Abstract
Purpose
Scant literature exists on the use of Complementary and Alternative Medicine (CAM) among patients with lung cancer. Preliminary data indicates that perceived control is an important factor leading patients to CAM. This study aimed to evaluate the relationship between perceived control and CAM use in patients with lung cancer.
Methods
We performed a cross sectional survey in patients with lung cancer under active treatment and follow-up at the oncology clinic of an academic medical center. Self-reported CAM use was the primary outcome. Multivariate logistic regression was performed to determine the relationship between perceived control and CAM use, controlling for other factors.
Results
Among 296 participants. 54.4% were female, 83.5% Caucasian, 57.6% ≤65 years old, 52.4% stage IV and 86.4% had Non-small cell lung cancer. 50.9% of patients had used CAM, most commonly vitamins (31.5%), herbs (19.3%), relaxation techniques (16%) and special diets (15.7%). In multivariate analysis, CAM use was associated with having greater perceived control over the cause of cancer (Adjusted Odds Ratio (AOR) 2.27, 95% CI 1.35–3.80), age ≤65 (AOR 1.64, 95% Confidence Interval (CI) 1.01–2.67), higher education (AOR 2.17, 95% CI 1.29–3.64), and never having smoked tobacco (AOR 2.39, 95% CI 1.25–4.54). Nearly 60% of patients who used CAM were receiving active treatment.
Conclusion
Over half of lung cancer patients have used CAM since diagnosis. Greater perceived control over the cause of cancer was associated with CAM use. Given the high prevalence of CAM, it is essential that oncologists caring for patients with lung cancer discuss its use.
Keywords: Integrative Medicine, Complementary and Alternative Medicine, Prevalence, Lung Cancer, Locus of Control, Perceived Control, Smoking
Background
Lung cancer remains the leading cause of cancer death in the United States and is a major source of symptom burden.[30, 36, 38] Compared with other common malignancies, the symptom distress experienced by patients with lung cancer is more severe and persistent, which has a detrimental effect on quality of life.[8, 33]. Impaired quality of life is associated with a worse prognosis in lung cancer,[26] and emerging data suggest interventions aimed solely at improving quality of life may prolong survival.[39] The mechanism underlying this survival advantage remains unclear, though this is an area of active research.[17] The potential for improved overall survival further emphasizes the importance of providing evidence-based supportive care. Unfortunately, the relative scarcity of supportive care research among patients with lung cancer makes such integration difficult. As a result, many patients turn to Complementary and Alternative Medicines (CAM) to alleviate their symptoms.
CAM utilization among patients with cancer has increased in recent years, and many patients use CAM to improve their quality of life.[24, 32] Depending upon the cancer population studied, estimates of CAM use range from 30–90%. CAM use has historically been associated with female gender, younger age, lack of tobacco exposure, and a higher level of education.[21, 29, 32, 35, 42]
CAM use has also been associated with a greater sense of perceived control among patients with breast cancer.[16] Perceived control is defined by the American Psychological Association as “the belief that one has the ability to make a difference in the course or consequence of some event or experience”.[15] When applied to cancer, this refers to control over why an individual got cancer as well as the treatment outcome.[40] Patients with cancer may utilize CAM as a way to regain control of issues both directly related to their cancer treatment as well as more global problems, which they may attribute to the cause of their cancer from a holistic perspective. [1, 31, 34]
Despite growing literature on CAM use in cancer, scant data exist among patients with lung cancer. Population studies do not include a proportional number of patients with lung cancer, and lung cancer focused studies have had small sample sizes.[25, 41] Amichai et al performed a qualitative assessment of twelve patients with lung cancer regarding their use of CAM, and noted that perceived control was a critical factor involved in CAM use in this population.[1] No study to date has quantitatively evaluated the impact of perceived control on CAM use in lung cancer. Tobacco use is the known cause of cancer in the majority of patients, so perceived control over cancer has different psychological implications compared to other malignancies where the cause is unknown.[11] Never smokers will likely have a very different perception of their control over their cancer as compared to former or current smokers, but this has never been evaluated.
The dearth of data regarding factors associated with CAM use among patients with lung cancer, along with the likely divergent nature of perceived control in this population, motivated us to quantify the prevalence of and identify the factors associated with CAM use in patients with lung cancer with a specific focus on perceived control. Our hypotheses were 1) Patients with lung cancer will have utilized CAM at a rate at least comparable to that seen in studies evaluating patients with multiple cancers, if not higher; and 2) Higher degree of perceived control over their cancer cause and treatment outcome will be associated with CAM use. As a secondary aim, we explored the relationship between tobacco exposure and perceived control.
Methods
Study design and patients
We conducted a cross-sectional survey study among a consecutive convenience sample of patients seen in the outpatient thoracic oncology clinic at the Hospital of the University of Pennsylvania between June 2010 and October 2011. Eligible participants were aged 18 years or older, had a primary diagnosis of lung cancer, and a Karnofsky score ≥ 60 (i.e. ambulatory). We did not exclude patients based on lung cancer stage or subtype (small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC)), cancer stage, cancer recurrence status, treatment types or status. Additional inclusion criteria stipulated the approval of the patient’s oncologist and the patient’s ability to understand and provide informed consent in English. Once the oncologist approved the patient’s enrollment, they had no access to the patient’s survey responses. Trained research assistants screened medical records and approached potential study subjects in the waiting area of the oncology clinic. After providing informed consent, each participant was given a self-report survey. The Institutional Review Board of the University of Pennsylvania approved the study.
CAM Use: Primary Outcome
To measure CAM use, we asked patients, “Have you used the following CAM therapies since your cancer diagnosis?” CAM modalities included acupuncture, chiropractic care, special diet, energy healing (e.g reiki, qi gong), expressive arts therapy, herbs, homeopathy, massage, relaxation techniques (e.g. mindfulness based meditation, deep breathing), vitamins (besides a daily multivitamin), yoga, tai chi, or other. Prayer for healing was not included since our group had previously found this had a different epidemiologic distribution from other CAM modalities.[23] Our group developed this instrument based upon CAM modalities commonly used in the 2002 National Health Interview Survey. The modalities included accounted for 92% of CAM usage in the survey.[22] Participants were dichotomized into two groups based on survey response: those who had used one or more of these modalities versus those who had not used any of these modalities.
Perceived Control – Primary independent variable
Perceived control was measured using the Cancer Locus of Control Scale.[40] Originally designed in Dutch to evaluate perceived control among cancer patients, it was then translated and validated in 68 English-speaking patients with cancer by Watson et al. This 17-item instrument was found to be reliable and separates perceived control into three subdomains: control over the cause of cancer, control over treatment outcome (course of cancer), and religious control. The Cronbach alpha for the subdomains ranged from 0.77 to 0.80. Because the distribution of the scores was not normal, we dichotomized the scores into no/low versus medium/high control for ease of interpretation, using methods described by Henderson et al.[16]
Covariates
We queried patients about their perceived health status. Patients were asked “How would you rate your health in general?” with five options ranging from “poor” to “excellent”. This single item question has been incorporated in multiple epidemiologic studies.[9]
Participants self-reported sociodemographic variables including gender, age, race/ethnicity, tobacco exposure history, and education level. Chart abstraction was performed using the electronic medical record to determine cancer subtype, cancer stage, and treatment status (not yet treated; receiving treatment; or post therapy). Staging was based upon the 7th edition of the American Joint Committee on Cancer Lung Cancer Staging Algorithm for both SCLC and NSCLC.
Statistical analysis
Sample size was estimated based upon an expected relative risk for CAM use in the setting of greater perceived control over cause of 1.4. This was a conservative estimate based upon the work of Henderson et al.[16] Based upon this, we would have 90% power to detect this size difference with a sample size of 242 patients. We performed statistical analyses using STATA software (Mac version 12.0, StataCorpLP, College Station, TX). Descriptive statistics were used to examine the distribution of the outcomes and covariates. Next, we used χ2 tests to identify which covariates were associated with CAM use. Multivariate logistic regression analyses were conducted to identify independent predictors of CAM use, using only variables that had a p value of ≤0.05 in the χ2 analyses. For the secondary analysis, χ2 tests were used to evaluate the association of smoking history with degree of perceived control. All analyses were two-sided at a significance level of 0.05.
Results
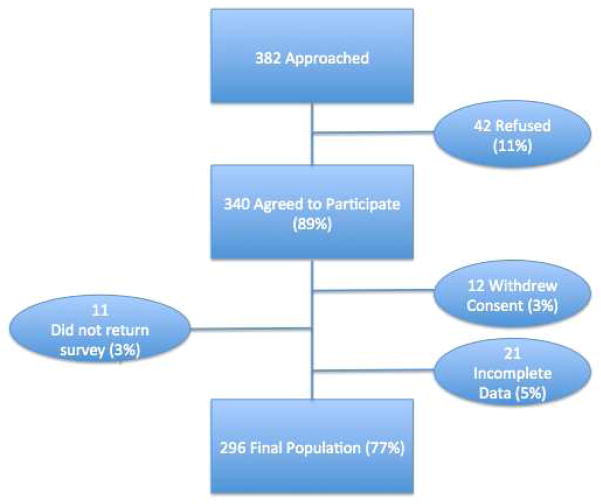
Of the 382 consecutive patients approached, 340 (89%) agreed to participate. The main reasons for patients to decline participation in the survey included lack of interest 35 (9.2%) or an inability to complete the survey due to time or sickness 7 (1.8%). Additionally, 12 subjects withdrew consent, 11 subjects did not return the survey, and 21 subjects were excluded from the analysis due to incomplete data, thus resulting in the final sample of 296 (see Figure I). This population reflected a response rate of 77.5% among eligible subjects.
Figure I.
Flow Diagram
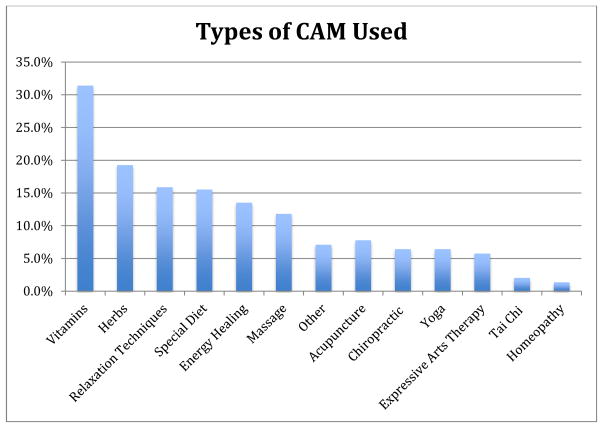
The mean age of participants was 63.1; 45.6% of patients were male. Caucasians made up 83.5% of the sample; the remainder included 12.5% African Americans, 2.7% Asian Americans and 0.34% Native Americans. NSCLC was the diagnosis in 86.4% of patients and 52.4% of patients had stage IV disease. 55.5% of patients surveyed were currently undergoing therapy. Of patients in our sample, 38.8% perceived a medium/high degree of control over the cause of their cancer, while 93.9% of patients perceived a medium/high degree of control over their treatment outcome (Table I). Among participants (n=296), 50.9% reported CAM use. The most common modalities included vitamins (31.5%), herbs (19.3%), relaxation techniques (16%), and special diets (15.7%) (Figure II).
Table I.
Demographics and Clinical Characteristics
| Number (%) | ||
|---|---|---|
| Gender | Male | 135 (45.6%) |
| Female | 161 (54.4%) | |
|
| ||
| Race | Caucasian | 247 (83.5%) |
| African American | 37 (12.5%) | |
| Asian | 8 (2.7%) | |
| Native American | 1 (0.34%) | |
| Other | 3 (1.0%) | |
|
| ||
| Age | Less than or Equal to 65 | 170 (57.6%) |
| Mean 63.1 (10.8) | Over 65 | 125 (42.4%) |
| Missing | 1 (0.34%) | |
|
| ||
| Education | Less than a College Degree | 190 (64.2%) |
| College Degree or more | 106 (35.8%) | |
|
| ||
| Smoking History | Current Smoker | 32 (10.9%) |
| Former Smoker | 201 (68.1%) | |
| Never Smoker | 62 (21.0%) | |
| Missing | 1 (0.34%) | |
|
| ||
| Cancer Type | NSCLC | 253 (86.4%) |
| SCLC | 33 (11.3%) | |
| Other | 7 (2.4%) | |
| Missing | 3 (1%) | |
|
| ||
| Cancer Stage | I | 36 (12.2%) |
| II | 28 (9.52%) | |
| III | 76 (25.9%) | |
| IV | 154 (52.4%) | |
| Missing | 2 (0.7%) | |
|
| ||
| Treatment History | Surgery to Remove Cancer | 118 (39.9%) |
| Chemotherapy | 248 (83.8%) | |
| Radiation Therapy | 160 (54.1%) | |
| Immune Therapies | 35 (11.8%) | |
|
| ||
| Treatment Phase | Prior to Therapy | 13 (4.5%) |
| Currently in Therapy | 162 (55.5%) | |
| Completed Therapy | 98 (33.6%) | |
| Other | 19 (6.5%) | |
| Missing | 4 (1.3%) | |
|
| ||
| CAM Utilization | Yes | 150 (50.9%) |
| No | 145 (49.2%) | |
| Missing | 1 (0.34%) | |
|
| ||
| Perceived Health Status | Fair/Poor | 69 (23.3%) |
| Good/Very Good/Excellent | 227 (76.7%) | |
|
| ||
| Control over Cause | No/Low Control | 181 (61.2%) |
| Medium/High Control | 115 (38.8%) | |
|
| ||
| Control over Outcome | No/Low Control | 18(6.1%) |
| Medium/High Control | 278 (93.9%) | |
|
| ||
| Religious Control | No/Low Control | 93 (31.4%) |
| Medium/High Control | 203 (68.6%) | |
Figure II.
Types of CAM Used
On univariate analysis, greater perceived control over the cause of cancer was associated with CAM use (p=0.02), but perceived control over treatment outcome (p=0.94) and religious control (p=0.75) were not. Younger age (p=0.02), higher education (p<0.001), and never having smoked tobacco (p=0.007) were also associated with CAM use. Gender (p=0.54) and race (p=0.78) were not significantly associated with CAM use (Table II). After adjusting for other covariates, a higher degree of perceived control (p=0.002), age ≤65 years old (p=0.047), having a minimum of a college degree (p=0.003), and never smoker status (p=0.008) were associated with CAM use (Table III). 57.8% of patients who used CAM were actively receiving therapy. On sub-analysis of patients who had taken herbs or vitamins (n=150), 96 (64%) were actively receiving therapy.
Table II.
Characteristics related to CAM Use
| CAM Use (%) | X2 p-value | ||
|---|---|---|---|
| Gender | Male | 66 (48.9%) | 0.54 |
| Female | 84 (52.5%) | ||
|
| |||
| Race | Caucasian | 127 (51.6%) | 0.78 |
| African American | 17 (46.0%) | ||
| Asian | 4 (50.0%) | ||
| Native American | 0 (0%) | ||
| Other | 2 (66.7%) | ||
|
| |||
| Age | Less than or Equal to 65 | 96 (56.5%) | 0.02 |
| Over 65 | 54 (43.2%) | ||
|
| |||
| Education | Less than College Degree | 82 (43.4%) | <0.001 |
| College Degree or more | 68 (64.2%) | ||
|
| |||
| Smoking History | Ever Smoker | 109 (46.8%) | 0.007 |
| Never Smoker | 41 (66.1%) | ||
|
| |||
| Cancer Type | NSCLC | 133 (52.6%) | 0.14 |
| SCLC | 12 (37.5%) | ||
| Other | 2 (28.6%) | ||
| Cancer Stage | I | 19 (54.3%) | 0.24 |
| II | 10 (35.7%) | ||
| III | 35 (46.1%) | ||
| IV | 84 (54.65) | ||
|
| |||
| Treatment Phase | Prior to Therapy | 5 (38.5%) | 0.07 |
| Currently in Therapy | 93 (57.8%) | ||
| Completed Therapy | 41 (41.8%) | ||
| Other | 10 (52.6%) | ||
|
| |||
| Perceived Health Status | Fair/Poor | 39 (56.5%) | 0.28 |
| Good/Very Good/Excellent | 111 (49.1%) | ||
|
| |||
| Control over Cause | No/Low Control | 82 (45.3%) | 0.02 |
| Medium/High Control | 68 (59.7%) | ||
|
| |||
| Control over Outcome | No/Low Control | 9 (50%) | 0.94 |
| Medium/High Control | 141 (50.9%) | ||
|
| |||
| Religious Control | No/Low Control | 46 (49.5%) | 0.75 |
| Medium/High Control | 104 (51.5%) | ||
Table III.
Multivariate Logistic Regression Predicting CAM Usage
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR* | 95% Confidence Interval | P value | aOR** | 95% Confidence Interval | P value | |
| Age | 0.024 | 0.047 | ||||
|
| ||||||
| Greater than 65 | 1 | 1 | ||||
| Less than or Equal to 65 | 1.71 | 1.07–2.72 | 1.64 | 1.01–2.67 | ||
|
| ||||||
| Education | 0.001 | 0.003 | ||||
|
| ||||||
| Less than college degree | 1 | 1 | ||||
| College degree or more | 2.33 | 1.43–3.81 | 2.17 | 1.29–3.64 | ||
|
| ||||||
| Control over Cause | 0.016 | 0.002 | ||||
|
| ||||||
| No/Low Control | 1 | 1 | ||||
| Medium/High Control | 1.78 | 1.11–2.87 | 2.27 | 1.35–3.80 | ||
|
| ||||||
| Tobacco History | 0.008 | 0.008 | ||||
|
| ||||||
| Ever Smoker | 1 | 1 | ||||
| Never Smoker | 2.22 | 1.24–3.99 | 2.39 | 1.25–4.54 | ||
OR – Odds Ratio
aOR- Adjusted Odds Ratio
Individuals who were former or current smokers more likely to perceive a higher degree of control over the cause of their cancer than patients without a tobacco use history (p<0.001, Table IV). There was no significant difference in perceived control over treatment outcome or degree of religious attribution of control between smokers and never-smokers.
Table IV.
Association of Perceived Control with Smoking History
| Ever Smokers (%) | Never Smokers (%) | X2 p value | ||
|---|---|---|---|---|
| Control over Cause | No/Low Control | 128 (54.7%) | 53 (85.5%) | <0.001 |
| Medium/High Control | 106 (45.3%) | 9 (14.5%) | ||
|
| ||||
| Control over outcome | No/Low Control | 14 (6%) | 4 (6.5%) | 0.89 |
| Medium/High Control | 220 (94%) | 58 (93.5%) | ||
|
| ||||
| Religious Control | No/Low Control | 71 (30.3%) | 22 (35.5%) | 0.44 |
| Medium/High Control | 163 (69.7%) | 40 (64.5%) | ||
Discussion
Interest in CAM use among cancer patients has surged in recent years, but scant literature exists regarding the use of CAM among patients with lung cancer. In the largest evaluation to date, we found that slightly over half of our patients with lung cancer had used CAM since diagnosis. While greater perceived control over the cause of cancer was associated with CAM use, greater perceived control over treatment outcome was not.
One previous study in Europe found that CAM use was relatively infrequent in patients with lung cancer,[25] but that study only had 111 patients with a relatively low level of education. Another prior study performed in the US revealed CAM use in 44% in patients with lung cancer, but that study focused exclusively on women and included prayer in their definition of CAM.[41] Our data indicate CAM use among patients with lung cancer mirrors rates seen in larger cancer population-based studies.[23, 24] It is also consistent with a prior population study which indicated a relatively high CAM utilization rate among patients with lung cancer compared with other cancers.[29] Contrary to what one may expect based solely on the demographic features most commonly associated with CAM use (e.g. female gender, never smoking status), patients with lung cancer are indeed using CAM modalities. This is an important topic for future prospective research.
The association of CAM use with younger age, greater education and a lack of an exposure to tobacco has been well established previously in population-based studies[13, 21, 32, 35, 42] and in a smaller previous study evaluating CAM use among patients with lung cancer.[41] In contrast, prior literature had indicated that women were more likely to utilize CAM,[13, 21, 32, 35, 42] which we did not find in our population. This distinction may be secondary to the inclusion of more breast cancer patients in prior population studies, given the high rate of CAM use in breast cancer relative to other cancers.[13, 23, 29, 35] Supporting our observation, a study from Sweden demonstrated no gender disparity in CAM use among patients with lung cancer.[20]
The lack of an association of CAM use with perceived control over treatment outcome is unexpected and novel. Among patients with breast cancer, one of the strongest predictors of CAM use is a perception that one will be able to influence treatment outcome.[16] The lesser association among patients with lung cancer may be related to prognosis. While over 50% of the patients in our study were incurable at diagnosis, less than 25% of patients with breast cancer present with incurable disease.[36] This may induce a type of therapeutic nihilism, whereby patients are less likely to undertake an intervention with a hope of affecting treatment outcome. Patients with breast cancer also tend to desire a high degree of control over their treatment decisions,[2] and this is amplified among CAM users.[4] As a result, the association of CAM use with control over treatment outcome may be a unique correlation in patients with breast cancer.
The strong association of CAM use with perceived control over the cause of cancer is also a new finding. In a prior study, a majority of patients with lung cancer admitted that their lung cancer was caused by smoking, but 81% then went on to qualify this response by stating that tobacco was only partially responsible. In addition, the degree of causal attribution varied over time, and a higher degree of causal attribution was associated with a worse quality of life.[11] This stands in stark contrast to breast and colon cancers, where the degree of perceived control over the cause of cancer remains constant over time and is associated with improved quality of life.[3, 6, 18] This distinction may be secondary to the societal stigma attached to lung cancer, which has been shown to have a profound negative impact on patients’ lives.[5] Patients may be using CAM modalities, which are widely viewed as health promoting,[1, 32] as a means to overcome the stigma attached to lung cancer.
In our secondary analysis, we found that former and current smokers have greater perceived control over the cause of their cancer. One hypothesis for the finding that both patients with a medium/high degree of perceived control over the cause of their cancer (largely current and former smokers) and never smokers (who had a low degree of perceived control over the cause of their cancer) were using CAM is that these represent two distinct groups. Both pathways, being a never smoker without perceived control and being a former smoker with higher perceived control, can lead to CAM use. This is consistent with prior qualitative work, which shows that patients with lung cancer may be driven to CAM use by multiple factors.[1] The relationship between perceived control over cause and CAM use requires further investigation in a prospective fashion to evaluate the direction of causality, if present.
Given the high rate of CAM use documented in our and other studies, communication about CAM use should be an essential aspect of oncologic care. Unfortunately, a majority of patients using CAM do not discuss its use with their oncologists.[10, 14] Some patients cited concern that their oncologist would judge their use of CAM harshly, but emerging data indicate that oncologists may be open to integration of CAM into their patients’ care.[19] A frank discussion is important to allow education of potential interactions and prevent toxicities.[12, 37] For example, despite the legitimate concerns among clinicians that some herbs and vitamins may affect chemotherapy levels or even be tumor protective from anti-cancer therapies,[7, 28] the most common forms of CAM used in our study were herbs and vitamins. Since over 60% of patients who used these modalities were actively receiving anti-cancer therapy, more research should focus on how to efficiently and effectively engage patients and providers to discuss CAM use in oncology setting.
Our study had a number of important limitations. First, the cross-sectional design limits any causal conclusions. In addition, our CAM measurement yielded a binary grouping of CAM users and CAM non-users. While this gave us the statistical power to answer the broader question as stated, it did not capture the more subtle nuances of CAM use (e.g. very brief exposure to CAM versus those who utilize multiple CAM modalities on a daily basis). Further study should more closely quantify CAM use in a prospective fashion. Next, our population contained more women than men, which is distinct from the epidemiology of lung cancer.[36] This is likely due to the improved prognosis women with lung cancer have relative to men.[27] Lastly, our sample was drawn from an urban academic cancer center therefore limiting its generalizability to community settings.
Despite these limitations, our study is the largest to date to evaluate CAM use among only lung cancer patients. Given the high degree of morbidity in this population and the fact that CAM is often used to improve quality of life, this population represents an important group to study. Over half the patients surveyed had used some form of CAM since their diagnosis, so it is clearly important for oncologists caring for patients with lung cancer to inquire about CAM use. In addition, this observation underscores the importance of continued research into evidence-based integration of these practices into usual clinical care. Such research represents an important opportunity to improve symptom management and quality of life for patients with lung cancer.
Acknowledgments
We would like to thank all of the patients who participated in this study. We thank the physicians, nurse practitioners, and staff for their support. We would like to thank our hard-working students, Eitan Frankel, Jonathan Burgess, Blake Freidman, Manuel Bramble, Neha Agarwal, and Tiffany Tan for their dedication to the data collection and management process.
Footnotes
Conflict of Interest: This study is partially funded by the Penn Institute of Aging Pilot Fund. Dr. Mao is supported National Institutes of Health [1K23 AT004112-05]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have full control of the primary data, which is available to the journal at their request for review.
References
- 1.Amichai T, Grossman M, Richard M. Lung cancer patients’ beliefs about complementary and alternative medicine in the promotion of their wellness. European journal of oncology nursing : the official journal of European Oncology Nursing Society. 2012;16:520–527. doi: 10.1016/j.ejon.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Balneaves LG, Kristjanson LJ, Tataryn D. Beyond convention: describing complementary therapy use by women living with breast cancer. Patient education and counseling. 1999;38:143–153. doi: 10.1016/s0738-3991(99)00061-0. [DOI] [PubMed] [Google Scholar]
- 3.Barez M, Blasco T, Fernandez-Castro J, Viladrich C. A structural model of the relationships between perceived control and adaptation to illness in women with breast cancer. Journal of psychosocial oncology. 2007;25:21–43. doi: 10.1300/J077v25n01_02. [DOI] [PubMed] [Google Scholar]
- 4.Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, McWilliam C, Gavin A, Baron RA, Aaron D, Haines-Kamka T. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18:2515–2521. doi: 10.1200/JCO.2000.18.13.2515. [DOI] [PubMed] [Google Scholar]
- 5.Chapple A, Ziebland S, McPherson A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. Bmj. 2004;328:1470. doi: 10.1136/bmj.38111.639734.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costanzo ES, Lutgendorf SK, Roeder SL. Common-sense beliefs about cancer and health practices among women completing treatment for breast cancer. Psychooncology. 2011;20:53–61. doi: 10.1002/pon.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Andrea GM. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J Clin. 2005;55:319–321. doi: 10.3322/canjclin.55.5.319. [DOI] [PubMed] [Google Scholar]
- 8.Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10:423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.DeSalvo KB, Bloser N, Reynolds K, He J, Muntner P. Mortality prediction with a single general self-rated health question. A meta-analysis. Journal of general internal medicine. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenberg DM, Kessler RC, Van Rompay MI, Kaptchuk TJ, Wilkey SA, Appel S, Davis RB. Perceptions about complementary therapies relative to conventional therapies among adults who use both: results from a national survey. Annals of internal medicine. 2001;135:344–351. doi: 10.7326/0003-4819-135-5-200109040-00011. [DOI] [PubMed] [Google Scholar]
- 11.Faller H, Schilling S, Lang H. Causal attribution and adaptation among lung cancer patients. Journal of psychosomatic research. 1995;39:619–627. doi: 10.1016/0022-3999(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel M, Abrams DI, Ladas EJ, Deng G, Hardy M, Capodice JL, Winegardner MF, Gubili JK, Yeung KS, Kussmann H, Block KI. Integrating Dietary Supplements Into Cancer Care. Integr Cancer Ther. 2013 doi: 10.1177/1534735412473642. [DOI] [PubMed] [Google Scholar]
- 13.Gansler T, Kaw C, Crammer C, Smith T. A population-based study of prevalence of complementary methods use by cancer survivors: a report from the American Cancer Society’s studies of cancer survivors. Cancer. 2008;113:1048–1057. doi: 10.1002/cncr.23659. [DOI] [PubMed] [Google Scholar]
- 14.Ge J, Fishman J, Vapiwala N, Li SQ, Desai K, Xie SX, Mao JJ. Patient-Physician Communication About Complementary and Alternative Medicine in a Radiation Oncology Setting. International Journal of Radiation Oncology, Biology, and Physics. 2013;85:e1–e6. doi: 10.1016/j.ijrobp.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerrig RJ, Zimbardo PG. Psychology and Life. Allyn and Bacon 2002 [Google Scholar]
- 16.Henderson JW, Donatelle RJ. The relationship between cancer locus of control and complementary and alternative medicine use by women diagnosed with breast cancer. Psychooncology. 2003;12:59–67. doi: 10.1002/pon.636. [DOI] [PubMed] [Google Scholar]
- 17.Irwin KE, Greer JA, Khatib J, Temel JS, Pirl WF. Early palliative care and metastatic non-small cell lung cancer: potential mechanisms of prolonged survival. Chronic respiratory disease. 2013;10:35–47. doi: 10.1177/1479972312471549. [DOI] [PubMed] [Google Scholar]
- 18.Kidd L, Hubbard G, O’Carroll R, Kearney N. Perceived control and involvement in self care in patients with colorectal cancer. Journal of clinical nursing. 2009;18:2292–2300. doi: 10.1111/j.1365-2702.2009.02802.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee RT, Hlubocky FJ, Hu JJ, Stafford RS, Daugherty CK. An international pilot study of oncology physicians’ opinions and practices on Complementary and Alternative Medicine (CAM) Integr Cancer Ther. 2008;7:70–75. doi: 10.1177/1534735408319059. [DOI] [PubMed] [Google Scholar]
- 20.Lövgren M, Wilde-Larsson B, Hök J. Push or pull? Relationships between lung cancer patients’ perceptions of quality of care and use of complementary and alternative medicine. European Journal of Oncology Nursing. 2011 doi: 10.1016/j.ejon.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Maggiore RJ, Gross CP, Togawa K, Tew WP, Mohile SG, Owusu C, Klepin HD, Lichtman SM, Gajra A, Ramani R, Katheria V, Klapper SM, Hansen K, Hurria A on behalf of the C, Aging Research G . Use of complementary medications among older adults with cancer. Cancer. 2012;118:4815–4823. doi: 10.1002/cncr.27427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao JJ, Cronholm PF, Stein E, Straton JB, Palmer SC, Barg FK. Positive changes, increased spiritual importance, and complementary and alternative medicine (CAM) use among cancer survivors. Integrative cancer therapies. 2010;9:339–347. doi: 10.1177/1534735410387419. [DOI] [PubMed] [Google Scholar]
- 23.Mao JJ, Farrar JT, Xie SX, Bowman MA, Armstrong K. Use of complementary and alternative medicine and prayer among a national sample of cancer survivors compared to other populations without cancer. Complementary therapies in medicine. 2007;15:21–29. doi: 10.1016/j.ctim.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J. Complementary and alternative medicine use among cancer survivors: a population-based study. Journal of cancer survivorship : research and practice. 2011;5:8–17. doi: 10.1007/s11764-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molassiotis A, Panteli V, Patiraki E, Ozden G, Platin N, Madsen E, Browall M, Fernandez-Ortega P, Pud D, Margulies A. Complementary and alternative medicine use in lung cancer patients in eight European countries. Complementary therapies in clinical practice. 2006;12:34–39. doi: 10.1016/j.ctcp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer. 2001;31:233–240. doi: 10.1016/s0169-5002(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 27.O’Connell JP, Kris MG, Gralla RJ, Groshen S, Trust A, Fiore JJ, Kelsen DP, Heelan RT, Golbey RB. Frequency and prognostic importance of pretreatment clinical characteristics in patients with advanced non-small-cell lung cancer treated with combination chemotherapy. J Clin Oncol. 1986;4:1604–1614. doi: 10.1200/JCO.1986.4.11.1604. [DOI] [PubMed] [Google Scholar]
- 28.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 29.Paltiel O, Avitzour M, Peretz T, Cherny N, Kaduri L, Pfeffer RM, Wagner N, Soskolne V. Determinants of the use of complementary therapies by patients with cancer. J Clin Oncol. 2001;19:2439–2448. doi: 10.1200/JCO.2001.19.9.2439. [DOI] [PubMed] [Google Scholar]
- 30.Paull DE, Thomas ML, Meade GE, Updyke GM, Arocho MA, Chin HW, Adebonojo SA, Little AG. Determinants of quality of life in patients following pulmonary resection for lung cancer. American journal of surgery. 2006;192:565–571. doi: 10.1016/j.amjsurg.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Polley MJ, Seers HE, Cooke HJ, Hoffman C, Paterson C. How to summarise and report written qualitative data from patients: a method for use in cancer support care. Support Care Cancer. 2007;15:963–971. doi: 10.1007/s00520-007-0283-2. [DOI] [PubMed] [Google Scholar]
- 32.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 33.Schag CA, Ganz PA, Wing DS, Sim MS, Lee JJ. Quality of life in adult survivors of lung, colon and prostate cancer. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 1994;3:127–141. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 34.Seers HE, Gale N, Paterson C, Cooke HJ, Tuffrey V, Polley MJ. Individualised and complex experiences of integrative cancer support care: combining qualitative and quantitative data. Support Care Cancer. 2009;17:1159–1167. doi: 10.1007/s00520-008-0565-3. [DOI] [PubMed] [Google Scholar]
- 35.Shumay DM, Maskarinec G, Gotay CC, Heiby EM, Kakai H. Determinants of the degree of complementary and alternative medicine use among patients with cancer. Journal of alternative and complementary medicine. 2002;8:661–671. doi: 10.1089/107555302320825183. [DOI] [PubMed] [Google Scholar]
- 36.Siegel R, Naishadham D, Jemal A. Cancer statistics. 2013 CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 37.Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Oncol. 2004;22:2489–2503. doi: 10.1200/JCO.2004.08.182. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Impact of dyspnea, pain, and fatigue on daily life activities in ambulatory patients with advanced lung cancer. J Pain Symptom Manage. 2002;23:417–423. doi: 10.1016/s0885-3924(02)00376-7. [DOI] [PubMed] [Google Scholar]
- 39.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 40.Watson M, Pruyn J, Greer S, van den Borne B. Locus of control and adjustment to cancer. Psychological reports. 1990;66:39–48. doi: 10.2466/pr0.1990.66.1.39. [DOI] [PubMed] [Google Scholar]
- 41.Wells M, Sarna L, Cooley ME, Brown JK, Chernecky C, Williams RD, Padilla G, Danao LL. Use of complementary and alternative medicine therapies to control symptoms in women living with lung cancer. Cancer Nurs. 2007;30:45–55. doi: 10.1097/00002820-200701000-00008. quiz 56-47. [DOI] [PubMed] [Google Scholar]
- 42.Yates JS, Mustian KM, Morrow GR, Gillies LJ, Padmanaban D, Atkins JN, Issell B, Kirshner JJ, Colman LK. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13:806–811. doi: 10.1007/s00520-004-0770-7. [DOI] [PubMed] [Google Scholar]