Abstract
Objectives
This study examines effects of daily use of adult day services (ADS) programs by caregivers of individuals with dementia (IWD) on a salivary biomarker of stress reactivity, dehydroepiandrosterone-sulfate (DHEA-S), and whether these effects on DHEA-S are associated with daily variability in positive mood and depressive symptoms.
Design
We used a daily diary design of 8 consecutive days with alternation of intervention (ADS) and non-intervention days to evaluate within- and between-person effects of the intervention.
Setting
Caregivers were interviewed daily by telephone at home.
Participants
151 family caregivers of IWD who were using ADS.
Measurements
Saliva samples were collected from caregivers 5 times a day for 8 consecutive days and were assayed for DHEA-S. Daily telephone interviews assessed daily stressors and mood.
Results
DHEA-S levels were significantly higher on days following ADS use. Daily DHEA-S levels covaried significantly with daily positive mood, but not depressive symptoms.
Conclusions
These results demonstrate an association of ADS use by family caregivers and higher DHEA-S levels on the next day. Prior research has found that higher DHEA-S levels are protective against the physiological damaging effects of stressor exposure and may reduce risks of illness. Regular use of ADS may help reduce depletion of DHEA-S and allow the body to mount a protective and restorative response to the physiological demands of caregiving. To our knowledge, this is the first study to examine DHEA-S levels across the day in connection with an intervention that affected daily exposure to stressors.
Keywords: Caregiving, stress, DHEA-S, adult day services, positive mood
Caregiving can be a highly stressful experience, particularly when assisting individuals with dementia (IWD). Daily and chronic caregiving stressors are associated with high levels of emotional distress, poor health outcomes, and increased mortality.1–3 Continual exposure to stressors exerts wear and tear on physiological homeostatic systems (e.g., hypothalamic-pituitary-adrenal axis (HPA), cardiovascular, and immune systems), and these effects increase risk of illness and mental health problems.4–6 Prior interventions have primarily targeted caregivers’ skill in managing stressors, but have not for the most part affected amount of exposure to stressors. By contrast, use of adult day service programs (ADS, also known as adult day care) lowers a caregiver’s exposure to care-related stressors on days their IWD attends ADS and leads to decreased subjective stress and depressive symptoms.7 The current study extends these findings by examining the effects of ADS use at a physiological level. Specifically, we focus on a marker of the caregiver’s HPA axis, dehydroepiandrosterone-sulfate (DHEA-S). DHEA-S is an appropriate target for study, because it is responsive to the effects of daily and chronic stressors.8,9 By lowering daily stressor exposure, ADS use may provide caregivers with opportunity for recovery from the effects of stressors at a physiological level as indicated by increased amounts of salivary DHEA-S.
Furthermore, DHEA-S has been found to be associated with positive mood and depressive symptoms.10,11 Several studies have explored whether treatment with DHEA-S leads to reduced depressive symptoms, though results have been mixed.11,12 The present study approaches this question in a different way. Specifically, since daily stressors are associated with both lower DHEA-S and poorer daily mood, we explore if daily fluctuations in salivary DHEA-S that result from reducing daily stressor exposure with ADS use covary with daily positive mood and depressive symptoms.
Recent studies of daily stressors of caregivers offer insight into the association of daily events, mood, and physiological markers of the stress response. Compared to numerous correlational reports, these studies use intensive repeated measures of individuals to understand the causal, temporal sequence of daily events, mood and the underlying physiological mechanisms through which stressors may affect health and emotional well-being. Mausbach and colleagues13 found associations between caregivers’ daily variability in pleasant activities and daily positive and negative mood, with stronger effects among individuals who reported higher initial burden. In a study of caregivers of persons with mild cognitive impairment, daily caregiving stressors were associated with elevated daily cortisol levels and flatter decline (i.e., less recovery at end of the day).14 In a sample of long-term caregivers, mothers of adolescents and adults with Autism Spectrum Disorders, cortisol response was found to be low across the day, indicating burnout of the HPA axis stress response.15 These studies suggest that the day-today stressors of caregiving have effects on daily mood and may not provide an opportunity for physiological recovery as indexed by salivary cortisol.
Much of the research on physiological responses to daily stressors has focused on cortisol.16 Other markers of the HPA axis may also provide insight into how the body handles stress. DHEA and its sulfated form, DHEA-S are anabolic steroids that protect the body from negative health consequences of stress exposure.10,17,18 Whereas cortisol is a catabolic hormone that supports the fight-or-flight stress response, DHEA-S contributes to recovery from stressors by mitigating the effects of inflammation and oxidative stress. 19 Higher levels of DHEA-S may have possible neuroprotective effects in aging and Alzheimer’s disease.20 In turn, low levels of DHEA-S among older people have been linked to cancer, insulin resistance, cardiovascular disease, and immune system deficiencies.21,22 While acute stress typically leads to increases in DHEA-S, prolonged exposure to stressors attenuates DHEA-S levels, thereby reducing its health protective effects.8,20 DHEA-S levels also decrease with age.10 Thus, older people experiencing chronic high levels of stressor exposure may be particularly vulnerable to depletion of DHEA-S.
To date, most research on DHEA-S has been conducted in laboratory settings and there are no published data on the daily effects of caregiving stressors on DHEA-S.23 There are also no field studies of DHEA-S where stressor exposure has been modified by an intervention during the observation period. Given findings of DHEA-S attenuation with chronic stress, there may be a time-lagged energy shift in adrenal cortex release of DHEA-S. Further, the estimated half-life of DHEA-S is long with estimates ranging from 13 to 28 hours,24 which means that it is cleared slowly from the blood. Thus, changes in salivary DHEA-S in response to a stress reducing intervention such as ADS use may occur over a longer period of time than what would be expected of a biomarker such as cortisol, which responds quickly to stressor exposure.
The current study has two goals. First, we examine the effects of an intervention, ADS use that lowers caregivers’ daily exposure to care-related stressors on daily levels of salivary DHEA-S. Specifically, we compare caregivers’ daily levels of DHEA-S on high stressor days where they spend most or all of their time with the IWD to low stressor days when their IWD attends ADS. Second, given prior findings of the association of DHEA-S and mood,10,12 and to expand our understanding of DHEA-S in response to the intervention, we examine how daily DHEA-S levels influenced positive mood and depressive symptoms. We hypothesized that there will be significant differences in DHEA-S levels across days, with higher levels found on days following ADS use, and that daily DHEA-S will positively covary with daily positive mood and negatively covary with depressive symptoms.
Methods
Design
We observed family caregivers of IWD who were enrolled in ADS programs across 8 consecutive days. The 8-day span allowed for collection of saliva and measures of mood on days when caregivers had primary responsibility for the IWD and days the IWD attended ADS. This classic within-person research design (A-B-A-B) allows us to determine efficacy by conducting within-person analyses that compare outcomes on days when participants do not receive treatment (A) with days they receive the intervention (B).25 This approach provides an opportunity to observe causally the effects of introducing and withdrawing the intervention (ADS use) on physiological and psychological recovery.
Participants
The sample consisted of caregivers assisting an IWD, who attended an ADS program at least two days a week to assure adequate treatment exposure. Caregivers had to be related to the IWD, live in the same household and indicate that they had primary responsibility for the IWD. We required that IWD and caregiver reside in the same household to assure differences in stressor exposure between types of day, specifically, that caregivers would spend more of their time with the IWD on non-ADS days and have reduced time with the IWD on ADS days. The IWD had a physician’s diagnosis of Alzheimer’s disease or another form of dementia. We did not include caregivers of people with MCI. Finally, caregivers were excluded if they had an endocrine disorder (e.g., Addison’s disease), which affects levels of DHEA-S, or if they were unable to produce saliva for any reason.
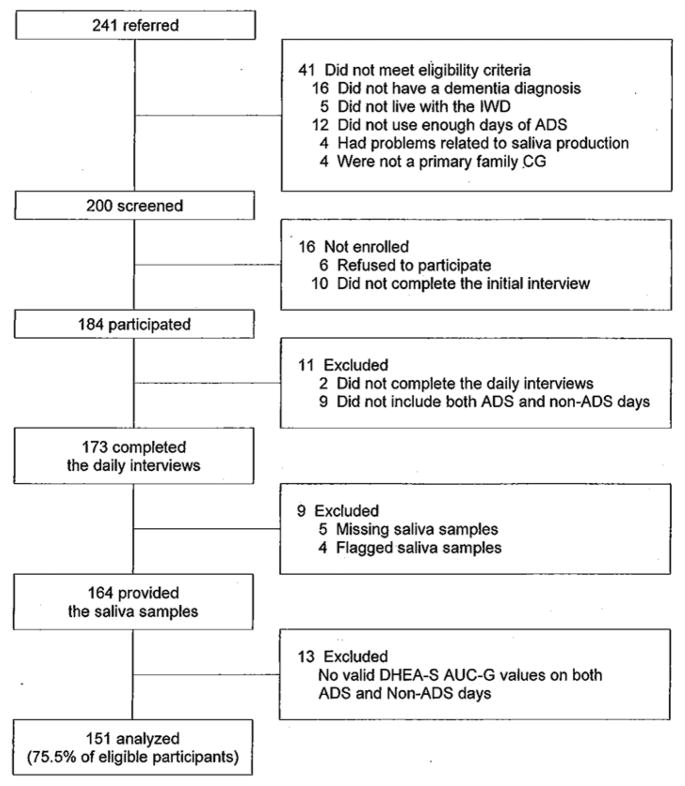
Recruitment was conducted at 57 ADS programs in Northern and Central New Jersey, the Philadelphia and Pittsburgh metropolitan areas, Northern Virginia, and Denver, Colorado. We screened 241 caregivers in an initial telephone interview, and found 41 (17%) who were not eligible for the study (Figure 1). The most frequent reasons were: the IWD did not have an eligible diagnosis dementia (n = 16); the IWD did not live with the caregiver (n = 5), and the IWD did not use enough days of ADS (n = 12). Sixteen of the 200 eligible participants (8%) subsequently decided not to enroll in the study. Another 33 (16.5%) caregivers were eliminated from the analysis because of missing interview days or missing or flagged saliva samples (i.e., out of range values or times of collection). The resulting sample was 151 people (75.5% of eligible participants). Sample characteristics are shown in Table 1.
Figure 1.
Flow diagram of participants.
Table 1.
Characteristics of Caregivers and Individuals with Dementia
| Mean | SD | Range | |
|---|---|---|---|
| CG’s characteristics | |||
| Age, years | 62.08 | 10.62 | 39–89 |
| Educationa | 4.39 | 1.22 | 1–6 |
| Household incomeb | 6.85 | 3.13 | 1–11 |
| Female, n (%) | 132 | (87.4) | – |
| White, n (%) | 107 | (70.9) | – |
| Married, n (%) | 107 | (70.9) | – |
| Relation to IWD | |||
| Spouse, n (%) | 59 | (39.1) | – |
| Child, n (%) | 86 | (57.0) | – |
| Employed, n (%) | 63 | (41.7) | – |
| Duration of care, months | 62.72 | 46.29 | 3–264 |
| Frequency of ADS use | 4.17 | 1.42 | 1–6 |
| Medication use, n (%) | 71 | (47.0) | – |
| IWD’s characteristics | |||
| Age, years | 81.85 | 8.61 | 57–100 |
| Female, n (%) | 89 | (58.9) | – |
| ADL impairmentc | 3.07 | 0.48 | 2–4 |
Notes: Participant N = 151.
CG = caregiver; IWD = individual with dementia; ADS = adult day services; ADL = activities of daily living.
Rated on a 6-point scale from 1 (less than high school) to 6 (post college degree).
Rated on a 11-point scale from 1 (less than $10,000) to 11 ($100,000 or over).
Rated on a 4-point scale from 1 (does not need help) to 4 (cannot do without help).
Procedures
An in-person interview was conducted to obtain signed consent and sociodemographic information. The interviewer also trained participants in the use of a home saliva kit. The kit contained 40 color-coded salivettes and a home collection diary. For the next 8 days, participants collected saliva 5 times a day (before getting out of bed, 30-minutes after getting out of bed, before lunch, before dinner, and before bed). Saliva collection times were recorded on salivettes and the home collection diary. Participants kept salivettes in the refrigerator until shipment back to the lab. In the evenings of each of the 8 days, interviewers from the Penn State Survey Research Center obtained information about daily stressors and mood and confirmed saliva sample times. Participants received payments for completing the daily interviews.
Measures
Salivary DHEA-S
Once received at the Penn State Biomarker Core Lab, saliva samples were weighed and stored at −80 degree C. Salivary DHEA-S was determined using commercially available enzyme immunoassay kits (DiaMetra, Italy). Samples were analyzed in duplicate and tests that were greater than 10% difference in their coefficient of variance (CV) were rerun for accuracy. DHEA-S data were converted to nmol/mL (i.e., ng/mL × 2.71).
DHEA-S area under the curve with respect to ground (AUC-G) was calculated for each day to estimate total output on that day. AUC-G uses a formula that adjusts for differences in the amount of time between samples.26
Type of Day
The IWD’s use of ADS was confirmed during each daily interview (1 = ADS day, 0 = non-ADS day).
Daily Mood
Depressive symptoms were assessed with four items (feeling worthless, hopeless, ashamed, so sad that nothing could cheer you up) from the Non-Specific Psychological Distress Scale.27 Positive mood was measured with nine items from the Non-Specific Psychological Distress Scale and the Positive and Negative Affect Schedule (PANAS).28 Respondents were asked how frequently they felt each emotion over the past day along a 5-point scale from 1 (none of the day) to 5 (all day). Mean scores were calculated (α = 0.77 for depressive symptoms; α = 0.92 for positive mood).
Daily Experiences
We included two measures of daily stressors, care-related stressors and non-care stressors and a measure of positive daily experiences. These measures were used to confirm that ADS days reduced exposure to care-related stressors and as controls in exploring the association of DHEA-S and daily mood.
Care-related stressors were assessed by the Daily Record of Behavior (DRB), a 19-item measure of Behavioral and Psychological Symptoms of Dementia.29 Caregivers reported the occurrence (1 = yes, 0 = no) of each behavior for three time periods in the previous 24 hours: waking to 9:00 a.m., 9:00 a.m. to 4:00 p.m., and 4:00 p.m. to bedtime. We summed the total number of behaviors reported each day.
Non-care stressors were assessed through the Daily Inventory of Stressful Events (DISE).30 Caregivers reported the occurrence (1 = yes, 0 = no) of eight non-overlapping items during the previous 24 hours (e.g., stressors affecting friends or family, work-related events). Total number of daily events was reported.
Positive events were assessed with a scale from the DISE. Caregivers reported the occurrence (1 = yes, 0 = no) of five non-overlapping items during the past 24 hours (e.g., share a laugh with someone, a positive experience at home). Total number of daily positive events was reported.
Covariates
Age and gender (1 = female, 0 = male), which could influence levels of DHEA-S, were included as between-person covariates. Specifically, DHEA-S levels decrease across the lifespan and men display higher DHEA-S levels than do women.31,32 We assessed medications likely to affect DHEA-S (e.g., steroidal medications, antidepressants). We also considered two variables representing chronicity of care: duration of caregiving (month) and IWD’s disability in 13 activities of daily living (ADL).33 Responses on ADL items ranged from 1 (does not need help) to 4 (cannot do without help). Another covariate was total number of days the IWD used ADS during the 8 interview days. Finally, we considered caregiver’s reported daily sleep quality as a within-person covariate of next day’s DHEA-S and mood.
Statistical Strategy
To analyze daily data nested within persons, we used two-level multilevel models (SAS PROC MIXED).34 We estimated the models via means of maximum likelihood, and specified unstructured covariance matrices for the error structures of our data. We first examined the effects of ADS use on DHEA-S by modeling levels of DHEA-S for dth day in the ith person at Level 1 (within-person):
We included concurrent use of ADS (today’s use, β1i) in this model to examine differences in daily levels of DHEA-S by ADS use, and then added yesterday’s use of ADS (β2i) to test for a lagged effect of ADS use. We also controlled for sleep quality at last night (β3i), which was centered at individual means to represent the within-person effects.35 At Level 2 (between-person), we controlled covariates for the intercept: caregiver’s age, gender, medication use, and duration of care, IWD’s ADL impairment, total ADS use during the 8 days, and individual-mean levels of sleep quality. Except for a dummy-coded covariate (gender), all continuous covariates were entered into the model after being centered at the group-mean.
We then conducted separate models to investigate the concurrent relationship of daily depressive symptoms and positive mood with daily DHEA-S levels. We parameterized mood for dth day in the ith person at Level 1 (within-person):
where β1i is the slope parameter for caregiver i’s change in the predicted mood for a one unit change in levels of DHEA-S. We controlled for daily experiences (care-related stressors, non-care stressors, and positive events; β2i, β3i, and β4i) and last night’s sleep quality (β5i) that could affect daily mood. The levels of DHEA-S, three daily experiences, and sleep quality included at Level 1 were centered at individual means. At Level 2, we controlled for the between-person covariates as well as individual-mean levels of DHEA-S, three daily experiences, and sleep quality. For effect sizes of the multilevel models, we computed Cohen’s d.36
Results
Participants completed an average of 6.7 days (SD = 1.5) of saliva collection. ADS use averaged 4.17 (SD = 1.42) days across the 8 days. Table 2 shows collection times and levels of DHEA-S. As in prior work,22 DHEA-S demonstrated a decreasing diurnal rhythm.
Table 2.
Summary of Daily Measures: Caregivers’ Salivary DHEA-S, Daily Experiences, Sleep Quality and Mood
| Mean | SD | ICC | |
|---|---|---|---|
| Daily salivary DHEA-S | |||
| Collection time (decimal hours) | |||
| a. Waking | 6.74 | 1.27 | 0.60 |
| b. 30 minutes after waking | 7.39 | 1.32 | 0.58 |
| c. Before lunch | 12.81 | 1.21 | 0.38 |
| d. Around 5pm | 17.46 | 1.16 | 0.52 |
| e. Before bed | 22.61 | 1.13 | 0.67 |
| Outcome level (nmol/L) | |||
| a. Waking | 15.65 | 11.29 | 0.79 |
| b. 30 minutes after waking | 10.33 | 5.34 | 0.74 |
| c. Before lunch | 8.28 | 4.68 | 0.56 |
| d. Around 5pm | 8.25 | 4.17 | 0.66 |
| e. Before bed | 9.35 | 5.56 | 0.54 |
| Total outcome: AUC-G | 143.10 | 67.67 | 0.82 |
| Daily experiencesa | |||
| Care-related stressors | 5.42 | 5.53 | 0.76 |
| Non-care stressors | 1.09 | 1.23 | 0.37 |
| Positive events | 2.47 | 1.31 | 0.44 |
| Daily sleep qualityb | |||
| Sleep quality | 2.97 | 1.04 | 0.39 |
| Daily moodc | |||
| Depressive symptoms | 1.14 | 0.41 | 0.59 |
| Positive mood | 3.06 | 0.93 | 0.73 |
Notes: Participant N = 151; Observation N = 1,011.
DHEA-S = dehydroepiandrosterone-sulfate; AUC-G = area under the curve with respect to ground; ICC = intraclass correlation.
Count of events or problems occurred during the 24-hour period.
Rated on a 5-point scale from 1 (poor) to 5 (excellent).
Rated on a 5-point scale from 1 (none of the day) to 5 (all day).
As an initial step (Table 3), we confirmed that care-related stressors were significantly lower on days the IWD attended ADS, compared to non-ADS days. We then examined differences between ADS and non-ADS days in AUC-G of DHEA-S (Table 4). ADS use was significantly associated with increased DHEA-S levels on days following ADS use. “Following” days included both days the IWD attended and did not attend ADS. Effect size was small (d = 0.11).
Table 3.
Multilevel Model for Daily Experiences of Family Caregivers by ADS Use
| ADS day | Non-ADS day | ADS effecta | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Mean | SD | Mean | SD | B | (SE) | p | |
| Care-related stressors | 3.76 | 5.26 | 4.83 | 5.75 | −1.18 | (0.20) | <0.001 |
| Non-care stressors | 1.20 | 1.25 | 1.00 | 1.20 | 0.20 | (0.07) | 0.003 |
| Positive events | 2.61 | 1.30 | 2.39 | 1.31 | 0.17 | (0.07) | 0.012 |
Notes. Participant N = 151; Observation N = 1,011.
ADS = adult day services; CG = caregiver; IWD = individual with dementia; ADL = activities of daily living; ICC = intraclass correlation.
Fixed effects of ADS use (1 = ADS day, 0 = non-ADS day; df = 859) for differences between ADS day and non-ADS day in multilevel models; p values are based on t values of the ADS coefficients (df = 859); CG’s age, gender, and employment, duration of care, IWD’s ADLs impairment, and number of ADS days were included as (between-person) control variables.
Table 4.
Multilevel Models for Daily Levels of DHEA-S (AUC-G) and Mood
| DHEA-S (AUC-G)
|
Depressive symptoms
|
Positive mood
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B | (SE) | p | B | (SE) | p | B | (SE) | p | |
| Fixed effects | |||||||||
| Intercept | 148.25 | (15.05) | <0.001 | 1.05 | (0.07) | <0.001 | 2.91 | (0.15) | <0.001 |
| Within-person predictorsa | |||||||||
| ADS use (1 = yes) | |||||||||
| Today use | 2.58 | (1.91) | 0.18 | – | – | ||||
| Yesterday use | 3.80 | (1.93) | 0.049 | – | – | ||||
| DHEA-S (AUC-G) | – | 0.00 | (0.00) | 0.71 | 0.00 | (0.00) | 0.006 | ||
| Daily experiences | |||||||||
| Care-related stressors | – | 0.02 | (0.00) | <0.001 | −0.01 | (0.01) | 0.018 | ||
| Non-care stressors | – | 0.04 | (0.01) | <0.001 | −0.09 | (0.02) | <0.001 | ||
| Positive events | – | −0.02 | (0.01) | 0.005 | 0.18 | (0.02) | <0.001 | ||
| Sleep quality (last night) | 1.81 | (1.20) | 0.13 | −0.01 | (0.01) | 0.47 | 0.04 | (0.02) | 0.017 |
| Between-person predictorsb | |||||||||
| Frequency of ADS use | 5.00 | (3.70) | 0.18 | 0.01 | (0.02) | 0.54 | 0.09 | (0.04) | 0.020 |
| CG Female (1 = yes) | −6.65 | (15.91) | 0.68 | 0.08 | (0.08) | 0.30 | 0.22 | (0.16) | 0.17 |
| CG Age, years | −0.89 | (0.50) | 0.08 | 0.00 | (0.00) | 0.14 | 0.00 | (0.01) | 0.80 |
| Medication use (1 = yes) | −5.32 | (10.46) | 0.61 | 0.04 | (0.05) | 0.41 | −0.11 | (0.10) | 0.30 |
| Duration of care, months | −0.27 | (0.11) | 0.018 | −0.00 | (0.00) | 0.35 | 0.00 | (0.00) | 0.15 |
| IWD ADL impairment | 18.75 | (11.12) | 0.09 | −0.02 | (0.05) | 0.69 | 0.00 | (0.11) | 0.99 |
| DHEA-S (AUC-G) | – | −0.00 | (0.00) | 0.53 | 0.00 | (0.00) | 0.36 | ||
| Daily experiences | |||||||||
| Care-related stressors | – | 0.02 | (0.01) | 0.003 | −0.01 | (0.01) | 0.31 | ||
| Non-care stressors | – | 0.06 | (0.03) | 0.08 | −0.09 | (0.07) | 0.23 | ||
| Positive events | – | −0.09 | (0.03) | 0.001 | 0.43 | (0.06) | <0.001 | ||
| Sleep quality | 1.36 | (7.27) | 0.85 | −0.02 | (0.04) | 0.53 | 0.33 | (0.08) | <0.001 |
| Random effects | |||||||||
| Intercept VAR | 3836.34 | (459.22) | <0.001 | 0.08 | (0.01) | <0.001 | 0.36 | (0.04) | <0.001 |
| Residual | 703.74 | (36.77) | <0.001 | 0.06 | (0.00) | <0.001 | 0.19 | (0.01) | <0.001 |
| −2 Log Likelihood | 8835.9 | 380.9 | 1584.5 | ||||||
| AIC | 8861.9 | 418.9 | 1622.5 | ||||||
Notes: Participant N = 151; Observation N = 1,011.
CG = caregiver; IWD = individual with dementia; ADS = adult day services; ADL = activities of daily living; DHEA-S = dehydroepiandrosterone-sulfate; AUC-G = area under the curve with respect to ground; VAR = variance; AIC = Akaike’s information criterion.
For fixed effects, p values are based on t values of the coefficients; For random effects, p values are based on z values of the coefficients.
Person-mean centered for continuous variables.
Group-mean centered for continuous variables.
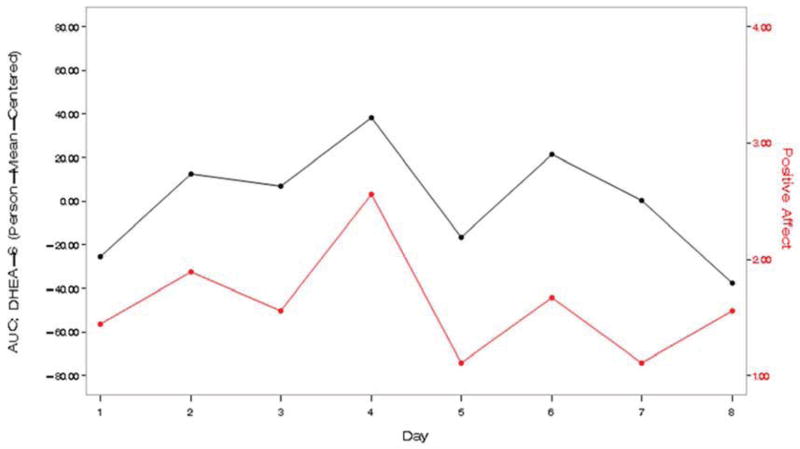
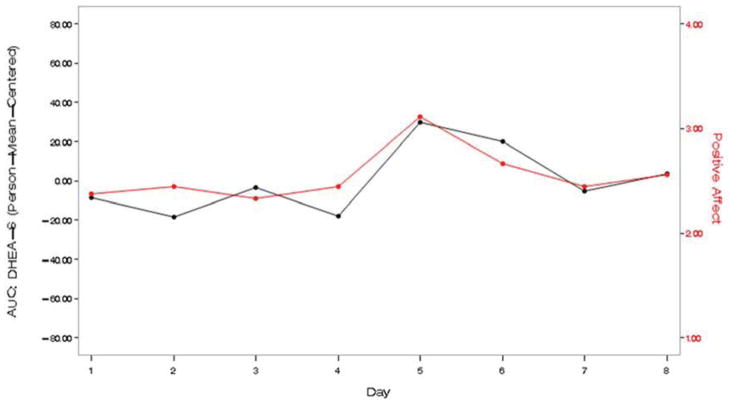
Turning to the relationship between levels of DHEA-S and daily mood (Table 4), caregivers’ positive mood showed a positive covariation with DHEA-S at the daily level, controlling for daily experiences. Effect size was medium (d = 0.43). Two examples (Figure 2) illustrate this association. Daily depressive symptoms were not associated with daily DHEA-S.
Figure 2.
Two individual examples of the positive covariation between caregivers’ self-reported positive mood and levels of salivary DHEA-S.
Notes: DHEA-S = dehydroepiandrosterone-sulfate; AUC-G = area under the curve with respect to ground.
Among covariates, longer duration of caregiving was associated with lower average levels of DHEA-S. Total ADS used during the 8 days was related to higher mean positive mood across the 8 days. Daily experiences were significantly associated with daily positive mood and depressive symptoms (within-person effects) and with average levels on these measures (between-person effects). Persons experiencing higher daily and average care-related and non-care stressors had higher depressive symptoms and lower positive mood, whereas positive events had the opposite associations with daily and average mood.
Discussion
With growing numbers of IWD and family caregivers assisting them, it is increasingly important to identify interventions that mitigate the effects of chronic stress and reduce risk of illness and mental health problems. This study uses promising approaches for the study of caregiver interventions, including the use of daily measurements, and, particularly, demonstrating that an intervention that alters daily stressor exposure, ADS has an effect on a physiological measure of the stress response, DHEA-S. These findings are noteworthy because DHEA-S levels have been found to decrease with prolonged exposure to stressors.8,9 To our knowledge, this is the first study to examine DHEA-S levels across the day in connection with an intervention that affected daily exposure to stressors, and to link DHEA-S levels to daily positive emotions. It is also one of the few studies demonstrating an effect of a caregiving intervention on physiological indicators of stress.37 Although effect size was small, these results suggest the value of broadening the focus of caregiver interventions to include their impact on relevant biological risk factors associated with chronic stress and disease.
The increases in DHEA-S levels on days following ADS use may allow the body to mount a protective and restorative response to the physiological demands of caregiving. Although the mechanism is not fully understood, it has been suggested that the anabolic and antiglucocorticoid effects of DHEA-S may buffer the health-damaging effects of stress and cortisol.38,39 The delayed increase in DHEA-S could be the product of a time-lagged energy shift in the adrenal cortex to release DHEA-S in order to compensate for stress-related cortisol dysregulation, or the result of the long half life of DHEA-S.24 An important next step is to determine the long-term health benefits of these DHEA-S elevations as a result of the intervention.
DHEA-S is part of a larger system of responses to stressors. Many studies have presented cortisol:DHEA-S ratios as a way of capturing a broader view of the biological process in stress responses.40 We decided not to take this approach because of concerns about the limitations of ratios as a way of representing associations of dynamic processes. Ratios restrict statistical variance and the same value may be produced by multiple combinations of the composite variables. A low cortisol:DHEA-S ratio could be the result of both values being high or both being low. Particularly, cortisol in chronic stress situations may be elevated or low14, which renders a cortisol:DHEA-S ratio uninterpretable. Furthermore, though related, cortisol and DHEA-S appear to respond to events at different rates, and demonstrate different morning rhythms, which further limit the utility of a ratio.
Daily DHEA-S levels covaried significantly with positive mood, though it had no association with depressive symptoms. This finding confirms prior research that posited an association between DHEA-S and positive mood.10 In a secondary analysis, however, we did not find an association of daily ADS use and positive mood, although total ADS days used was significantly related to higher mean positive mood. Thus, although higher levels of DHEA-S were associated with increased positive mood, the influence of ADS on positive mood appears indirect. We also do not know the direction of the association of DHEA-S and positive mood. Prior work has viewed affect as both a precursor and consequence of HPA axis markers.16 As with DHEA-S, higher positive mood is a possible protective factor for caregivers, leading to improved ability to cope with stressors,41 and to better health and reduced mortality.42 Whether these effects are the result of or complementary to the associations with DHEA-S remains to be determined. We also note that other analyses from the current study identified that daily and cumulative ADS use had a broad impact on emotional well-being, including same day reductions of feelings of anger, and buffering the effects of stressors on daily and average levels of depressive symptoms.7
This study has several limitations. First, the sample consisted of volunteers who were already using ADS. We cannot rule out that the sample might have selectively included people experiencing a positive response to ADS. We also did not use a typical control group, but rather utilized within-person comparisons of days when an intervention (ADS use) was delivered and not delivered. Though not widely used, this within-person design provides a different type of control than randomized trials by demonstrating covariation at the individual level between introduction and withdrawal of an intervention and outcomes.25 Third, ADS use had a smaller effect on care stressor exposure than in prior work,29 though this may be due to using a count of problems, rather than duration of episodes. Fourth, the short period of observation did not allow us to determine if improvements in DHEA-S levels with ADS use are cumulative over time, or if benefits are found only in response to each specific usage. Fifth, we hypothesized that the next day effects on DHEA-S and positive mood are due to reduced exposure to care stressors on the previous day, but we cannot rule other possible reasons for this delayed response. Finally, although gender was included as a covariate, a significant portion of our participants were women. Furthermore, the effects of DHEA-S levels differ by gender. 31,32 DHEA-S plays a key role in testosterone production in males and estrogen production in females. In as much as estrogen is protective against the development of certain diseases (e.g., cardiovascular), DHEA-S changes in response to stress may alter disease risk for women who disproportionately outnumber men among caregivers.43
Acknowledgments
This research was supported by grant RO1 AG031758 “Daily Stress and Health of Family Caregivers” from the National Institute on Aging (NIA).
Footnotes
This research was presented in part at the annual meetings of the Gerontological Society of America (GSA), San Diego, CA, November 2012.
No disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capistrant BD, Moon JR, Berkman LF, et al. Current and long-term spousal caregiving and onset of cardiovascular disease. J Epidemiol Commun H. 2011;66:951–956. doi: 10.1136/jech-2011-200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins M, Howard VJ, Wadley VG, et al. Caregiver strain and all-cause mortality: evidence from the REGARDS study. J Gerontol B Psychol Sci Soc Sci. 2013;68:504–512. doi: 10.1093/geronb/gbs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodaty H, Woodward M, Boundy K, et al. Prevalence and predictors of burdehn in caregivers of people with dementia. Am J Geriatr Psychiatry. doi: 10.1016/j.jagp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Fantidis P. The role of the stress-related anti-inflammatory hormones ACTH and cortisol in atherosclerosis. Curr Vasc Pharmacol. 2010;8:517–525. doi: 10.2174/157016110791330889. [DOI] [PubMed] [Google Scholar]
- 5.Kiecolt-Glaser JK, McGuire L, Robles TF, et al. Psychoneuroimmunology: psychological influences on immune function and health. J Consult Clin Psychol. 2002;70:537–547. doi: 10.1037//0022-006x.70.3.537. [DOI] [PubMed] [Google Scholar]
- 6.von Känel R, Mills PJ, Mausbach BT, et al. Effect of Alzheimer caregiving on circulating levels of c-reactive protein and other biomarkers relevant to cardiovascular disease risk: a longitudinal study. Gerontology. 2012;58:354–365. doi: 10.1159/000334219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarit SH, Kim K, Femia EE, et al. The effects of adult day services on family caregivers’ daily stress, affect and health: outcomes from the Daily Stress and Health (DaSH) study. Gerontologist. 2013 Jun 19; doi: 10.1093/geront/gnt045. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennartsson A-K, Theorell T, Kushnir MM, et al. Perceived stress at work is associated with attenuated DHEA-S response during acute psychosocial stress. Psychoneuroendocrinology. 2013 Jul 25; doi: 10.1016/j.psyneuen.2013.01.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Izawa S, Saito K, Shirotsuki K, et al. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: a study of a two-week teaching practice. Psychoneuroendocrinology. 2012;37:852–858. doi: 10.1016/j.psyneuen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Rev. 1999;30:264–288. doi: 10.1016/s0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 11.Van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- 12.Rabkin JG, McElhiney MC, Rabkin R, et al. Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 2006:59–66. doi: 10.1176/appi.ajp.163.1.59. [DOI] [PubMed] [Google Scholar]
- 13.Mausbach BT, Harmell AL, Moore RC, et al. Influence of caregiver burden on the association between daily fluctuations in pleasant activities and mood: a daily diary analysis. Behav Res Ther. 2011;49:74–79. doi: 10.1016/j.brat.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savla J, Granger DA, Roberto KA, et al. Cortisol, alpha amylase, and daily stressors in spouses of persons with mild cognitive impairment. Psychol Aging. 2013 Jun 17; doi: 10.1037/a0032654. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord. 2012;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piazza JR, Almeida DM, Dmitrieva NO, et al. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65B:513–525. doi: 10.1093/geronb/gbq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawata H, Yanase T, Goto K, et al. Mechanism of action of anti-aging DHEA-S and the replacement of DHEA-S. Mech Ageing Dev. 2002;123:1101–1106. doi: 10.1016/s0047-6374(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 18.Obut TA, Ovsyukova MV, Cherkasova OP. Stress-limiting effect of dehydroepiandrosterone sulfate and its mechanism. Bull Exp Biol Med. 2003;135:231–233. doi: 10.1023/a:1024168510925. [DOI] [PubMed] [Google Scholar]
- 19.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 20.Luppi C, Fioravanti B, Bertolini M, et al. Growth factors decrease in subjects with mild to moderate Alzheimer’s disease (AD): potential correction with dehydroepiandrosterone sulfate (DHEAS) Arch Gerontol Geriatr Suppl. 2009;1:173–184. doi: 10.1016/j.archger.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Izawa S, Saito K, Shirotsuki K, et al. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: a study of a two-week teaching practice. Psychoneuroendocrinology. 2012;37:852–858. doi: 10.1016/j.psyneuen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Ritsner MS. The clinical and therapeutic potentials of dehydroepiandrosterone and pregnenolone in schizophrenia. Neuroscience. 2011;191:91–100. doi: 10.1016/j.neuroscience.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Harmell AL, Chattillion EA, Roepke SK, et al. A review on the psychogiology of dementia caregiving: a focus on resilience factors. Curr Psychiatry Rep. 2011;13:219–224. doi: 10.1007/s11920-011-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Legrain S, Massien C, Lahlou N, et al. Dehydroepiandrosterone replacement administration: pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. J Clin Endocrinol Metab. 2000;85:3208–3217. doi: 10.1210/jcem.85.9.6805. [DOI] [PubMed] [Google Scholar]
- 25.Barlow DH, Nock MK, Hersen M. Single case experimental designs: strategies for studying behavior change. 3. Boston, MA: Pearson Allyn & Bacon; 1999. [Google Scholar]
- 26.Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 27.Kessler RC, Andrews G, Colpe LJ, et al. Short screening scales to monitor population prevalences and trends in non-specific psyshological distress. Psychol Med. 2002;32:959–976. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 28.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 29.Femia EE, Zarit SH, Stephens MAP, et al. Impact of adult day services on behavioral and psychological symptoms of dementia. Gerontologist. 2007;47:775–788. doi: 10.1093/geront/47.6.775. [DOI] [PubMed] [Google Scholar]
- 30.Almeida DM, Wethington E, Kessler RC. The daily inventory of stressful events: an interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- 31.Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 32.Whetzel CA, Klein LC. Measuring DHEA-S in saliva: methodological considerations in biobehavioral health research. BMC Res Notes. 2010;3:204–208. doi: 10.1186/1756-0500-3-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 34.Littell RC, Miliken GA, Stroup WW, et al. SAS systems for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- 35.Hoffman L, Stawski RS. Person as contexts: evaluating between-person and within-person effects in longitudinal analysis. Res Hum Dev. 2009;6:97–120. [Google Scholar]
- 36.Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 37.Moore RC, Chattillion EA, Ceglowski J, et al. A randomized clinical trial of Behavioral Activation (BA) therapy for improving psychological and physical health in dementia caregivers: results of the Pleasant Events Program (PEP) Behav Res Ther. 2013;51:623–632. doi: 10.1016/j.brat.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalimi M, Shafagoj Y, Loria R, et al. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA) Mol Cell Biochem. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- 39.Morgan CA, Southwick S, Hazlett G, et al. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Arch Gen Psychiatry. 2004;61:819–825. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- 40.Bauer ME. Chronic stress and immunosenescence: a review. Neuroimmunomodulat. 2008;15:241–250. doi: 10.1159/000156467. [DOI] [PubMed] [Google Scholar]
- 41.Folkman S. Positive psychological states and coping with severe stress. Soc Sci Med. 1997;45:1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 42.Damen NL, Pelle AJ, Boersma E, et al. Reduced positive affect (anhedonia) is independently associated with 7-year mortality in patients treated with percutaneous coronary intervention: results from the RESEARCH registry. Eur J Prev Cardiol. 2012;20:127–134. doi: 10.1177/2047487312436452. [DOI] [PubMed] [Google Scholar]
- 43.Glei DA, Goldman N, Weinstein M, et al. Dehydroepiandrosterone sulfate (DHEAS) and health: does the relationship differ by sex? Exp Gerontol. 2004;39:321–331. doi: 10.1016/j.exger.2003.11.003. [DOI] [PubMed] [Google Scholar]