Abstract
Human pluripotent stem cells represent an unlimited source of skeletal tissue progenitors for studies of bone biology, pathogenesis, and the development of new approaches for bone reconstruction and therapies. In order to construct in vitro models of bone tissue development and to grow functional, clinical-size bone substitutes for transplantation, cell cultivation in three-dimensional environments composed of porous osteoconductive scaffolds and dynamic culture systems—bioreactors—has been studied. Here, we describe a stepwise procedure for the induction of human embryonic and induced pluripotent stem cells (collectively termed PSCs) into mesenchymal-like progenitors, and their subsequent cultivation on decellularized bovine bone scaffolds in perfusion bioreactors, to support the development of viable, stable bone-like tissue in defined geometries.
Keywords: Human embryonic stem cells, Human induced pluripotent stem cells, Mesenchymal-like progenitors, Osteogenic differentiation, Osteogenic medium, Bone scaffolds, Cell seeding, Perfusion bioreactor, Medium flow rate, Bone tissue development
1 Introduction
Bioengineered human tissues have a broad range of applications in regenerative medicine, including reconstructive therapies, studies of development, disease modeling, and drug development and screening (1, 2). A variety of human stem cells from embryonic, fetal, and adult tissues are under investigation for the bioengineering of functional bone tissue substitutes (3). Among these, human pluripotent stem cells (hPSCs) with their unlimited growth and the potential to differentiate into any cell type of the human body, and immunocompatibility represent an unprecedented resource (4, 5). However, pluripotency also represents a challenge for reproducible, stable, and efficient induction of cells into the desired lineages.
For most mesodermal lineages, stepwise differentiation protocols were developed to reproduce the signals encountered by the pluripotent cells during early embryonic patterning, and guide the cell development in vitro (6). Similarly, osteogenic- and mesenchymal stem cell-like progenitors (MPs) were derived from hPSCs by a variety of protocols, involving two- or three-dimensional (3D) embryoid body cell cultures in the presence of inductive signals such as serum, growth factors, osteogenic factors, and coculture with primary stromal/osteogenic cells (reviewed in (1)). In these studies, osteogenic marker expression and limited (<1 mm) bone-like tissue formation were demonstrated either in vitro or in vivo. In some cases, cells formed teratomas after extended in vivo transplantation, suggesting the need for further optimization of the hPSC induction/ tissue engineering protocols (7, 8).
Bone tissue engineering has been extensively studied with adult mesenchymal stem cells (MSCs) (reviewed in (3, 9)). The influences of various tissue engineering and in vitro culture parameters on bone development have been elucidated, such as delivery of osteoinductive factors, the properties of osteoconductive scaffolds (composition, structure, mechanical properties), and the culture in static or dynamic environments, with their specific mass transport and gas exchange properties, and the possibilities to deliver select biochemical and biophysical stimuli (3).
Our group has developed a biomimetic tissue-engineering strategy for the cultivation of functional, anatomically shaped bone substitutes, by culturing MSCs from the bone marrow on 3D porous scaffolds resembling the matrix of native bone in bioreactors providing interstitial flow of culture medium (10). For smaller (<1 cm) cylindrical bone constructs, we have defined the influences of initial cell seeding density, fluid perfusion rate, and bone scaffold architecture on in vitro bone development (11–13).
Herein, we describe this in vitro bone development model adjusted for growing bone-like tissue from hPSCs (14, 15). Based on previous studies (16, 17), we hypothesized that hPSCs-derived MPs will form bone-like tissue under conditions optimized with MSCs (11, 12), and that the osteogenic induction of MPs should thus be followed by the conditions supporting bone formation. Indeed, we found that the stepwise induction of MPs, and their subsequent seeding and culture on decellularized bovine bone scaffolds in osteogenic medium under constant perfusion resulted in the formation of viable, stable bone-like tissue of defined geometries (14, 15).
2 Materials
Dulbecco’s modified eagle medium (DMEM), KnockOut DMEM (KO-DMEM), KnockOut Serum Replacement (KO-SR), Dulbecco’s phosphate buffered saline solution (DPBS), GlutaMAX solution (100×), nonessential amino acids (100×), beta-mercaptoethanol, penicillin–streptomycin solution (100×, 100 U/ml), trypsin/ethylenediaminetetraacetic acid (EDTA; 0.25 %), bovine serum albumin fraction V (BSA), fetal bovine serum (FBS), insulin, sodium pyruvate (100×), and basic fibroblast growth factor (bFGF) were purchased from Life Technologies. HyClone FBS, Tris buffer, proteinase K and phosphate buffered saline (PBS, 10×) were purchased from Fisher Scientific. Antibodies for flow cytometry were from BD Biosciences. Bovine bone joints were from Green Village Packing. All other chemicals were purchased from Sigma-Aldrich unless otherwise noted.
Prepare all solutions using tissue-culture grade water, unless otherwise instructed. Prepare culture media fresh for weekly media changes, and differentiation media fresh prior to each medium change. Culture media are prepared complete with the addition of serum/serum replacement, antimicrobials, cytokines, growth factors, and other supplements, filter-sterilized and stored at 4–8 °C. Culture and differentiation media are warmed to 37 °C prior to media changes and scaffold and bioreactor conditioning.
2.1 hPSC Cultivation and Induction into Mesenchymal-Progenitors
-
hPSC culture medium:
Prepare medium by combining 80 % KO-DMEM with 20 % KO-SR (vol/vol), and adding 10 ng/ml bFGF, 2 mM Gluta-MAX, 0.1 mM nonessential amino acids, 0.1 mM beta-mercaptoethanol, and 100 U/ml (1×) penicillin–streptomycin (see Note 1).
-
Gelatin-coated plates:
Dissolve gelatin 0.1 % (wt/vol) in tissue culture water, filter-sterilize, and store at room temperature. Pipet 2 ml of 0.1 % gelatin solution per well of 6-well tissue culture plates (Nunclon) or 0.5 ml of gelatin solution per 4-well/24-well plates and incubate at least 2 h at room temperature. Gelatin-coated tissue culture plates can be stored for several weeks in the incubator at 37 °C.
-
Mouse embryonic fibroblasts (MEF) medium:
Make-up MEF medium by combining 90 % DMEM with 10 % FBS (vol/vol), and adding 100 U/ml penicillin–streptomycin.
-
MEF feeder layers:
Thaw one vial of MEF (irradiated, strain CF1, GlobalStem) and transfer into a tube with 5–10 ml of MEF medium at 37 °C. Centrifuge the cell suspension at 250 × g for 5 min, resuspend in MEF medium and seed at 150,000–300,000 cells/well of gelatin-coated 6 well plate or 40,000–75,000 cells/well of gelatin-coated 4 well plate. MEF feeder layers are used after 1–3 days of culture (at 37 °C in a humidified atmosphere containing 5 % CO2—standard conditions) for seeding undifferentiated hPSC cells (see Note 2).
-
Mesenchymal induction medium:
Prepare induction medium by combining 80 % KO-DMEM with 20 % HyClone FBS (vol/vol), and adding 2 mM Gluta- MAX, 0.1 mM nonessential amino acids, 0.1 mM beta-mercaptoethanol, and 100 U/ml penicillin–streptomycin.
2.2 Characterization of hPSC-Mesenchymal Progenitor Surface Antigen Expression and Osteogenic Potential
-
Flow cytometry buffer and antibodies:
Prepare flow cytometry buffer by combining DPBS with 0.5 % BSA (vol/vol), 100 U/ml penicillin–streptomycin, 2 mM EDTA, and 20 mM glucose, sterile filter and store at 4–8 °C. Use a combination of antibodies to assess the mesenchymal-like surface antigen profile of progenitors derived from hPSC (14), for example: Tra1-60 PE (catalog no. 560193), SSEA1 PE (560142), SSEA4 V450 (561156), CD14 PE (561707), CD31 PE (555446), CD34 PE (555822), CD44 PE (561858), CD45 PE (560975), CD73 FITC (561254), CD90 PE (555596), and appropriate isotype controls (BD Biosciences). Dilute each antibody to a final concentration of 2 µl per 100 µl of flow cytometry buffer prior to staining. Alternatively, prepare several antibody dilutions to determine the lowest antibody concentration resulting in strong fluorescent signal for the staining.
-
Osteogenic medium:
Prepare osteogenic medium by combining 90 % DMEM with 10 % HyClone FBS (vol/vol), and adding 100 U/ml penicillin–streptomycin, 1 µM dexamethasone, 10 mM beta-glycerophosphate, and 50 µM ascorbic acid-2-phosphate.
-
Control medium:
Prepare control medium by combining 90 % DMEM with 10 % HyClone FBS (vol/vol), and adding 100 U/ml penicillin–streptomycin.
-
Alkaline phosphatase staining components:
The components of Fast blue RR Salt staining kit (Sigma-Aldrich) are prepared according to the manufacturer’s instructions.
Prepare citrate working solution by diluting 2 ml citrate concentrate solution with 98 ml with deionized H2O.
Prepare acetone fixative solution (citrate buffered acetone, 60 %) by adding 2 volumes of room temperature citrate working solution to 3 volumes of acetone under constant stirring (see Note 3).
Dissolve one capsule of Fast blue RR Salt in 48 ml of room temperature H2O, and add 2 ml of Naphthol AS-MX Phosphate Alkaline Solution (see Note 4).
Prepare fresh filtered Mayor’s Hematoxylin Solution.
-
Von Kossa staining components:
Prepare 3.7 % formaldehyde in PBS (vol/vol) and store at room temperature.
Dissolve AgNO3 in distilled H2O to make a 2 % (w/vol) staining solution (see Note 5).
2.3 Decellularized Bovine Bone Scaffolds
-
Bovine trabecular bone:
Obtain metacarpal joints of 2-week to 4-month-old calves from local butcher. Remove the soft tissues from the joint using scalpel and drill into the subchondral trabecular bone region to obtain cylinders of appropriate diameter (4–8 mm) (see Note 6).
-
0.1 % EDTA solution in PBS:
Dissolve 1 g of EDTA in 1,000 ml PBS and store at room temperature.
-
0.1 % EDTA solution in Tris:
Dissolve 1 g of EDTA in 1,000 ml of 10 mM Tris buffer and store at room temperature.
-
0.5 % SDS solution in Tris:
Dissolve 0.5 ml of SDS in 1,000 ml of 10 mM Tris buffer and store at room temperature.
-
DNAse/RNase solution in Tris:
Dissolve one vial (2,000 Kunitz, ≥0.5 mg total protein) of freeze-dried DNAse (Sigma Aldrich) and one piece (0.1–0.15 mg) of dust RNAse A (Sigma Aldrich) per each 40 ml of 10 mM Tris in sterile H2O.
2.4 Cultivation in Perfusion Bioreactors
-
Perfusion bioreactor components and tubing:
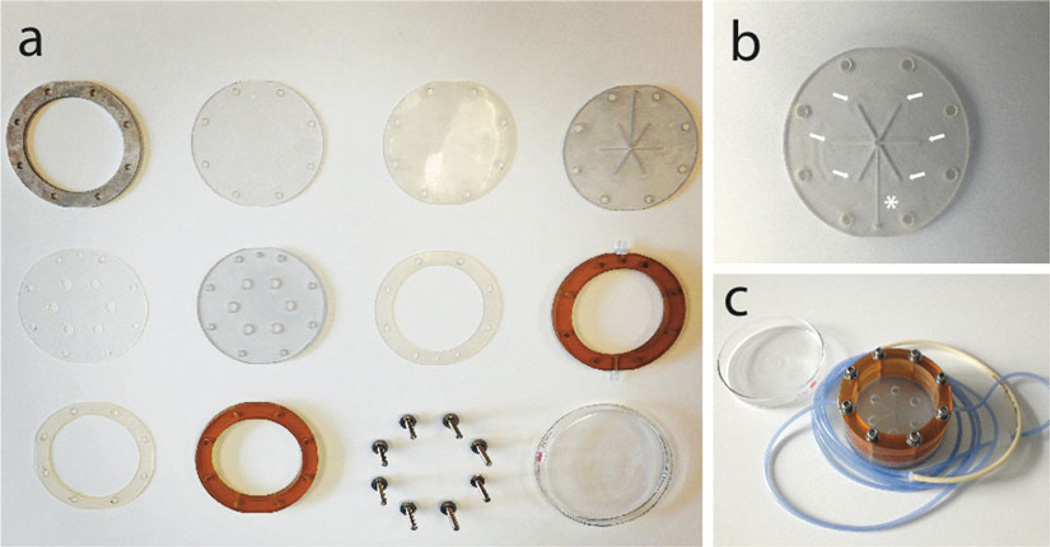
Gather the components of specific perfusion bioreactor system selected for the study. For instance, the components of custom-made round bioreactors used in our studies (14, 15) and the assembled bioreactor system are presented in Fig. 1 (11). For reusable bioreactor systems, disinfect the culture chambers/cassettes at the end of each experiment in 10 % hydrogen peroxide in water (vol/vol) for 30 min, wash thoroughly under stream of water, rinse with deionized water, and air-dry. Prior to the experiment, loosely assemble the bioreactors, connect new (medical-grade) tubing of appropriate length, fit the connectors for the stopcocks/syringes and sterilize by autoclaving.
-
Tools for bioreactor assembly and peristaltic pump components:
Prepare screws, hex-L-key, forceps, scissors, scalpels and other stainless-steel tools, and sterilize by autoclaving.
Assemble the peristaltic pump and transfer to the top/inside the incubator (depending on the selected bioreactor/pump system).
Osteogenic medium (prepared as in Section 2.2, item 2).
Fig. 1.
Round bioreactor system for simultaneous perfusion of six cell-scaffold constructs. Components of the reusable round bioreactor system are cleaned, loosely assembled, and autoclaved prior to each use (a). The system holds six wells into which cell-scaffold constructs are tightly fitted. The culture medium flows to the central channel (asterisk) and splits six ways (arrows) to assure equal perfusion of the constructs (b). The bioreactor is covered with a glass cover and connected to a single perfusion loop (c) (11, 14, 15)
3 Methods
3.1 Culture and Induction of Pluripotent Stem Cells into Mesenchymal Progenitors
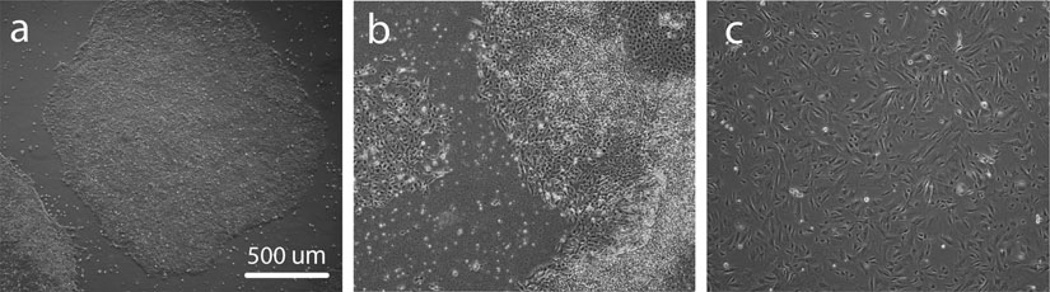
Prepare gelatin-coated plates, MEF medium, hPSC medium, and MEF feeder layers. Thaw hPSCs on fresh feeder layers (typically 1 frozen well of cells per 1 well of the culture plate) and culture in hPSC medium in the incubator at standard conditions. Manually remove the differentiated areas and split the colonies 1:1 to 1:3 for the first passages (depending on the growth of particular hPSC line) using standard techniques (mechanical splitting or enzymatic treatment). Expand the hPSCs to a fully confluent 6-well plate of colonies in hPSC medium (Fig. 2a).
Change the hPSC medium in confluent cultures to mesenchymal induction medium and incubate for 1 week in the incubator at standard conditions (Fig. 2b). Change media on days 3 and 6.
After 7 days, aspirate the culture medium, wash the cultures with DPBS, and detach the cells by incubating in trypsin/EDTA for 5 min at 37 °C. Count and seed the cells at high density (100,000 cells/cm2) in gelatin-coated plates. Incubate the cultures in mesenchymal induction medium in standard conditions and change media twice a week.
Upon reaching confluence, passage cells using trypsin/EDTA in mesenchymal induction medium at a density of 10,000/cm2 until they become homogenous for a fibroblastic-like morphology (passages 3–5 after induction, Fig. 2c) (see Note 7).
To assess the genomic stability of MPs following in vitro expansion, perform karyotpye analysis (for instance using a commercial provider, such as Cell Line Genetics).
Fig. 2.
Induction of hPSCs into MPs. Typical morphologies of undifferentiated hPSC cultures (a), overgrown hPSC cultures after 1 week in mesenchymal induction medium (b) and MPs after passaging in mesenchymal medium (c)
3.2 Characterization of hPSC-Mesenchymal Progenitor Surface Antigen Expression
Detach expanded MPs with trypsin/EDTA, wash with mesenchymal induction medium and filter through a 70 mm cell strainer (BD Biosciences) to obtain a single-cell suspension.
Count the cells and resuspend 5 × 106 cells in 3 ml of a sterile flow cytometry buffer and transfer on ice.
Prepare 50 µl aliquots of diluted primary antibodies and dispense them into black 96-well plates (Corning), followed by the addition of 50 µl aliquots containing 8 × 104 cells for a total of 100 µl per well.
Incubate cells on ice in the dark for 30 min, wash in staining buffer, and analyze immediately on flow cytometer/sorter (BD Biosciences ARIA-IIu SOU Cell Sorter) configured with a 100 µm ceramic nozzle and operating at 20-psi sheath fluid pressure.
Collect and analyze data using the software provided by the system (BD Biosciences Diva 6.0 software), using a combination of standard procedures with fluorescence minus one and isotype controls.
3.3 Characterization of hPSC-Mesenchymal Progenitor Osteogenic Potential
Seed MPs at passages 3–5 (or any other selected passage) at a density of 10,000/cm2 in 24-well gelatin-coated plates in osteogenic and control media. Culture in standard conditions and change media twice a week.
At 2 and 4 weeks after seeding, evaluate alkaline phosphatase activity and matrix mineralization by von Kossa staining.
For alkaline phosphatase activity, rinse sample wells in PBS and then fix by immersing in citrate buffered acetone for 30 s. Rinse gently in deionized H2O for 45 s. Do not allow the wells to dry. Then add alkaline-dye mixture and incubate at room temperature for 30 min in the dark. Rinse thoroughly with deionized H2O and counterstain with Mayer’s Hematoxylin solution for 10 min. Rinse abundantly with deionized H2O and evaluate microscopically (see Note 8).
For assessing matrix mineralization by von Kossa staining, rinse sample wells in PBS and then fix in 3.7 % formaldehyde in PBS (vol/vol) for 30 min. Rinse twice with deionized H2O, add 2 % AgNO3 solution to cover the well surface and keep in the dark for 10 min. Rinse with deionized H2O and then expose the samples to bright light (100 W) for 15–30 min. Then rinse again with deionized H2O and either dehydrate quickly adding 95–100 % ethanol or store the samples in PBS at 4–8 °C (see Note 9).
Observe and document the positive blue-purple staining of alkaline phosphatase and black staining of accumulated minerals in osteogenic cultures by light microscopy (see Note 10).
3.4 Preparation of Decellularized Bovine Bone Scaffolds
Wash the bone cylinders under high-pressure water stream to remove the bone marrow (see Note 11).
Place bone cylinders into 50 ml centrifuge tubes to fill approximately one half of the tube volume. Wash the bone cylinders with 0.1 % solution of EDTA in PBS for 1 h, then with 0.1 % EDTA solution in 10 mM Tris for 12 h at 10–15 °C under agitation, and finally with 0.5 % SDS solution in 10mMTris for 18–24 h at room temperature under agitation. Rinse the samples in PBS 20–30 times until all bubbles disappear under agitation (15 min each wash).
Subsequently, incubate the bone cylinders in a solution of DNase and RNase in 10 mM Tris for 6 h at room temperature.
Rinse the decellularized bone cylinders two times with PBS, freeze-dry and cut to 4–5 mm thickness. Polish the bone disks to obtain scaffolds of 4 × 4 mm in diameter and thickness (see Note 12).
Weigh and measure each individual scaffold using a caliper to calculate the scaffold density. Select scaffolds in the range of 0.37–0.45 mg/mm3 for bone engineering.
Sterilize scaffolds in 70 % ethanol (vol/vol) overnight at room temperature.
Condition the scaffolds in osteogenic medium overnight in the incubator before cell seeding.
3.5 Cell Seeding of Decellularized Bovine Bone Scaffolds
Expand MPs expressing mesenchymal surface markers and exhibiting osteogenic differentiation potential on gelatin-coated plates in mesenchymal medium (see Note 13).
Detach expanded MPs with trypsin/EDTA, wash with mesenchymal induction medium, determine the total cell number, centrifuge at 250 × g for 5 min, and resuspend the cells to a seeding density of 30 × 106 cells/ml in osteogenic medium (see Note 14).
Place sterilized, conditioned scaffolds onto sterile gauze to blot the culture medium, and quickly transfer each scaffold per well of low attachment 6-well plates (Corning).
Pipet a 40 µl aliquot of the cell suspension (a total of 1.5 × 106 cells) onto each 4 × 4 mm bone scaffold carefully, allowing the cell suspension to penetrate the scaffold pores.
To facilitate uniform cell distribution, flip the scaffolds every 15 min for 1 h, and each time add 5 µl of the osteogenic medium to prevent the cells from drying out.
After 1 h, add 6 ml of osteogenic medium to each well and transfer the seeded scaffolds to the incubator. Incubate without disturbing in standard conditions for 3 days.
3.6 Cultivation of Cell-Seeded Scaffolds in Perfusion Bioreactors
On days 1–2 after cell seeding, prepare the bioreactor chambers. Tighten the assembled, sterilized perfusion bioreactors with sterilized tools working in the aseptic conditions (laminar flow hood) and fit the three-way stopcocks with syringes into the perfusion loop connectors. Fill the chambers with medium and place them in the incubator for overnight conditioning. Attach to the peristaltic pump and start the medium flow.
On day 3 after seeding, harvest some of the seeded constructs for the evaluation of cell viability, cell seeding efficiency, cell distribution, and other analyses at the start of perfusion culture. Transfer some of the constructs in new low attachment 6-well plates for static culture controls, and add to each 6 ml of fresh osteogenic medium (see Note 15).
On day 3 after seeding, transfer the constructs to each of the perfusion bioreactors for culture in osteogenic medium for up to 5 weeks. Check the bioreactors for potential leaks, and tighten loose connections. Transfer the seeded bone constructs into culture chambers (1 construct per culture chamber, 6 constructs per round bioreactor presented in Fig. 1) using sterile forceps. Cover the culture chamber with small amount of DPBS to prevent drying, and check the fluid flow through the constructs by attaching a syringe with fresh osteogenic medium into the perfusion loop and pushing the medium toward the bioreactor chamber with constructs (see Note 16). Fit the constructs into the chambers tightly to allow the flow through all parallel chambers, and remove any bubbles blocking the flow using forceps and syringes.
Aspirate the DPBS and add an appropriate amount of osteogenic medium into the bioreactor medium reservoir (~6 ml per each construct, a total of 40 ml per round bioreactors in Fig. 1).
Cover/close the bioreactor chambers, wrap with parafilm, transfer to the incubators, and attach to the peristaltic pump to start perfusion.
Adjust the medium flow rate according to the selected study parameters. For instance, a uniform flow rate of 3.6 ml/min corresponding to an interstitial velocity of 0.8 mm/s was used in our studies (14, 15). The flow rate was calibrated and set using a digital, low-flow, multichannel Masterflex peristaltic pump (Cole Palmer). Culture medium in the bioreactors is recirculated and maintained in equilibrium with the atmosphere in the incubator (standard conditions).
Monitor the bioreactors daily for any potential leakage or flow obstruction.
Along the experimental period, exchange 50 % of the osteogenic medium volume twice weekly with fresh medium, and collect medium aliquots for biochemical analyses (see Note 17).
Harvest perfused bone constructs and statically-cultured control constructs after 3 and 5 weeks of culture (or other selected time points), weigh, cut, and process the samples depending on the selected biochemical and histological analyses (14, 15).
Acknowledgements
This work was supported by the New York Stem Cell Foundation-Helmsley Investigator Award (to D.M.); the Leona M. and Harry B. Helmsley Charitable Trust; Robin Chemers Neustein; Goldman Sachs Gives, at the recommendation of Alan and Deborah Cohen; New York State Stem Cell Science Shared Facility, Grant C024179; National Institutes of Health Grants DE016525 and EB002520, (to G.V.-N.); and the New York Stem Cell Foundation.
Footnotes
During initial expansion of hPSC stocks for storage in liquid nitrogen, we usually omit the antibiotics from the culture medium.
We usually test one vial of MEFs from each lot to assess the cell survival after thawing, and the ability to support undifferentiated hPSC growth.
Acetone fixative solution is prepared fresh prior to each staining.
Faxt blue RR Salt solution is prepared fresh prior to each staining.
AgNO3 solution can be stored at room temperature protected from light.
Use drills of appropriate strength to avoid tip breakage. We used drills made of Copper 40–50 %, Iron 40–43 %,Chromium1–3 %, and Tin <1 % (purchased from Sampson Diamond Tool).
Differences in derivation efficiency and quality of derived progenitors can be observed when using different hPSC lines. 1 ng/ml of basic fibroblast growth factor can be used to boost MP expansion.
Do not let the samples dry. Samples can be stored in PBS at 4–8 °C for later evaluation.
Use a white background to reflect light. When exposing to light, keep the samples in H2O to prevent drying.
Control cultures typically present with minimal or absent staining.
Make sure no traces of bone marrow are left in the bone matrix before continuing.
Polishing assures the scaffolds fit tightly into the bioreactor chambers, forcing the medium flow through the interior of the scaffolds. Slightly less-perfect scaffolds can be used for static culture controls.
During initial MP expansion, we usually note the cell growth so we can plan accordingly to expand sufficient numbers of cells until the start of bioreactor experiment. Per each culture/control scaffold, 1.5 million cells are needed.
At this high cell density, the cell volume is significant. We usually estimate the volume of cell pellet after centrifugation, and then add an appropriate volume of medium to achieve the correct final seeding density.
When planning a bioreactor experiment, an appropriate number of scaffolds and cells is dedicated to various controls.
Use medium with phenol-red as an indicator of the fluid flow.
Due to evaporation, total volume of the medium in the bioreactor chamber is measured each time by aspirating into a 50 ml pipet. 20 ml of the spent medium is returned into the chamber, and 20 ml of the fresh medium is added.
References
- 1.de Peppo GM, Marolt D. Modulating the biochemical and biophysical culture environment to enhance osteogenic differentiation and maturation of human pluripotent stem cell-derived mesenchymal progenitors. Stem Cell Res Ther. 2013;5:106. doi: 10.1186/scrt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandon N, Marolt D, Cimetta E, Vunjak-Novakovic G. Bioreactor engineering of stem cell environments. Biotechnol Adv. 2013;7:1020–1031. doi: 10.1016/j.biotechadv.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marolt D, Knezevic M, Vunjak-Novakovic G. Bone tissue engineering with human stem cells. Stem Cell Res Ther. 2010;2:10. doi: 10.1186/scrt10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedgesm R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;6:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;5:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;4:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Kuznetsov SA, Cherman N, Robey PG. In vivo bone formation by progeny of human embryonic stem cells. Stem Cells Dev. 2011;2:269–287. doi: 10.1089/scd.2009.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levi B, Hyun JS, Montoro DT, Lo DD, Chan CK, Hu S, Sun N, Lee M, Grova M, Connolly AJ, Wu JC, Gurtner GC, Weissman IL, Wan DC, Longaker MT. In vivo directed differentiation of pluripotent stem cells for skeletal regeneration. Proc Natl Acad Sci U S A. 2012;50:20379–20384. doi: 10.1073/pnas.1218052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fröhlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G. Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther. 2008;4:254–264. doi: 10.2174/157488808786733962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proc Natl Acad Sci U S A. 2010;8:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grayson WL, Bhumiratana S, Cannizzaro C, Chao PH, Lennon DP, Caplan AI, Vunjak-Novakovic G. Effects of initial seeding density and fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008;11:1809–1820. doi: 10.1089/ten.tea.2007.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grayson WL, Marolt D, Bhumiratana S, Fröhlich M, Guo XE, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;5:1159–1170. doi: 10.1002/bit.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcos-Campos I, Marolt D, Petridis P, Bhumiratana S, Schmidt D, Vunjak-Novakovic G. Bone scaffold architecture modulates the development of mineralized bone matrix by human embryonic stem cells. Biomaterials. 2012;33:8329–8342. doi: 10.1016/j.biomaterials.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Peppo GM, Marcos-Campos I, Kahler DJ, Alsalman D, Shang L, Vunjak-Novakovic G, Marolt D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;21:8680–8685. doi: 10.1073/pnas.1301190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marolt D, Campos IM, Bhumiratana S, Koren A, Petridis P, Zhang G, Spitalnik PF, Grayson WL, Vunjak-Novakovic G. Engineering bone tissue from human embryonic stem cells. Proc Natl Acad Sci U S A. 2012;22:8705–8709. doi: 10.1073/pnas.1201830109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Peppo GM, Sjovall P, Lennerås M, Strehl R, Hyllner J, Thomsen P, Karlsson C. Osteogenic potential of human mesenchymal stem cells and human embryonic stem cell-derived mesodermal progenitors: a tissue engineering perspective. Tissue Eng Part A. 2010;11:3413–3426. doi: 10.1089/ten.TEA.2010.0052. [DOI] [PubMed] [Google Scholar]
- 17.de Peppo GM, Svensson S, Lennerås M, Synnergren J, Stenberg J, Strehl R, Hyllner J, Thomsen P, Karlsson C. Human embryonic mesodermal progenitors highly resemble human mesenchymal stem cells and display high potential for tissue engineering applications. Tissue Eng Part A. 2010;7:2161–2182. doi: 10.1089/ten.TEA.2009.0629. [DOI] [PubMed] [Google Scholar]