over 50%, with colonoscopies constituting more than three quarters of those screening tests.(1) Patient-oriented strategies that have been reported to increase CRC screening include mailings and reminders, (2) as well as navigation.(3,4) The role of patient preferences regarding CRC screening modalities is less clear, although such preferences differ across racial and ethnic groups, and may help to account for disparities in CRC screening rates.(5-9) A 2010 NIH State-of-the-Science Conference called for studies on patient preferences and other factors influencing the choice of a CRC screening modality, and for research to develop strategies that can reduce barriers and increase access to screening.(10) However, to our knowledge, no studies have examined the interplay of test preference, test access, and navigation in CRC screening.
We used data from a randomized controlled trial of CRC screening interventions in primary care,(11) to investigate the independent effects of test preference, test access, and navigation on CRC screening. We addressed four questions: (1) Does the likelihood of undergoing CRC screening vary with individual test preference? (2) How does increasing access to stool blood testing and colonoscopy affect CRC screening? (3) How does providing telephone navigation for stool blood testing and colonoscopy affect CRC screening? and (4) Does tailoring access and navigation to each individual’s preference have a different impact compared to providing access and navigation for both stool blood test and colonoscopy to all individuals?
MATERIALS AND METHODS
Study design
Between 2007 and 2011, we conducted a randomized controlled trial of screening interventions among patients at 10 primary care practices affiliated with the Christiana Care Health System (CCHS) in Delaware. Participants aged 50 to 79 who were not up to date with CRC screening were randomized into one of three groups: (1) a usual care control (C) group; (2) a standard intervention (SI) group that received non-tailored mailed access to both stool blood test and colonoscopy; and (3) a tailored navigation intervention (TNI) group that was provided mailed access and navigation based on self-reported screening test preference.(11) The study was approved by institutional review boards at Thomas Jefferson University and CCHS.
The control group had limited access to stool blood tests, as colonoscopy (CX) was the overwhelming CRC screening modality of choice at the participating practices. In the SI group, all participants were mailed a fecal immunochemical test (FIT) kit with instructions, along with a letter that included a telephone number to call for information on how to schedule a CX appointment. Callers were given the telephone number of an approved colonoscopy provider. In the TNI group, interventions were tailored to each participant’s CRC screening test preference reported on the baseline survey. Only the FIT kit was mailed to participants who preferred FIT, while only the CX information letter was mailed to participants who preferred CX. Participants who reported an equal FIT/CX preference received both the FIT kit and the CX information letter. A patient navigator then attempted to contact all TNI participants to encourage and assist them in completing CRC screening.
Study measures
Data were collected through baseline and endpoint surveys, and a medical records review. Information was obtained on participant sociodemographic characteristics, perceptions of CRC and screening, and screening decision stage related to FIT and CX screening.(11,12) Screening decision stage was assessed for FIT and CX separately (decided against, never heard of, not considering, undecided, or decided to do), and the highest of the two test-specific decision stages was defined the participant’s overall screening decision stage.(11,13,14) The screening test with the highest decision stage was considered the preferred test (prefer FIT, equal FIT/CX preference, or prefer CX). In addition, study participants were categorized in terms of access to screening tests and navigation. Test access was classified as: usual care (i.e., screening tests as offered by the practice), mailed FIT kit, mailed CX information number, or mailed FIT kit plus CX information number. Navigation status was also classified as: no navigation, navigation for FIT only, navigation for CX only, and navigation for both FIT and CX.
CRC screening was defined as the performance of any test recommended by American Cancer Society guidelines that applied at the start of the study in 2007. Evidence of screening was obtained from laboratory reports and medical records reviews, as well as from participant endpoint surveys. Screening recorded in any of those sources was counted, as long as it was performed within a 12-months following study randomization.
Data analyses
The main trial results regarding the intervention effects have been reported elsewhere.(11) In this paper, our main objective was to estimate the independent impact of test preference, test access, and navigation, as well as preference-tailoring, on overall and test-specific (FIT and CX) CRC screening:
Preference: comparison of different test preferences: FIT, equal FIT/CX, CX.
Access: comparison of different types of test access: usual care, FIT only, CX only, or FIT+CX (primarily, the contrast of access to both FIT and CX versus usual care).
Navigation: comparison of different levels of navigation: no navigation, FIT, CX, or FIT and CX (primarily, the contrast of navigation to both FIT and CX versus no navigation).
Tailoring: comparison of access and navigation to both FIT and CX versus tailored access and navigation (FIT only, CX only, or FIT+CX, depending on preference).
These effects were not directly estimable through simple comparisons of the trial arms. First, preference was not a randomization factor. Second, to completely evaluate access (usual care, FIT only, CX only, or FIT+CX) and navigation (none, FIT only, CX only, FIT+CX), we would need a 4x4 factorial trial design, and to evaluate tailoring we would need 2 additional arms (tailored access without navigation, or tailored access with navigation). Obviously, this was not feasible, and the trial randomized only a few combinations of elements, i.e., non-tailored access to both FIT and CX without navigation (SI) versus tailored access to FIT or CX with navigation (TNI). Consequently, only certain intervention elements can be evaluated directly through the randomized trial results (most notably, the main effect of access to FIT+CX versus usual care can be obtained by contrasting the SI and UC groups). Other effects of individual intervention elements can only be evaluated indirectly through observational data analyses, and those are the ones we present in this paper. Finally, certain effects of individual intervention elements are not estimable at all because of complete colinearity between them (e.g., the effect of FIT-only access cannot be disentangled from the effect of FIT-only navigation since the two are either both present or both absent for each study participant).
We used logistic regression to analyze overall CRC screening (yes versus no) and polytomous logistic regression for test-specific screening (no screening, FIT screening, or CX screening). Preference, access, and navigation were the main variables of interest. Final analyses controlled for primary care practice, age, sex, race, education, marital status, perceptions of CRC and screening, and baseline screening decision stage.
Our analyses focused on estimating the separate effects of access and navigation with respect to both tests (see Supplementary Material for technical details). More specifically, we estimated the contrast between mailed FIT kit plus the CX information number versus usual care (“access” effect), and the contrast of navigation for both tests versus no navigation (“navigation” effect). The access effect was estimable among all three preference categories, and therefore we also tested the interaction between preference and access. In contrast, the navigation effect was estimable only among participants with an equal FIT/CX preference, and therefore we could not assess the interaction between preference and navigation. Finally, we also estimated the preference-based tailoring effect, which corresponds to the comparison of a hypothetical intervention that provided access and navigation for both tests, irrespective of preference, versus tailored access and navigation only for the preferred test (TNI group). The estimation of the tailoring effect relied on the appropriate combinations of access and navigation effects as estimated in the previous analyses (see Supplementary Material).
RESULTS
Table 1 summarizes the baseline characteristics of the 945 study participants.(11) CRC screening was completed by 305 (32%) participants, with screening tests being either an FIT or a CX (n = 164 and 141, respectively). Table 2 summarizes overall and test-specific screening rates (FIT and CX) according to preference, access, and navigation status.
Table 1. Baseline characteristics of study participants (N = 945).
| Age (years), n (%) | ||
| 50-59 | 658 | (69.6) |
| 60-79 | 287 | (30.4) |
| Sex, n (%) | ||
| Female | 589 | (62.3) |
| Male | 356 | (37.7) |
| Race, n (%) | ||
| White | 740 | (78.3) |
| Non-white | 205 | (21.7) |
| Education, n (%) | ||
| High school or less | 401 | (42.8) |
| Greater than high school | 535 | (57.2) |
| Marital status, n (%) | ||
| Married (or living as married) | 586 | (62.2) |
| Single/divorced/widowed | 356 | (37.8) |
| CRC and screening perceptions, n (%) | ||
| Unfavorable | 135 | (14.3) |
| Favorable | 806 | (85.7) |
| Screening decision stage, n (%) | ||
| Decided against / Never heard of | 42 | (4.4) |
| Not considering / Undecided about | 191 | (20.2) |
| Decided to do | 712 | (75.3) |
| Screening test preference, n (%) | ||
| Prefer FIT | 185 | (19.6) |
| Equal preference for FIT and CX | 395 | (41.8) |
| Prefer CX | 365 | (38.6) |
| Test access, n (%) | ||
| Usual care | 317 | (33.5) |
| Mailed access to FIT | 52 | (5.5) |
| Mailed access to FIT and CX | 441 | (46.7) |
| Mailed access to CX | 135 | (14.3) |
| Navigation, n (%) | ||
| None | 633 | (67.0) |
| Navigation for FIT | 52 | (5.5) |
| Navigation for FIT and CX | 125 | (13.2) |
| Navigation for CX | 135 | (14.3) |
FIT: fecal immunochemical test. CX: colonoscopy.
Counts may not sum to 945 because of occasional missing data.
Table 2. Summary of CRC screening rates by participant test preference, test access category, and navigation status (N = 945).
| Group | Preference | Access | Navigation | Screening adherence (%) |
||||
|---|---|---|---|---|---|---|---|---|
| N | Overall | FIT | CX | |||||
| 1. | C | FIT | Usual care | No | 58 | 10% | 3% | 7% |
| 2. | C | Equal FIT/CX | Usual care | No | 151 | 18% | 3% | 15% |
| 3. | C | CX | Usual care | No | 108 | 22% | 0% | 22% |
|
| ||||||||
| 4. | SI | FIT | FIT kit + CX number | No | 75 | 43% | 37% | 5% |
| 5. | SI | Equal FIT/CX | FIT kit + CX number | No | 119 | 34% | 29% | 5% |
| 6. | SI | CX | FIT kit + CX number | No | 122 | 35% | 17% | 18% |
|
| ||||||||
| 7. | TNI | FIT | FIT kit | FIT | 52 | 48% | 46% | 2% |
| 8. | TNI | Equal FIT/CX | FIT kit + CX number | FIT+CX | 125 | 51% | 34% | 17% |
| 9. | TNI | CX | CX number | CX | 135 | 33% | 6% | 27% |
C: control (usual care). SI: standard intervention. TNI: tailored navigation intervention. FIT: fecal immunochemical test. CX: colonoscopy.
Test preference
Within subgroups that were provided the same access and navigation (i.e., C and SI groups), overall screening rates did not differ significantly across test preference categories (Table 2, C: 10%, 18%, and 22%, p = 0.166; SI: 43%, 34%, 35%, p = 0.433). However, the type of test performed was significantly associated with test preference. More specifically, FIT screening rates tended to be higher among participants who preferred FIT than among those who had an equal preference for FIT and CX or those who preferred CX. Similarly, CX screening rates tended to be higher among participants who preferred CX than among those who had an equal preference for FIT and CX or those who preferred FIT. The multivariable analyses confirmed that test preference was significantly associated with both FIT and CX screening (Table 3, p = 0.005 and 0.032, respectively).
Table 3. Independent effects of test preference, test access, and navigation on CRC screening adherence (N = 933*).
| Overall screening | FIT screening vs. none | CX screening vs. none | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | |
| Test preference | 0.940 | 0.005 | 0.032 | ||||||
| FIT | 1.02 | (0.61, 1.71) | 0.930 | 1.33 | (0.70, 2.53) | 0.384 | 0.59 | (0.25, 1.39) | 0.226 |
| Equal FIT/CX | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | |||
| CX | 0.94 | (0.60, 1.46) | 0.787 | 0.44 | (0.23, 0.84) | 0.013 | 1.61 | (0.93, 2.81) | 0.091 |
|
| |||||||||
| Test access | |||||||||
| Usual care | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | |||
| Mailed FIT kit + CX number | 2.64 | (1.79, 3.90) | 0.001 | 29.1 | (10.3, 82.0) | 0.001 | 0.71 | (0.42, 1.19) | 0.194 |
|
| |||||||||
| Navigation | |||||||||
| No navigation | 1.00 | Ref | 1.00 | Ref | 1.00 | Ref | |||
| Navigation FIT + CX | 2.09 | (1.26, 3.49) | 0.005 | 1.53 | (0.85, 2.76) | 0.157 | 3.22 | (1.52, 6.82) | 0.002 |
Final multivariable results based on 933 participants with full covariate data. The model included the three variables shown in the table, and controlled for primary care practice, age, sex, race, education, marital status, perceptions of CRC and screening, and baseline screening decision stage
FIT: fecal immunochemical test. CX: colonoscopy.
Mailed access to both FIT and CX
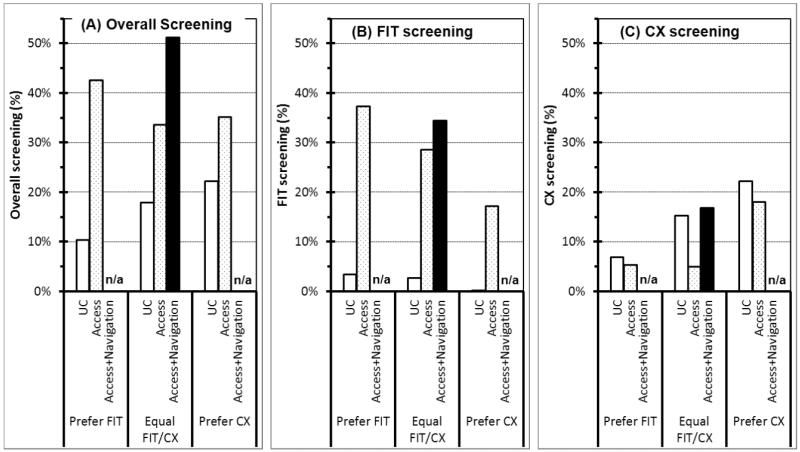
Compared to usual care access, mailed access to both FIT and CX was associated with a strong increase in overall screening (Figure 1A; “access” versus “usual care” bars within each of the test preference categories). This was due to a substantial increase in FIT screening, although there was also a small decrease in CX screening (Figure 1B and 1C, respectively). Multivariable analyses (Table 3) confirmed that providing mailed access to both tests led to a strong and statistically significant increase in overall screening (odds ratio, OR = 2.64). This effect was due to significantly increased FIT screening (OR = 29.1), while CX screening was somewhat decreased, although not significantly so (OR = 0.71).
Figure 1.
Colorectal cancer screening adherence by test preference, test access, and navigation (N = 945). (A) Overall screening; (B) FIT screening; and (C) CX screening.
UC: usual care. Access: mailed access for both FIT (mailed kit) and CX (study telephone number). Navigation: telephone navigation for both FIT and CX.
Navigation for both FIT and CX
Navigation for both tests was associated with an increase in overall screening, as well as both FIT and CX (Figure 1A, 1B, and 1C, respectively; “access+navigation” versus “access” bars among individuals with an equal preference for FIT and CX). Multivariable analyses (Table 3) confirmed that navigation was associated with a significant increase in overall screening (OR = 2.09), and that this effect was due to increases mainly in CX (OR = 3.22) and less so in FIT (OR = 1.53). Although the navigation impact on CX screening was significant and that on FIT screening was not, the magnitude of these two effects was not statistically different from each other (p = 0.080).
Non-tailored access and navigation for both tests versus tailored access and navigation
To estimate the independent effect of tailoring access and navigation, we would need to contrast the TNI group, which provided access and navigation for the preferred test(s), with a non-tailored intervention that would have provided access and navigation to both tests, irrespective of test preference. Since the latter intervention was not implemented in our study, we estimated the impact of tailored access and navigation indirectly.
This indirect estimation of the tailoring effect was based on an assumption. We found that navigation among participants with an equal FIT/CX preference led to increases in both FIT and CX screening (Table 3, OR = 1.53 and 3.22, respectively), but could not estimate the effect among participants with either FIT or CX preference. Therefore, our assumption entailed an extrapolation that the same screening benefit would also apply to these latter individuals, i.e., that there is no interaction between preference and navigation. This assumption is partly supported by the fact that we found no significant interaction between preference and access. The substantial increase in FIT screening and modest decrease in CX screening was evident among each of the three test preference categories (Figure 1, “access” versus “usual care” bars within each of the three test preference categories; adjusted p for the interaction = 0.751 for FIT screening and 0.318 for CX screening). The assumption is also consistent with the possibility that navigation for both tests may increase not only the performance of the preferred test, but also that of the non-preferred test, as some individuals change their preference during navigation. In our study, test preference did indeed change during the navigation telephone call for 8% of those initially preferring FIT and 17% of those initially preferring CX.
For the estimation of the tailoring effect, the non-tailored and the preference-tailored interventions are by definition identical among individuals with equal FIT/CX preference. However, among individuals who prefer FIT, we estimated that the non-tailored intervention will lead to slightly higher overall screening than the tailored intervention (Table 4, OR = 1.12, p = 0.799), largely due to somewhat increased performance of the non-preferred test (CX). Similarly, among individuals who prefer CX screening, we estimated that the non-tailored intervention will lead to substantially higher overall screening than the tailored intervention (Table 4, OR = 2.70, p = 0.005), again due to substantially higher performance of the non-preferred test (FIT).
Table 4. Comparison of hypothetical strategy of non-tailored access and navigation for both FIT and CX versus TNI strategy of tailored access and navigation for preferred test(s) only (N = 933*).
| Overall screening | FIT screening vs. none | CX screening vs. none | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | |
| Among those with: | |||||||||
| FIT preference | 1.12 | (0.47, 2.66) | 0.799 | 0.95 | (0.36, 2.51) | 0.918 | 5.39 | (0.57, 51.2) | 0.143 |
| Equal FIT/CX preference | 1.00 | N/A | 1.00 | N/A | 1.00 | N/A | |||
| CX preference | 2.70 | (1.36, 5.37) | 0.005 | 5.62 | (1.81, 17.4) | 0.001 | 1.78 | (0.75, 4.20) | 0.190 |
Final multivariable results based on 933 participants with full covariate data. The model included terms for preference, access, and navigation (from which the above tailoring effects were estimated), and controlled for primary care practice, age, sex, race, education, marital status, perceptions of CRC and screening, and baseline screening decision stage.
FIT: fecal immunochemical test. CX: colonoscopy.
N/A: not applicable (interventions are identical by design for this preference group).
DISCUSSION
In our study, more participants preferred CX or had similar preference for the two tests than preferred SBT. By itself, participant preference for a given CRC screening test was not associated with the likelihood of screening. However, having a preference for a particular screening test increased the likelihood of completing that test.
Our study was conducted in a diverse set of practices with patterns regarding modalities of CRC screening that mirror patterns that exist in many health systems in the United States.(10) Yet, our results regarding access, navigation, and tailoring may not generalize to settings where screening modalities other than colonoscopy are predominant.
In terms of access, we found that the mailing of both an FIT kit and a CX information number led to doubling of overall screening, which was due mainly to a thirty-fold increase in FIT screening. This result is consistent with prior conclusions that mailed stool blood test contacts are an effective way to increase CRC screening.(15,16) However, providing mailed access to both tests also led to a small and non-significant decrease in CX screening. These findings are in very good agreement with the results of a recent randomized trial.(17)
In our study, the addition of patient navigation to a mailed FIT kit and CX information number led to a doubling of overall screening, which reflected a 50% increase in FIT screening and a three-fold increase in CX screening. Our findings are again similar to those reported by a recent randomized trial.(17) In our study, the effect of navigation on CX screening is particularly notable, given that participants were only provided a telephone number to use in scheduling CX screening. The impact of navigation might be enhanced if the navigators were authorized to schedule the screening procedure for interested patients, as has been the case in some prior studies.(18,19) However, in a recent randomized trial, after a provider recommendation during an office visit for either stool blood test or colonoscopy, the latter strategy performed worse, even with direct scheduling and other support offered.(20)
Preference-tailoring of screening options has been partly motivated by the concern that too many options may lead to patient confusion and inaction.(21) However, results of a recent randomized trial suggest that restricting the choice regarding screening tests might actually have a negative impact on screening, particularly if the test offered is colonoscopy.(20,22) In our analyses, we estimated that providing access and navigation to both FIT and CX would generally have higher overall screening than providing limited access and navigation tailored to self-reported preference. The key in this difference is the performance of a test other than the one reported to be preferred. Our results rely on the assumption of no interaction between preference and navigation. However, if the effect of navigation is stronger among individuals with equal FIT/CX preference than among individuals who have a preference for either test, then our results overestimate the difference between the tailored and non-tailored strategies. On the other hand, if the navigation impact is stronger among individuals with a clear preference for either FIT or CX than among individuals with an equal preference for those tests, then our results underestimate that difference.
Our findings regarding tailoring agrees with a previous report that patients do not necessarily complete the screening plan that they deem most preferable.(23) Therefore, to maximize overall CRC screening, it may be advisable to provide access to both FIT and CX screening initially, irrespective of reported preference, and deliver broad navigation that may support the completion of either test (particularly if the patient is having trouble completing his/her preferred test). Given that the logistics of performing an FIT will probably always be somewhat easier than those for undergoing a CX, a non-tailored approach would likely lead to somewhat fewer colonoscopies (and more stool blood tests) than a tailored approach, and therefore require more efforts to achieve annual follow-up of individuals.(24) So, in order for the non-tailored strategy to preserve those screening benefits in the long run, it should incorporate an element of periodic reminders and possibly support and navigation activities for the individuals that opt for stool blood testing. This can be accomplished via automated reminders linked to electronic medical records systems which have been reported to influence patients’ CRC screening.(2,10,25,26) Finally, provider preferences and differential support for one or the other test, the influence of practice and provider performance metrics, and insurance and reimbursement issues may also affect the relative impact of each screening strategy.
Although one-fifth of our study participants were members of minority populations, our findings may not apply to settings that serve more diverse patients. Finally, study enrollment did not take place at the time of a primary care office visit, nor did study contacts involve direct contact between patients and their primary care providers. Although this study design allowed us to reach patients who may not see a provider very often, it did not have the benefit of a face-to-face provider recommendation and referral.(10,27)
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT: All authors received support through a grant from the National Institutes of Health, National Cancer Institute (R01 CA116576, PI: R.E. Myers). Dr. R.E. Myers also received support through a small grant from Olympus America. Stool blood tests were donated by Quest Diagnostics.
Footnotes
CONFLICTS OF INTEREST: The authors declare no conflicts of interest.
Authors’ contributions
Conception and design: Daskalakis, Vernon, Myers.
Acquisition of data: Daskalakis, Sifri, DiCarlo, Cocroft, Myers.
Analyses and interpretation of data: Daskalakis, Cocroft, Sendecki.
Writing and review of manuscript: Daskalakis, Vernon, Sifri, Myers.
Data were collected during the course of a clinical trial with clinicaltrials.gov registration NCT00617071.
REFERENCES
- 1.American Cancer Society . Colorectal Cancer Facts & Figures, 2011-2013. American Cancer Society; Atlanta: [accessed 3/20/2014]. 2011. Available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-028323.pdf. [Google Scholar]
- 2.Stone EG, Morton SC, Hulscher ME, Maglione MA, Roth EA, Grimshaw JM, et al. Interventions that increase use of adult immunization and cancer screening services: a meta-analysis. Ann Intern Med. 2002;136:641–51. doi: 10.7326/0003-4819-136-9-200205070-00006. [DOI] [PubMed] [Google Scholar]
- 3.Jandorf L, Gutierrez Y, Lopez J, Christie J, Itzkowitz SH. Use of a patient navigator to increase colorectal cancers screening in an urban neighborhood health clinic. J Urban Health. 2005;82:216–24. doi: 10.1093/jurban/jti046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasser KE, Murillo J, Lisboa S, Casimir AN, Valley-Shah L, Emmons KM, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171:906–12. doi: 10.1001/archinternmed.2011.201. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher RH. Personalized Screening for Colorectal Cancer. Med Care. 2008;46:S5–S9. doi: 10.1097/MLR.0b013e31817d930b. [DOI] [PubMed] [Google Scholar]
- 6.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46:S10–6. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 7.Nelson RL, Schwartz A. A survey of individual preference for colorectal cancer screening technique. BMC Cancer. 2004;4:76. doi: 10.1186/1471-2407-4-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shokar NK, Carlson CA, Weller SC. Informed decision making changes test preferences for colorectal cancer screening in a diverse population. Ann Fam Med. 2010;8:141–50. doi: 10.1370/afm.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf RL, Basch CE, Brouse CH, Shmukler C, Shea S. Patient preferences and adherence to colorectal cancer screening in an urban population. Am J Public Health. 2006;96:809–11. doi: 10.2105/AJPH.2004.049684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al. NIH State-of-the-Science Statement on Enhancing Use and Quality of Colorectal Cancer Screening. NIH Consens State Sci Statements. 2010 Feb 2-4;27:1–31. [PubMed] [Google Scholar]
- 11.Myers RE, Bittner-Fagan H, Daskalakis C, Sifri R, Vernon SW, Cocroft J, et al. A Randomized Controlled Trial of Tailored Navigation and Standard Intervention in Colorectal Cancer Screening. Cancer Epidemiol Biomarkers Prev. 2013;22:109–17. doi: 10.1158/1055-9965.EPI-12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial Validity and Invariance of a Survey Measuring Psychosocial Correlates of Colorectal Cancer Screening among African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005;14:2855–61. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- 13.Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–91. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 14.Myers RE, Hyslop T, Sifri R, Bittner-Fagan H, Katurakes NC, Cocroft J, et al. Tailored navigation in colorectal cancer screening. Med Care. 2008;46:S123–31. doi: 10.1097/MLR.0b013e31817fdf46. [DOI] [PubMed] [Google Scholar]
- 15.Community Preventive Services Task Force Updated Recommendations for Client- and Provider-Oriented Interventions to Increase Breast, Cervical, and Colorectal Cancer Screening. Am J Prev Med. 2012;43:92–6. doi: 10.1016/j.amepre.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Sabatino SA, Habarta N, Baron RC, Coates RJ, Rimer BK, Kerner J, et al. Task Force on Community Preventive Services Interventions to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers. Am J Prev Med. 2008;35:S67–S74. doi: 10.1016/j.amepre.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Green BB, Wang C-Y, Anderson ML, Chubak J, Meenan RT, Vernon SW, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening. Ann Intern Med. 2013;158:301–11. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–84. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 19.Percac-Lima S, Grant RW, Green AR, Ashburner JM, Gamba G, Oo S, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: A randomized, controlled trial. J Gen Intern Med. 2009;24:211–17. doi: 10.1007/s11606-008-0864-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, et al. Adherence to colorectal cancer screening: A randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–82. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones RM, Vernon SW, Woolf SH. Is discussion of colorectal cancer screening options associated with heightened patient confusion? Cancer Epidemiol Biomarkers Prev. 2010;19:2821–25. doi: 10.1158/1055-9965.EPI-10-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin TR. The importance of choosing colorectal cancer screening tests: Comment on “Adherence to colorectal cancer screening.”. Arch Intern Med. 2012;172:582–3. doi: 10.1001/archinternmed.2012.349. [DOI] [PubMed] [Google Scholar]
- 23.Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Med Decis Making. 2002;22:125–39. doi: 10.1177/0272989X0202200210. [DOI] [PubMed] [Google Scholar]
- 24.Myers RE, Balshem AM, Wolf TA, Ross EA, Millner L. Adherence to continuous screening for colorectal neoplasia. Med Care. 1993;31:508–19. doi: 10.1097/00005650-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Atassi K. Strategies to increase colorectal cancer screening. The Nurse Practitioner. 2012;37:21–6. doi: 10.1097/01.NPR.0000415240.16601.d1. [DOI] [PubMed] [Google Scholar]
- 26.Sarfaty M. How to Increase Colorectal Cancer Screening Rates in Practice: A Primary Care Clinician’s Evidence-Based Toolbox and Guide, 2008. The National Colorectal Cancer Roundtable, American Cancer Society and Centers for Disease Control and Prevention; [accessed 3/20/2014]. 2006. (revised 2008). Available at http://www.cancer.org/acs/groups/content/documents/document/acspc-024588.pdf. [Google Scholar]
- 27.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare System Factors and Colorectal Cancer Screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.