Abstract
Objective
Basic studies have demonstrated that optimal levels of prefrontal cortical dopamine are critical to various executive functions such working memory, attention, inhibitory control and risk/reward decisions--all of which are impaired in addictive disorders such as alcoholism. Based on this and imaging studies in alcoholics that have demonstrated less dopamine in the striatum, we hypothesized decreased dopamine transmission in the prefrontal cortex in alcoholism. To test this hypothesis, we used amphetamine and [11C]FLB 457 positron emission tomography (PET) to measure cortical dopamine transmission in a group of 21 recently abstinent alcoholics and matched healthy controls.
Methods
[11C]FLB 457 binding potential (BPND) was measured in subjects with kinetic analysis using the arterial input function both before and after 0.5 mg kg−1 of d-amphetamine.
Results
Amphetamine-induced displacement of [11C]FLB 457 binding potential (Δ BPND) was significantly smaller in the cortical regions in alcoholics compared to healthy controls. Cortical regions that demonstrated lower dopamine transmission in alcoholics included the dorsolateral prefrontal cortex, medial prefrontal cortex, orbital frontal cortex, temporal cortex and medial temporal lobe.
Conclusions
The results of this study for the first time unambiguously demonstrate decreased dopamine transmission in the cortex in alcoholism. Further research is necessary to understand the clinical relevance of decreased cortical dopamine as to whether it is related to impaired executive function, relapse, and outcome in alcoholism.
Keywords: 11C]FLB 457, amphetamine, alcoholism, D2/3, dopamine, PET
INTRODUCTION
Prefrontal cortical dopamine modulates executive functions such as attention, working memory, and risk/reward decision making (1, 2)--all of which are impaired in alcoholism (3-6). Based on this, it is tempting to postulate decreased dopamine transmission in the prefrontal cortex in alcoholism. Unfortunately, the preclinical literature on this topic is mixed and inconclusive with some studies suggesting increased (7), decreased (8, 9) and no change (10-12) in prefrontal cortical dopamine transmission in alcoholism. Nevertheless, the ability of prefrontal cortical dopamine to modulate alcohol consumption has been demonstrated in microinjection studies using dopamine D2/3 antagonist and agonist drugs (13, 14). In humans, the displacement of the D2/3 specific PET radiotracer [11C]raclopride following an acute amphetamine (or methylphenidate) challenge has been validated as a noninvasive measure of the change in extracellular dopamine concentration induced by the challenge (15). Using this approach, two groups have reported decreased striatal dopamine transmission in alcohol dependent subjects compared to healthy controls (16, 17). A limitation of these studies was that measurements of dopamine transmission were restricted to the striatum and its subdivisions, i.e., caudate, putamen and ventral striatum. Studies were limited to the striatum, because [11C]raclopride does not provide sufficient signal-to-noise ratio to quantify D2/3 receptors in extrastriatal areas, such as the cortex, where the concentration of D2/3 receptors is much lower than in the striatum. Thus, no previous studies have reported on the in vivo status of dopamine in the prefrontal cortex in alcoholism.
We recently validated the high affinity D2/3 PET radioligand [11C]FLB 457 as a tool to image amphetamine-induced dopamine transmission in the human cortex (18). The results of these validation studies demonstrate: low test-retest variability (≤ 15%) for [11C]FLB 457 binding potential (BPND) under both baseline and post-amphetamine conditions (19, 20); no carryover mass-induced decrease in BPND in the imaging paradigm used to measure dopamine (19); a relatively small fraction of D2/3 receptor specific binding for [11C]FLB 457 in the cerebellar reference region compared to cortical regions of interest (21); and a linear relationship between the amphetamine-induced decreases in [11C]FLB 457 BPND and increases in extracellular dopamine as measured with microdialysis (22). Here, we used amphetamine and [11C]FLB 457 PET to contrast cortical dopamine transmission in 21 recently abstinent subjects with alcohol dependence and 21 healthy comparison subjects matched for age, gender, race, and nicotine smoking status.
MATERIALS AND METHODS
Human Subjects
Seventy-seven alcohol dependent subjects and 36 healthy controls were enrolled in the study to arrive at 21 completers/group. The study was conducted following the approvals of the University of Pittsburgh Institutional Review Board and Radioactive Drug Research Committee. All subjects provided written informed consent. Alcohol dependent subjects and healthy controls were largely recruited through advertisements displayed at local community centers, buses, newspapers and web sites. In addition, addiction medicine clinics and hospital emergency rooms in the community also referred alcohol dependent subjects. Study criteria for alcohol dependence were [1] males or females between 18 and 40 years old of all ethnic and racial origins; [2] fulfill DSM-IV criteria for alcohol dependence as assessed by SCID; [3] no current or past DSM-IV Axis I disorder other than alcohol abuse or dependence, including abuse or dependence to other recreational drugs (nicotine dependence was allowed); [4] no current (as confirmed by urine drug screen at screening) use of cocaine, opiates, cannabis, sedative-hypnotics, amphetamines, 3,4-methylenedioxy-N-methylamphetamine, and phencyclidine; [5] not currently on any prescription or over the counter medications; [6] no current or past chronic medical or neurological illnesses (including glaucoma, seizure disorders, a focal finding on MRI such as stroke or tumor, chronic obstructive pulmonary disease or respiratory disease, renal problems, and liver problems) as assessed by a complete physical exam and labs; [7] no resting systolic blood pressure > 140 and diastolic blood pressure > 90; [8] no more than one risk factor for coronary artery disease (e.g., smoking, obesity, cholesterol > 240 mg dl−1, sedentary life style etc.); [9] no first-degree relative with a psychotic or mood disorder; [10] not currently pregnant; [11] no history of radioactivity exposure from nuclear medicine studies or occupation; [12] no metallic objects in the body that are contraindicated for magnetic resonance imaging (MRI).
Alcohol dependent subjects completed a minimum of 14-days of outpatient abstinence monitored with witnessed urine toxicology. Subjects were monitored for alcohol and recreational drug use with urine alcohol metabolite (ethyl glucuronide and ethyl sulfate) and urine drug screens three times/week for two consecutive weeks. Since alcohol metabolites and common drugs of abuse can be detected for 2 to 3 days after use, subjects were informed that they should not use alcohol or street drugs for the 14 days prior to the PET study. In order to promote abstinence from alcohol during this two-week period, subjects were paid $75 for each urine sample that was negative for ethyl glucuronide and ethyl sulfate. Alcohol dependent subjects were scheduled for the PET scans after successful completion of the abstinence monitoring protocol. Subjects who tested positive for ethyl glucuronide and ethyl sulfate were offered up to three attempts to re-start the abstinence monitoring protocol. This abstinence monitoring protocol ensured that all subjects were abstinent for a minimum of two weeks prior to the PET scan. Alcohol dependent subjects were also monitored for alcohol withdrawal signs and symptoms three times/week during the first week of abstinence using the Clinical Institute Withdrawal Assessment of Alcohol Scale (23). Alcohol dependent subjects who were at risk of severe withdrawal, i.e., scored greater than 19 on the Clinical Institute Withdrawal Assessment of Alcohol Scale and had prior history of alcohol withdrawal seizures or delirium tremens were excluded from the research protocol. The severity of alcohol dependence was assessed in with the Michigan Alcohol Screening Test (24) and Alcohol Dependence Scale (25).
Healthy control subjects matched for age, gender, ethnicity and smoking status had no past or present neurological or psychiatric illnesses including substance abuse (confirmed by urine drug screen both at screening and the day of the PET scan). Healthy controls and alcohol dependent subjects underwent the PET scans as outpatients. Following the completion of the PET scans, alcohol dependent subjects were scheduled for a follow up visit during which they were debriefed of the risks of alcohol abuse and provided a referral for outpatient treatment.
Image acquisition and analysis
Following a structural MRI, subjects underwent a baseline and a post-amphetamine [11C]FLB 457 PET scan in the same experimental session using procedures described in (18).
Briefly, [11C]FLB 457 was synthesized using the methodology reported by Halldin, et al. (26). PET imaging sessions were conducted with the ECAT EXACT HR+ camera. Following a transmission scan, subjects received an intravenous bolus injection of [11C]FLB 457 and emission data was collected for 90 min. Arterial blood samples were collected to measure the plasma free fraction (fP) for [11C]FLB 457 and derive a metabolite corrected arterial input function for modeling using methods described previously (18). The maximum injected mass for [11C]FLB 457 was restricted to 0.6 μg (27). The post-amphetamine [11C]FLB 457 scan was performed 3 hours after the administration of 0.5 mg kg−1 of oral d-amphetamine. During this scan, amphetamine blood levels were measured in three arterial samples drawn at time 0 min, 45 min and 90 min and analyzed using methods described in (28).
PET data were reconstructed and processed with the image analysis software MEDx (Sensor Systems, Inc., Sterling, Virginia) and SPM2 (www.fil.ion.ucl.ac.uk/spm) as described in (18). Frame-to-frame motion correction for head movement and MR-PET image alignment were performed using a mutual information algorithm implemented in SPM2. MRI segmentation was performed using the automated segmentation tool in Functional MRI of the Brain Software Library (29). Cortical (medial temporal lobe, dorsolateral prefrontal cortex, orbital frontal cortex, medial prefrontal cortex, anterior cingulate cortex, temporal cortex, parietal cortex, and occipital cortex) and subcortical (midbrain and cerebellum) regions of interest were defined on the MRI using a segmentation-based and direct identification method described in (19, 30, 31). Regional volumes and time activity curves were then generated in MEDx as outlined in (30, 31). Primary analysis included the eight cortical regions that had been validated in our previous [11C]FLB 457 human studies (18-21). Secondary analysis included the midbrain as a region of interest to test if there is convergence between the midbrain dopamine cells and terminal fields. Derivation of [11C]FLB 457 distribution volume (VT) in the regions of interest (VT ROI) and cerebellum (VT CER) was performed using a two-tissue compartment kinetic analysis using the arterial input function as described in (18).
PET outcome variables are defined in accordance to the consensus nomenclature for in vivo imaging of reversibly binding radioligands (32). D2/3 receptor availability at baseline and post-amphetamine was estimated using BPND, i.e., binding potential relative to non-displaceable uptake, which was derived as
| Eq 1 |
where, fND (=fP/VT CER) is the free fraction of [11C]FLB 457 in the non-displaceable compartment, Bavail is the density of D2/3 receptors (nmol L−1) available to bind to [11C]FLB 457 in vivo, KD is the in vivo equilibrium dissociation constant of [11C]FLB 457 (nmol L−1)
The amphetamine-induced change in BPND (Δ BPND) was calculated as the difference between BPND measured in the post-amphetamine condition (BPND AMPH) and BPND measured in the baseline condition (BPND BASE), and expressed as a percentage of BPND BASE:
| Eq 2 |
Finally, as our validation work with [11C]FLB 457 demonstrated D2/3 specific binding in the cerebellum there was concern that any amphetamine-induced change in VT CER could bias the dopamine-release outcome measure, Δ BPND (BPND is dependent on VT CER, see Eq 1). Therefore, to arrive at a dopamine release measurement in the cortex (i.e., D2/3 receptor occupancy following amphetamine) that is independent of VT CER, we analyzed the baseline and post-amphetamine VT values from the eight cortical regions of interest with Lassen plots as described in (33). Briefly, the equation for the line [y=mx+b], where y=[VT BASELINE –VT AMPHETAMINE], and x=VT BASELINE, produced a linear relationship with slope of line equal to receptor occupancy (m). This approach assumed that there is uniform receptor occupancy across the cortical regions.
Statistical analysis
Comparison between scan conditions (baseline vs. post-amphetamine) was performed with paired t-tests. Comparisons between groups (alcohol dependence vs. healthy controls) were performed with unpaired t-tests (regions of interest level) and repeated measures of ANOVA (amphetamine blood levels). Furthermore, to test for a global effect of diagnosis (alcohol dependence vs. healthy controls) on baseline cortical BPND and Δ BPND, a linear mixed model analysis was performed with cortical regions of interest as a repeated measure and diagnostic group as the fixed factor (IBM SPSS Statistics). Relationship between PET data and clinical characteristics (years of drinking, amount of drinks/day, Alcohol Dependence Scale and Michigan Alcohol Screening Test) were assessed by Pearson product moment correlation coefficient. A two-tailed probability value of p < 0.05 was selected as the significance level for all analyses. A false discovery rate correction with α = 0.05 was applied to correct for multiple comparisons in the regions of interest (34).
RESULTS
Twenty-one alcohol dependent subjects were matched with 21 healthy controls on age, gender, ethnicity and smoking status (including the number of cigarettes smoked per day rounded to the nearest one-half pack, i.e., 10 cigarettes). Table 1 lists demographics variables and measures of alcohol use in alcohol dependent subjects.
Table 1.
Demographic and clinical parameters for Healthy controls and Alcohol dependent subjects (n= 21/group)
| Healthy Controls | Alcohol dependence | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Gender | ||||
| Male | 16 | 16 | ||
| Female | 5 | 5 | ||
| Ethnicity | ||||
| African American | 2 | 4 | ||
| Asian | 2 | 0 | ||
| Caucasian | 17 | 17 | ||
| Smoking status | ||||
| Yes | 12 | 12 | ||
| No | 9 | 9 | ||
| Positive family history for alcoholism | 0 | 18 | ||
| Age | 28 | 4 | 28 | 5 |
| Weight (Kg) | 74 | 12 | 77 | 14 |
| Duration of abuse (years) | - | - | 11 | 6 |
| Alcohol frequency (days/week) | - | - | 5.7 | 1.5 |
| Alcohol amount (standard drinks/day) | - | - | 13 | 5 |
| Michigan Alcohol Screening Test (scoring range 0 to 22) | - | - | 13 | 4 |
| Alcohol dependence scale (scoring range 0 to 47) | - | - | 21 | 6 |
| Abstinence before scans (days) | - | - | 33 | 18 |
Scan parameters
Table 2 shows the [11C]FLB 457 scan parameters for healthy controls and alcohol dependent subjects under baseline and post-amphetamine conditions. No significant differences between the baseline and post-amphetamine condition were observed in any of these scan parameters in healthy controls and alcohol dependent subjects. Notably, there was no significant change in VT CER following amphetamine in both groups (ΔVT CER, healthy controls = −2.5 ± 15.1%; alcohol dependence = −0.9 ± 10.8%, t=0.41, df= 40, p=0.68). This justified the use of Δ BPND to contrast differences in amphetamine-induced dopamine transmission between the healthy controls and alcohol dependence group (see Eq 1, refer to 35).
Table 2.
[11C]FLB 457 PET scan parameters
| Condition | Injected dose (mCi) | Specific activity (Ci mmol−1) | Injected mass (ug) | Free Fraction in plasma (%) | VND (mL cm−3) | ||
|---|---|---|---|---|---|---|---|
| Healthy Controls | Baseline | Mean | 7.1 | 8891 | 0.39 | 34.7 | 4.27 |
| SD | 1.7 | 5689 | 0.17 | 5.9 | 1.23 | ||
| Amphetamine | Mean | 7.7 | 8741 | 0.39 | 34.3 | 4.04 | |
| SD | 1.4 | 4307 | 0.13 | 5.1 | 0.86 | ||
| Alcohol Dependence | Baseline | Mean | 7.6 | 11369 | 0.36 | 38.9 | 4.43 |
| SD | 1.4 | 8871 | 0.17 | 7.6 | 1.01 | ||
| Amphetamine | Mean | 6.8 | 6995 | 0.46 | 39.3 | 4.37 | |
| SD | 1.9 | 4736 | 0.16 | 7.3 | 0.97 |
Values are mean and standard deviation (SD), n = 21 per group
* p ≤ 0.05, paired t-tests (baseline vs. amphetamine)
The amphetamine blood levels measured at time, t =0, 45 and 90 min relative to post-amphetamine [11C]FLB 457 scan in healthy controls (80 ± 10, 73 ± 9 and 70 ± 10 ng mL−1) was not significantly different from that measured in alcohol dependent subjects (75 ± 8, 69 ± 7 and 66 ± 7 ng mL−1; repeated measures of ANOVA, effect of diagnosis: F=3.08, df=1, p =0.09; effect of time: F = 57.20, df=2 , p <0.001; diagnosis x time interaction: F=0.07, df=2, p =0.93).
Regional volumes
No between-group differences were found in the cortical regions, midbrain and cerebellum volumes determined from the MRI scans (data not shown, all p-values ≥ 0.2), suggesting lack of measurable volumetric changes in alcohol dependence.
Cortex
D2/3 receptor availability (BPND) under baseline conditions
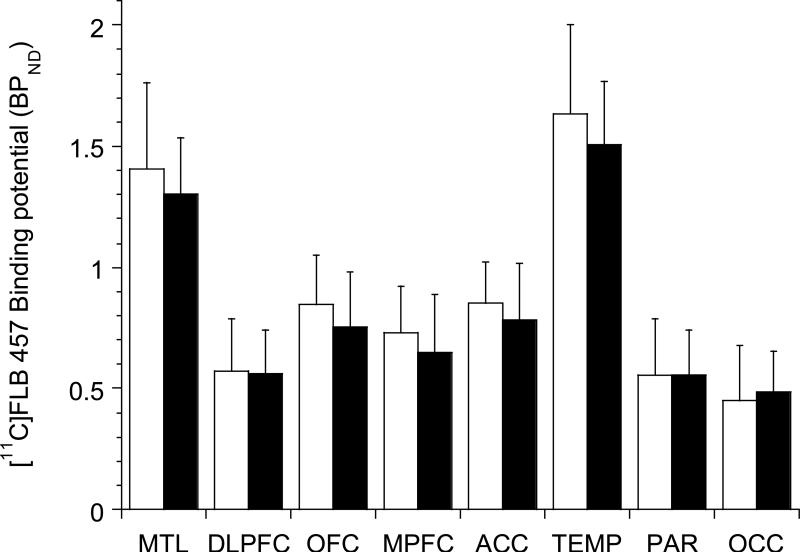
As shown in Figure 1, no differences in baseline [11C]FLB 457 BPND were observed in alcohol dependence compared to healthy controls (linear mixed model, effect of diagnosis, F (1, 40) = 0.89, p =0.35; effect of region, F (7, 280) = 332.65, p < 0.001; region x diagnosis interaction, F (7, 280) = 1.71, p = 0.11). In addition, unpaired t-tests conducted at the level of the individual regions of interest failed to show any significant differences between the two groups.
Figure 1.
shows the lack of difference in D2/3 receptor availability in cortical regions of interest in alcohol dependent subjects (black bars) compared to healthy controls (white bars). MTL: medial temporal lobe, DLPFC: dorsolateral prefrontal cortex, OFC: orbital frontal cortex, MPFC: medial prefrontal cortex, ACC: anterior cingulate cortex, TEMP: temporal cortex, PAR: parietal cortex, and OCC: occipital cortex
Amphetamine-induced reduction in D2/3 receptor availability (Δ BPND)
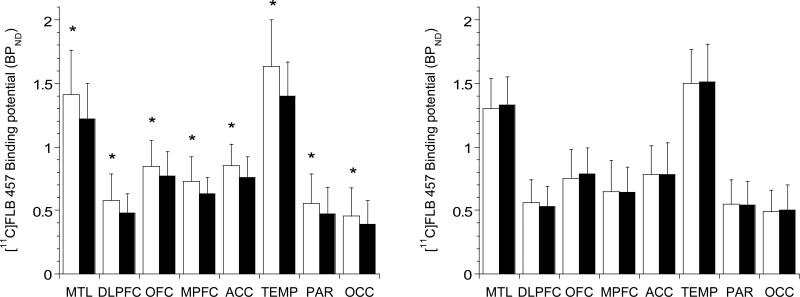
Amphetamine led to a significant reduction in [11C]FLB 457 BPND in healthy controls (Figure 2, left panel), but not in alcohol dependence (Figure 2, right panel). The amphetamine-induced Δ [11C]FLB 457 BPND was significantly lower in alcohol dependence compared to healthy controls (linear mixed model, effect of diagnosis, F (1, 40) = 11.03, p =0.002; effect of region, F (7, 280) = 1.99, p < 0.056; region x diagnosis interaction, F (7, 280) = 0.65, p = 0.71). The inclusion of mean amphetamine blood levels as a co-variate in the model did not change the significance of the results (linear mixed model, effect of diagnosis (F (1, 39) = 9.44, p=0.004). Unpaired t-tests conducted at the level of the individual regions of interest were significant in all of the cortical regions except the anterior cingulate cortex (see Table 3). All significant comparisons, except in the parietal and occipital cortex survived the false discovery rate correction. Also, consistent with the Δ BPND results, the amphetamine-induced dopamine release's occupancy of D2/3 receptors (derived using Lassen plots that is independent of VT CER) was significantly lower in alcohol dependent subjects compared to healthy controls (healthy controls = 16.0 ± 15.6%; alcohol dependence = −1.2 ± 19.5%, t= −3.16, df=40, p=0.003).
Figure 2.
shows [11C]FLB 457 BPND under baseline (white bars) and post-amphetamine (black bars) conditions in healthy controls (left panel) and alcohol dependent subjects (right panel). Amphetamine led to a significant decrease in [11C]FLB 457 BPND in healthy controls, but not in alcohol dependence (* represents p< 0.05, following the false discovery rate correction for multiple comparisons). MTL: medial temporal lobe, DLPFC: dorsolateral prefrontal cortex, OFC: orbital frontal cortex, MPFC: medial prefrontal cortex, ACC: anterior cingulate cortex, TEMP: temporal cortex, PAR: parietal cortex, and OCC: occipital cortex
Table 3.
Amphetamine-induced displacement of [11C]FLB 457 binding potential (BPND)
| Region | Healthy controls | Alcohol dependence | Two-tailed, unpaired t-test | p | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | ||
| Medial temporal lobe | −11.1 | 16.2 | 3.1 | 15.9 | 2.88 | 39.98 | 0.006* |
| Dorsolateral prefrontal cortex | −13.7 | 14.7 | −3.9 | 12.7 | 2.30 | 39.12 | 0.027* |
| Orbital frontal cortex | −8.6 | 12.4 | 8.5 | 18.6 | 3.50 | 34.87 | 0.001* |
| Medial prefrontal cortex | −11.6 | 15.1 | 5.4 | 27.3 | 2.48 | 31.62 | 0.018* |
| Anterior cingulate cortex | −9.0 | 17.7 | 1.1 | 19.6 | 1.75 | 39.61 | 0.089 |
| Temporal cortex | −12.2 | 13.4 | 0.4 | 10.4 | 3.39 | 37.80 | 0.002* |
| Parietal cortex | −11.7 | 18.6 | −1.6 | 12.2 | 2.09 | 34.65 | 0.044 |
| Occipital cortex | −10.6 | 19.2 | 1.3 | 17.2 | 2.11 | 39.52 | 0.041 |
Values are mean and standard deviation (SD), n = 21 per group
significant following multiple corrections using false discovery rate
Midbrain
D2/3 receptor availability (BPND) under baseline conditions
No significant differences were observed in midbrain [11C]FLB 457 BPND in alcohol dependence compared to healthy controls (BPND: healthy controls = 2.50 ± 0.62; alcohol dependence = 2.23 ± 0.38, t= −1.67, df= 40, p=0.10).
Amphetamine-induced reduction in D2/3 receptor availability (Δ BPND)
Amphetamine led to a significant reduction in [11C]FLB 457 BPND in the midbrain in healthy controls (baseline = 2.50 ± 0.62; post-amphetamine = 2.09 ± 0.37, t=3.16, df =20, p=0.005), but not in alcohol dependence (baseline = 2.23 ± 0.38; post-amphetamine = 2.35 ± 0.63, t= −1.49, df =20, p=0.15). The amphetamine-induced Δ [11C]FLB 457 BPND in the midbrain was significantly lower in alcohol dependence compared to healthy controls (ΔBPND, healthy controls = −13.2 ± 15.2%; alcohol dependence = 5.0 ± 14.6%, t = 3.91, df= 40, p=0.0003). This comparison survived the false discovery rate correction.
Clinical correlations
Correlation analyses revealed no significant associations between Δ BPND in the regions of interest (cortex and midbrain) and mean amphetamine blood levels in healthy controls and alcohol dependence. In addition, there was no significant associations between Δ BPND and any of the clinical measures (alcohol frequency, amount, duration of abuse, Michigan Alcohol Screening Test, or Alcohol Dependence Scale scores) in alcohol dependence.
DISCUSSION
In this PET study, we found less displacement of [11C]FLB 457 BPND in the cortex and midbrain after amphetamine in recently abstinent alcoholics compared to healthy controls. In a previous study using PET and microdialysis, it was shown that 1% displacement of [11C]FLB 457 BPND in the cortex corresponds to a 57% increase in extracellular dopamine concentration (22). Extending this relationship to the current dataset (mean ΔBPND in healthy controls = −9 to −14%; alcohol dependence = +9 to −4% in Table 4) suggests that cortical dopamine in healthy controls and alcohol dependent subjects increases by ~513-798% and 0-228% respectively following the same dose of amphetamine. This result for the first time unequivocally demonstrates that there is decreased dopamine transmission in the cortex in alcoholism. These data also for the first time show convergence between the midbrain dopamine cells and terminal fields with respect to decreased dopamine transmission in alcoholism. Such a blunting in mesocortical dopamine transmission in alcoholics is consistent with what has been previously reported in the nigrostriatal system that includes the limbic-related nucleus accumbens (16, 36). Decreased dopamine transmission in the mesolimbic regions, such as the ventral striatum and medial temporal lobe, likely contributes to anhedonia, amotivation, and decreased reward sensitivity in alcohol dependence. This has led to the conceptualization of alcohol dependence as a reward-deficit disorder with a higher reward threshold for both natural and drug/alcohol reinforcers (37, 38). The fact that there is also less dopamine in the prefrontal cortex, which governs executive functions, is important because it could impair the addict's ability to learn and utilize informational/behavioral strategies critical to relapse prevention. This is supported by literature that links prefrontal cortical dopamine with executive functions, such as attention, working-memory, behavioral flexibility and risk/reward decision-making --all of which are impaired in addictive disorders such as alcoholism (3, 39). Floresco and Magyar, in a study using a rodent version of the Iowa Gambling Task (a task that measures risk preference decision-making), demonstrated that blocking dopamine transmission in the prefrontal cortex leads to a response decision that fails to integrate the consequences of conditioned punishment (39). Based on this study, it is tempting to speculate that the failure to incorporate past negative consequences in a decision to drink alcohol during abstinence is related to decreased prefrontal cortical dopamine in alcoholism. If this hypothesis were confirmed, it would support a role for medications that increase prefrontal cortical dopamine to prevent relapse in alcoholism.
Alcohol-induced potentiation of GABA, the major inhibitory transmitter in the brain, inhibits GABA-ergic interneurons in the ventral tegmental area and substantia nigra, and leads to increased phasic (or synaptic) dopamine transmission (40, 41). However, chronic and repeated use of alcohol leads to decreased phasic dopamine via adaptations in the tonic (or extracelluar) dopamine and glutamatergic systems in the cortico-limbic pathways (for detailed review, refer to 40). If decreased dopamine transmission is the result of an adaptation in the cortico-limbic circuits, it might be possible to reverse this deficit in alcoholics with prolonged abstinence. On the other hand, alcoholics also demonstrate signs of inflammation (i.e., greater activated microglia and pro-inflammatory cytokines) and a reduction of dopamine neuronal markers in the brain (42, 43). Therefore, the possibility of a toxic irreversible loss of dopamine neurons in alcoholism cannot be ruled out. This may explain the persistent and enduring cognitive impairments that have been reported in abstinent alcoholics (44). Also unclear is whether decreased dopamine transmission in alcoholism represents a premorbid trait or alcohol-induced state. Future dopamine imaging studies in recovering alcoholics with prolonged periods of abstinence and non-human primates that can be imaged both pre- and post- alcohol exposure are necessary to evaluate these issues.
Another interesting observation in this study is the lack of differences in baseline D2/3 receptor binding potential in both the cortex and midbrain in alcoholics compared to controls (Figure 1). This is in contrast to previous [11C]raclopride imaging studies that have reported ~10-20% decrease in D2/3 receptor BPND in the striatal subdivisions in alcoholics (16, 36). The exact reason and physiological relevance for decreased D2/3 receptor BPND in the striatum, but not in the extrastriatal regions in alcoholism is not clear. Reasons that may have contributed to the inability to detect group differences in this study include diminished power due to greater between-subject variability in cortical D2/3 receptor binding potential and/or a more pronounced reduction in baseline dopamine levels (i.e., prior to amphetamine stimulation) in the cortex compared to striatum in alcoholics. PET studies in alcoholism with alpha-methyl-para-tyrosine that can deplete baseline dopamine in the striatal and extrastriatal regions are necessary to further understand this issue.
The strengths of this study are: inclusion of relatively young individuals (≤ 40 years) with mild to moderate alcohol dependence; exclusion of individuals with comorbid medical, psychiatric or drug abuse; monitored abstinence prior to imaging; use of a validated imaging paradigm to measure cortical dopamine transmission; use of compartmental modeling with an arterial input function to derive PET outcome measures; ruled out changes in [11C]FLB 457 non-specific binding (VND) as a significant contributor to ΔBPND in patients and controls; and measurement of amphetamine blood levels. The limitations of the study are: the exclusion of older individuals with severe alcohol dependence; and no relationship between ΔBPND and alcohol measures such as frequency, amount, severity and duration of abuse. One possible reason for the failure to demonstrate a relationship between ΔBPND and alcohol measures is the limited range of values observed in the alcohol abuse measurements (see Table 1). This is likely an unintended consequence of excluding individuals with more severe alcoholism and comorbid disorders. In conclusion, we found decreased dopamine transmission in abstinent alcoholics in several of the cortical regions that have been implicated in addiction including the prefrontal cortex and medial temporal lobe. The results of these studies suggest that dopamine dysfunction in alcohol dependence is more widespread than previously conceptualized and not restricted to the striatum. Further studies are necessary to understand the mechanisms that contribute to blunted dopamine transmission and its clinical relevance in alcoholism.
ACKNOWLEDGEMENTS
The project described was supported by Award Numbers R01AA018330 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or the National Institutes of Health.
Footnotes
DISCLOSURES
Drs. Narendran, Mason and Frankle have done contractual research work at the University of Pittsburgh for ONO Pharmaceuticals Co., LTD
REFERENCES
- 1.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205(4409):929–32. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 2.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251(4996):947–50. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 3.Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl) 2012;221(3):361–87. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longmore BE, Knight RG. The effect of intellectual deterioration on retention deficits in amnesic alcoholics. J Abnorm Psychol. 1988;97(4):448–54. doi: 10.1037//0021-843x.97.4.448. [DOI] [PubMed] [Google Scholar]
- 5.Goldman MS. Cognitive impairment in chronic alcoholics. Some cause for optimism. Am Psychol. 1983;38(10):1045–54. doi: 10.1037//0003-066x.38.10.1045. [DOI] [PubMed] [Google Scholar]
- 6.Oscar-Berman M, Shagrin B, Evert DL, Epstein C. Impairments of brain and behavior: the neurological effects of alcohol. Alcohol Health Res World. 1997;21(1):65–75. [PMC free article] [PubMed] [Google Scholar]
- 7.De Montis MG, Grappi S, Gambarana C, Leggio B, Nanni G, Scheggi S, Tagliamonte A. Sardinian alcohol-preferring rats show low 5-HT extraneuronal levels in the mPFC and no habituation in monoaminergic response to repeated ethanol consumption in the NAcS. Brain Res. 2004;1006(1):18–27. doi: 10.1016/j.brainres.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38(1):5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32(5):363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- 10.Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G. Non-psychostimulant drugs of abuse and anxiogenic drugs activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology (Berl) 1996;124(4):293–9. doi: 10.1007/BF02247433. [DOI] [PubMed] [Google Scholar]
- 11.Hegarty AA, Vogel WH. Modulation of the stress response by ethanol in the rat frontal cortex. Pharmacol Biochem Behav. 1993;45(2):327–34. doi: 10.1016/0091-3057(93)90247-q. [DOI] [PubMed] [Google Scholar]
- 12.Murphy JM, McBride WJ, Gatto GJ, Lumeng L, Li TK. Effects of acute ethanol administration on monoamine and metabolite content in forebrain regions of ethanol-tolerant and -nontolerant alcohol-preferring (P) rats. Pharmacol Biochem Behav. 1988;29(1):169–74. doi: 10.1016/0091-3057(88)90291-2. [DOI] [PubMed] [Google Scholar]
- 13.Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20(9):1631–8. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 14.Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79(4-5):581–90. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- 15.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, deBartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc Natl Acad Sci USA. 1997;94(6):2569–74. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27(46):12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biological psychiatry. 2005;58(10):779–86. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, Vora S, Litschge M, Kendro S, Cooper TB, Mathis CA, Laruelle M. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63(6):447–61. doi: 10.1002/syn.20628. [DOI] [PubMed] [Google Scholar]
- 19.Narendran R, Mason NS, May MA, Chen CM, Kendro S, Ridler K, Rabiner EA, Laruelle M, Mathis CA, Frankle WG. Positron emission tomography imaging of dopamine D/ receptors in the human cortex with [(1)(1)C]FLB 457: reproducibility studies. Synapse. 2011;65(1):35–40. doi: 10.1002/syn.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narendran R, Himes M, Mason NS. Reproducibility of Post-Amphetamine [(11)C]FLB 457 Binding to Cortical D2/3 Receptors. PLoS One. 2013;8(9):e76905. doi: 10.1371/journal.pone.0076905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narendran R, Mason NS, Chen CM, Himes M, Keating P, May MA, Rabiner EA, Laruelle M, Mathis CA, Frankle WG. Evaluation of dopamine D/ specific binding in the cerebellum for the positron emission tomography radiotracer [(1)(1)C]FLB 457: implications for measuring cortical dopamine release. Synapse. 2011;65(10):991–7. doi: 10.1002/syn.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narendran R, Jedema HP, Lopresti B, Mason NS, Gurnsey K, Ruskiewicz J, Chen C-M, Deuitch L, Frankle G, Bradberry C. Imaging dopamine transmission in the frontal cortex: a simultaneous microdialysis and [11C]FLB 457 PET study. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353–7. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 24.Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1976;127:1653–8. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- 25.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 26.Halldin C, Farde L, Hogberg T, Mohell N, Hall H, Suhara T, Karlsson P, Nakashima Y, Swahn CG. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med. 1995;36(7):1275–81. [PubMed] [Google Scholar]
- 27.Sudo Y, Suhara T, Inoue M, Ito H, Suzuki K, Saijo T, Halldin C, Farde L. Reproducibility of [11 C]FLB 457 binding in extrastriatal regions. Nucl Med Commun. 2001;22(11):1215–21. doi: 10.1097/00006231-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Reimer ML, Mamer OA, Zavitsanos AP, Siddiqui AW, Dadgar D. Determination of amphetamine, methamphetamine and desmethyldeprenyl in human plasma by gas chromatography/negative ion chemical ionization mass spectrometry. Biol Mass Spectrom. 1993;22(4):235–42. doi: 10.1002/bms.1200220404. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 30.Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R, Foged C, Halldin C, Laruelle M. Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab. 2000;20(2):225–43. doi: 10.1097/00004647-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708–19. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 33.Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab. 2010;30(1):46–50. doi: 10.1038/jcbfm.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;(57):289–300. [Google Scholar]
- 35.Narendran R, Mason NS, Laymon C, Lopresti B, Velasquez N, May M, Kendro S, Martinez D, Mathis C, Frankle G. A comparative evaluation of the dopamine D2/3 agonist radiotracer [11C]NPA and antagonist [11C]raclopride to measure amphetamine-induced dopamine release in the human striatum. Journal of Pharmacology and Experimental Therapeutics. 2010;63(7):574–84. doi: 10.1124/jpet.109.163501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–86. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 37.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188(4):567–85. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- 40.Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–28. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- 41.Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O'Malley SS, Krystal JH, Abi-Dargham A. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biological psychiatry. 2010;68(8):689–96. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210(2):349–58. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilman S, Koeppe RA, Adams KM, Junck L, Kluin KJ, Johnson-Greene D, Martorello S, Heumann M, Bandekar R. Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography. Ann Neurol. 1998;44(3):326–33. doi: 10.1002/ana.410440307. [DOI] [PubMed] [Google Scholar]
- 44.Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, Szelenberger W. Cognitive functions in abstinent alcohol-dependent patients. Alcohol. 2012;46(7):665–71. doi: 10.1016/j.alcohol.2012.04.005. [DOI] [PubMed] [Google Scholar]