Summary
Chimeric antigen receptors re-direct T cells to surface antigens. Discovery and validation of appropriate target antigens expands the possible indications for CAR-T cells. CS1 is expressed at high levels by multiple myeloma cells, but also to some extent on other lymphocytes. CS1 may be a viable target for CAR-T cells in multiple myeloma.
In this issue of Clinical Cancer Research, Chu and colleagues (1) explore the potential of targeting the CS1 glycoprotein antigen with chimeric-antigen receptor (CAR)-transduced T cells, with the goal of developing a clinical T cell therapy to treat multiple myeloma.
The early and impressive success of CAR-transduced T cells targeting the CD19 antigen in B cell malignancies has spurred a great deal of interest in broadening this type of technology to other malignancies. Briefly, chimeric antigen receptors are engineered proteins that fuse the antigen-binding domains of antibodies to T cell signaling molecules such as CD3 zeta, with or without additional signaling domains derived from costimulatory molecules such as CD27, CD28 or 4-1BB (2). The technology to molecularly engineer the constructs is readily available, and producing retroviral vectors and transducing T cells with the construct of interest is rapid and reliable. Obtaining or generating an antibody sequence on which to base the antigen-binding moiety can take time, but the greatest challenge in developing a new CAR remains finding a suitable antigen to target.
CS1 is a glycoprotein expressed on the cell surface of nearly all myeloma cells. However, it is also expressed at lower levels on the majority of lymphocytes, including NK cells and subsets of T cells and B cells, but not hematopoietic stem cells (3). Though testing is underway to determine the exact number of molecules that a CAR T cell can respond to (4), clinically, CAR T cells detect are known to detect and target cells expressing even low levels of cognate antigen: CD19-directed T cells cause B cell aplasia, carbonic-anhydrase IX-directed T cells targeted bile duct epithelium and caused cholangitic liver toxicity (5), and Her2/neu-directed T cells caused rapid death due to low-level expression of Her2 on the pulmonary vascular endothelium (6). Fortunately for CAR investigators targeting myeloma, extensive immunohistochemistry-based testing of CS1 expression on normal tissues has already been performed and published as part of the development of the CS1-directed antibody elotuzumab (3). The function of CS1 is not completely understood, and most of its signaling function has been described in lymphocytes (Figure). Elotuzumab is known to inhibit myeloma cell adhesion to marrow stromal cells (7), but its principal mechanism of action is to induce NK-mediated ADCC (8). Given its nearly universal expression on myeloma cells, it is tempting to speculate that CS1 performs an essential function for the maintenance of the tumor.
Figure.
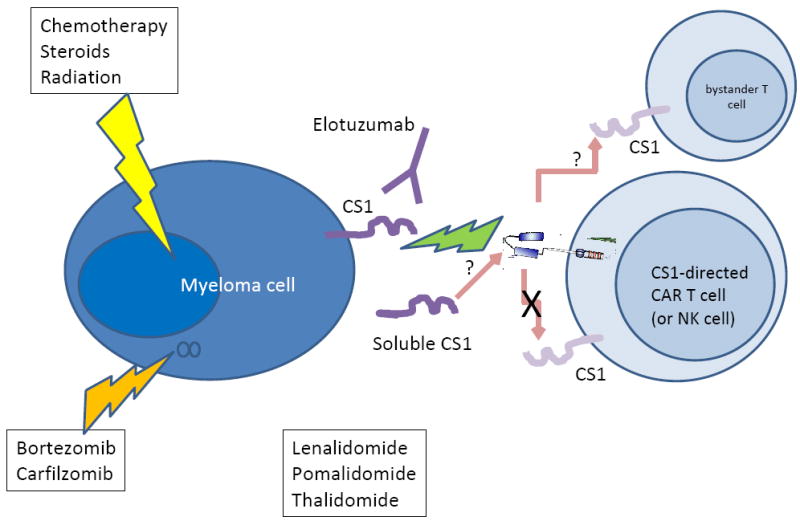
Myeloma is currently treated with combinations of chemotherapy, steroids, and radiation. Novel drugs include proteasome inhibitors such as bortezomib and carfilzomib, and immunomodulatory agents such as thalidomide, lenalidomide, and pomalidomide. Elotuzumab is a monoclonal antibody targeting the glycoprotein CS1 that has been shown to have activity in combination with novel drugs. CS1 is expressed on myeloma cells and on other lymphocyte subsets, including T cells and NK cells; it is also shed into the circulation. Chimeric antigen receptors (CARs) targeting CS1 and introduced into T cells (or NK cells) can kill CS1-expressing myeloma cells, but do not appear to mediate T cell intrinsic suicide. Outstanding questions are whether CS1-directed T cells will have subtle fratricide effects on bystander T cells, and whether soluble CS1 will inactivate the CS1 CARs.
The authors show that a second generation CAR based on a single chain variable fragment of an antibody related to elotuzumab effectively redirects T cells to secrete cytokines, degranulate, and exhibit cytotoxic activity in response to myeloma cell lines and primary human myeloma cells in vitro. CS1-directed T cells also inhibited tumor growth and prolonged survival in orthotopic xenograft mouse models of myeloma. However, at issue is whether the mice were actually cured by the CS1 CAR T cells, because follow up of the mice was short in the reported experiment (1). The authors demonstrated that CS1-directed T cell activity correlated with the expression level of CS1 on myeloma cells. One clinical question that will emerge is whether previous treatment with CS1-specific antibodies (i.e. elotuzumab), binding the same target as the CAR T cells, will select for escape variants that may or may not be visible to CAR-T cells. Interestingly, CS1 is detectable as a soluble form in the serum of patients with multiple myeloma, and the serum level of CS1 correlates with disease stage (7). We would predict that CAR T cells may be inhibited by soluble versions of the same target if the binding epitope is preserved in the soluble form compared to the membrane-bound form; experiments to address this could be performed in vitro, and correlative studies to address this question could be included in the first trials.
An interesting question is why T cells, which also express CS1, don’t seem to commit suicide or ‘fratricide.’ Elotuzumab cytotoxicity occurs via antibody-dependent, NK-cell mediated cytotoxicity (ADCC), and is specifically directed to CS1-bearing myeloma tumor cells. There is no apparent cytotoxicity directed to fraternal CS1-bearing NK cells, as demonstrated both in vitro and in vivo (8, 9). In this paper, CS1-directed T cells did not seem to degranulate in the absence of myeloma target cells, though there was increased expression of the activation marker CD69, suggesting some low-level recognition of fraternal CS1-bearing T cells. Significant fratricide could impair CAR-T cell expansion, and therefore reduce the feasibility of manufacturing the target cell dose. Moreover, immune deficiencies could occur in vivo if a specific subset of T cells, such as CMV-specific T cells, were subject to CS1-directed elimination. Although some of these safety questions could be addressed in the pre-clinical setting, the ultimate determination of safety and efficacy can only occur in clinical trials. In this paper, the authors transduce T cells; the same group previously transduced the same type of CAR into NK cells (10). Recent studies have shown that NK and T cells can exert cytotoxic activity with remarkably different contact dynamics (11). Therefore, it would be interesting to evaluate the two CAR cell-types side-by-side in vitro, and even perhaps in vivo in a competitive re-population trial design.
Finally, the CS1-directed antibody elotuzumab is safe, and though it has almost no single agent activity, it does improve response rates when administered in combination with agents commonly used in the treatment of myeloma. Elotuzumab in combination with the immunomodulatory drug lenalidomide and low-dose dexamethasone yielded an 82% objective response rate (12); elotuzumab in combination with the proteasome inhibitor bortezomib yielded a 48% objective response rate (13). The mechanism of these drugs is thought to be synergistic with the postulated mechanisms of elotuzumab. Although investigators often use lymphodepleting drugs with CAR-T cells, it would be interesting to integrate this new platform more closely with standard myeloma therapies.
Given the knowledge that antibody targeting of CS1 is safe, and that CS1-directed CAR T cells effectively eliminate myeloma in vitro and in xenogeneic mouse models, CS1-CAR T cells have significant potential to change the landscape of myeloma treatment.
Acknowledgments
MVM is supported by NCI K08CA166039 and has sponsored research from Novartis. CHJ has sponsored research and royalty income from Novartis.
References
- 1.Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, et al. Genetic Modification of T Cells Redirected towards CS1 Enhances Eradication of Myeloma Cells. Clinical Cancer Research. 2014:XXX. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annual Review of Immunology. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clinical Cancer Research. 2008;14:2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terakura S, Goto T, Hanajiri R, Imahashi N, Shimada K, Nishida T, et al. Anti-CD20 Chimeric Antigen Receptor Transduced T Cells Can Recognize Very Low Antigen Expression: Determination Of The Lower Threshold Required To Activate The CAR-T Cells. Blood. 2013;122:4493. [Google Scholar]
- 5.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. Journal of Clinical Oncology. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tai YT, Dillon M, Song W, Leiba M, Li XF, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112:1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins SM, Bakan CE, Swartzel GD, Hofmeister CC, Efebera YA, Kwon H, et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: evidence for augmented NK cell function complementing ADCC. Cancer Immunology, Immunotherapy. 2013;62:1841–9. doi: 10.1007/s00262-013-1493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–6. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 10.Chu J, Deng Y, Benson DM, Jr, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance In Vitro and In Vivo anti-tumor activity against human multiple myeloma. Leukemia. 2014;28:917–27. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deguine J, Breart B, Lemaitre F, Di Santo JP, Bousso P. Intravital imaging reveals distinct dynamics for natural killer and CD8(+) T cells during tumor regression. Immunity. 2010;33:632–644. doi: 10.1016/j.immuni.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Lonial S, Vij R, Harousseau JL, Facon T, Moreau P, Mazumder A, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. Journal of Clinical Oncology. 2012;30:1953–9. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 13.Jakubowiak AJ, Benson DM, Bensinger W, Siegel DS, Zimmerman TM, Mohrbacher A, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. Journal of Clinical Oncology. 2012;30:1960–5. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]


