Abstract
The light/dark (LD) test is a commonly used rodent test of unconditioned anxiety-like behavior that is based on an approach/avoidance conflict between the drive to explore novel areas and an aversion to brightly lit, open spaces. We used the LD test to investigate developmental differences in behavior between adolescent (postnatal day (PN) 28–34) and adult (PN67–74) male rats. We investigated whether LD behavioral measures reflect anxiety-like behavior similarly in each age group using factor analysis and multiple regression. These analyses showed that time in the light compartment, percent distance in the light, rearing, and latency to emerge into the light compartment were measures of anxiety-like behavior in each age group, while total distance traveled and distance in the dark compartment provided indices of locomotor activity. We then used these measures to assess developmental differences in baseline LD behavior and the response to anxiogenic drugs. Adolescent rats emerged into the light compartment more quickly than adults and made fewer pokes into the light compartment. These age differences could reflect greater risk taking and less risk assessment in adolescent rats than adults. Adolescent rats were less sensitive than adults to the anxiogenic effects of the benzodiazepine inverse agonist N-methyl-β-carboline-3-carboxamide (FG-7142) and the α2 adrenergic antagonist yohimbine on anxiety-like behaviors validated by factor analysis, but locomotor variables were similarly affected. These data support the results of the factor analysis and indicate that GABAergic and noradrenergic modulation of LD anxiety-like behavior may be immature during adolescence.
Keywords: Adolescence, Anxiety, Light/dark test, Factor analysis, FG-7142, Yohimbine
1. Introduction
The light/dark (LD) test is based on an approach-avoidance conflict between exploration of novel environments and avoidance of brightly lit, open spaces [1]. The test was developed in mice by Crawley and colleagues, who observed that anxiolytic drugs increased the number of crossings between compartments [2,3]. Later studies showed that time in the light compartment and distance traveled in the light also reflect anxiety-like behavior and expanded the use of the LD test to rats [4–10].
The LD test has been widely used to assess anxiety-like behavior in adult rodents, and a few studies have utilized this test in younger animals. Risk taking behavior peaks during adolescence and contributes to most of the major causes of adolescent injury and mortality [11,12]. Immature function of corticolimbic and neuromodulatory systems mediating avoidance of aversive stimuli is thought to contribute to these changes in adolescent behavior [13,14]. Behavior in unconditioned anxiety tests such as the LD test is thought to reflect impulsivity or risk taking in addition to anxiety, so the LD test may be a useful model to investigate neural systems relevant to adolescent risk taking [15–17].
Anxiety-like behavior in adolescents has been investigated with the LD test and other unconditioned tests of anxiety-like behavior such as the elevated plus maze (EPM) and social interaction test (SI), but no consensus has emerged about whether adolescents exhibit more, less, or similar levels of anxiety-like behavior as adults [16,18–24]. Species, strain, and methodological differences may account for discrepancies between studies. Adolescent male rats (around PN28) have been reported as having either more or less anxiety-like behavior than adults in the LD test, and a study of adolescent male mice found greater anxiety-like behavior than adults [18–21]. Early adolescent male rats (PN28) do not exhibit anxiety-like behavior in the SI test, a behavior that does not mature until after the onset of puberty [22]. The effects of adolescence and puberty on EPM behavior are less clear, but age differences in behavior may also be influenced by pre-test injections and other experimental manipulations, as adults are more sensitive to such effects on EPM behavior [26].
Assessing developmental differences in the response to aversive stimuli or drug treatments in unconditioned tests for anxiety-like behavior may be a useful method for investigating maturation of neural systems relevant to risk taking during adolescence [15–17]. The LD test has been used to assess age differences in the response to stress and anxiogenic drugs in adolescent versus adult male rats [19–21]. Adolescents have been reported as exhibiting greater anxiogenic responses to stress, but are less sensitive to the anxiogenic effects of drugs targeting the endocannabinoid and serotonergic systems [19–21].
An important consideration in interpreting LD behavior in adolescents is whether behavioral measures of anxiety-like behavior in adults are also valid in adolescents. Some studies report that adolescent rodents exhibit greater novelty-seeking behavior, greater locomotion in novel open fields, and greater novel object preference than adults [27–30]. This developmental difference in the response to novelty could affect exploration of the novel, aversive area of the LD box and alter the relationship between locomotor activity and anxiety-like behavior. Factor analysis has proven to be a useful tool in investigating the contribution of behavioral factors such as locomotor activity and anxiety to the behaviors measured in rodent anxiety tests. For example, factor analysis of male and female rats in the EPM revealed sex differences in the contribution of locomotor activity and anxiety-like behavior to overall EPM behavior [31]. The EPM has been validated for use in male and female adolescents by factor analysis, showing that similar behavioral measures reflect anxiety-like behavior or locomotion in each age group [32]. However, no such validation has been performed for the LD test. The EPM and LD are both based upon approach/avoidance conflict but do not produce identical behavior [7,33–35]. It will therefore be important to determine which LD test measures provide the best comparison of anxiety-like behavior in adult and adolescent rats.
The purpose of this study was to determine which LD test behavioral variables provide a valid measure of anxiety-like behavior in both adolescent (PN28–34) and adult (PN67–73) male rats. We used factor analysis to classify behavioral measures as reflecting anxiety-like or locomotor behavior in each age group, then used multiple regression to determine whether the relationship between locomotor and anxiety-like behavior differed between age groups. We then used the measures of anxiety-like and locomotor behavior validated by factor analysis to investigate developmental differences in baseline behavior and the anxiogenic effects of the benzodiazepine inverse agonist N-methyl-β-carboline-3-carboxamide (FG-7142) and the α2 adrenergic receptor antagonist yohimbine.
2. Methods
2.1. Animals
Young adult (PN60–63) and juvenile (PN21) male Sprague-Dawley (CD) rats were purchased from Charles River Laboratories (Raleigh, NC). The rats were housed in ventilated plastic cages (Techniplast USA, Exton, PA) or standard rat cages (Allentown Caging, Allentown, NJ) with corn cob bedding on a 12:12 h light/dark cycle with lights on at 06:00 and lights off at 18:00. All rats were allowed to acclimate to our AALAC accredited facility for at least 7 days before behavior testing. The acclimation period did not exceed 13 days to ensure that adolescent rats were tested during the first week of adolescence (PN28–34). Animals were not handled prior to testing, as adults and adolescents may respond differently to pretest manipulations in tests for anxiety-like behavior [26]. All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
2.2. Drugs
N-methyl-β-carboline-3-carboxamide (FG-7142) and yohimbine hydrochloride were purchased from Tocris Bioscience (Bristol, UK). FG-7142 was dissolved in 40% propylene glycol, 10% ethanol and injected at a volume of 1–2 mL/kg. Yohimbine was dissolved in saline and injected at a volume of 2 mL/kg. All drug solutions were prepared the morning of testing.
2.3. Light/dark test
Rats were tested in Kinder locomotor boxes (Kinder Scientific, Inc. Poway, CA) (40 cm × 40 cm × 40 cm) with black plastic inserts (20 cm × 40 cm × 40 cm) that occupied half of the locomotor box. Both age groups were tested in the same locomotor boxes. The two compartments were connected by a small opening (7.5 cm × 8.5 cm) that was covered by a sliding door. The room was lit by two incandescent lamps so that brightness of the light side of each box averaged 65 lx. Rats were placed in the dark half of the box and testing began as the door to the light side of the box was raised. Time (s) and distance traveled (cm) in each compartment were measured for 15 min using infrared photobeams and software from the manufacturer. We have successfully used Kinder locomotor boxes to measure activity in adolescent and adult rats as well as adult mice, and do not anticipate any confounding effect of the size difference in adult and adolescent rats on measurement of activity [36–38]. The latency to emerge into the light compartment was determined by dividing the session into 5 s bins and determining the bin of first light entry. The following behavioral measures were recorded: time spent in the light compartment, distance traveled in the light and in the dark compartments, total distance traveled, the percent of total distance traveled in the light compartment, the number of entries into the light compartment, the number of pokes into the light compartment, the latency to emerge into the light compartment, and total rearing across both compartments.
Two separate experiments were conducted to determine the effects of FG-7142 (2.5, 7.5, and 10 mg/kg), or yohimbine (1, 2.5, and 5 mg/kg) on LD behavior in each age group. In each experiment, animals were injected with vehicle or drug 30 min prior to testing. Doses and pretreatment times were based on previous studies of drug-induced anxiety in adult rats [9,39,40]. Vehicle-treated animals were used as a control group to determine drug effects on behavior, and drug-treated animals were compared to age-matched vehicle groups. The number of animals used for the FG-7142 experiment was as follows: vehicle, n = 20 per age group, 2.5 mg/kg, n = 8 per age group, 7.5 mg/kg, n = 8 per age group, 10 mg/kg, n = 11–12 per age group. Sample sizes for the yohimbine experiment were: vehicle, n = 15 adolescents, 14 adults, 1 mg/kg, n = 8 per age group, 2.5 mg/kg, n = 8 per age group, 5 mg/kg n = 9 adolescents, 8 adults.
2.4. Factor analysis
Factor analysis was performed on pooled data from vehicle-treated (saline or distilled water) animals from multiple experiments that were run under the conditions described above (n = 88 adults, 93 adolescents) [21]. This data set also included a few animals used in experiments that received no injection (10 out of 88 total adults and 13 out of 93 total adolescents). Behavior of these non-injected animals did not differ from vehicle-injected animals, so they were included in the factor analysis. These animals were from over 20 separate cohorts run on different days over a three year period. Each cohort included adult and adolescent rats that arrived in our animal facilities on the same day and had the same acclimation period prior to testing. This was done to minimize any confounding effects of shipping stress or other environmental variables upon anxiety-like behavior. These animals had no prior experience with the LD test. The analysis was performed using JMP 8.0 (SAS, Cary, NC). The latency to emerge into the light was log transformed prior to analysis due to its non-Gaussian distribution. Data from adult and adolescent rats were analyzed separately to compare factor loading between age groups. The correlation matrices (Supplementary Table 1) for each age group were analyzed by principle components analysis, and factors with eigenvalues greater than 1 were kept for factor rotation. Factor rotation was performed by the Varimax method. Factor loadings >0.5 were reported.
2.5. Multiple regression analysis of LD behavior
Multiple regression was performed using JMP 8.0 to compare the relationship of total distance traveled (cm) to other LD measures in each age group. Each LD behavior was regressed against total distance traveled, with age as a covariate. The interaction between age and total distance traveled was used to compare the regression between age groups. Interactions were considered significant at p < 0.05.
2.6. Comparison of baseline behavior
Animals from the control data set described above (n = 88 adults, 93 adolescents) were used to compare baseline behavior of each age group. These animals were run in over 20 cohorts over a three year period, and had no prior experience with the LD test. Baseline behavior in each age group was compared by two-tailed t-test using Graphpad Prism 5.0 (Graphpad, La Jolla, CA), with the exception of the latency to emerge into the light compartment, which was analyzed by Mann–Whitney test using Graphpad Prism 5.0 due to non-Gaussian distribution of the data.
2.7. Analysis of FG-7142 and yohimbine effects
The effects of FG-7142 and yohimbine were analyzed by ANOVA with age and treatment as factors using NCSS 2004 software (NCSS, Kayesville, UT). Significant age × treatment interactions were followed by Newman–Keuls post hoc testing with significance set at p < 0.05.
3. Results
3.1. Factor analysis of behavior in the light/dark test
Age differences in the underlying behaviors contributing to LD test performance were investigated by factor analysis. The analysis produced two factors in adult rats that accounted for 70.2% of the total variance. Factor 1 (41.7% of the variance) contained anxiety-like behaviors, and factor 2 (28.5% of the variance) included locomotor and exploratory behaviors (Table 1). Analysis of adolescent behavior produced three factors that accounted for 68.7% of the variance. As seen in adults, factor 1 (39.7% of the variance) included anxiety-like behaviors. However, locomotor and exploratory behaviors were split between two factors. Factor 2 (20.6% of the variance) included locomotor behavior, and factor 3 (8.4% of the variance) only contained light pokes, an exploratory or potential risk-assessment behavior.
Table 1.
Results of factor analysis of LD behavior in adult (n = 88) and adolescent (n = 93) rats. The percent of variance accounted for by each factor in principle components analysis and the total variance accounted for in each age group are listed. Factor loadings greater than 0.5 are shown.
| Adults (70.2% variance) | Adolescents (68.7% variance) | ||||
|---|---|---|---|---|---|
| Factor 1 (anxiety-like behavior) (41.7%) |
Factor 2 (locomotion/exploration) (28.5%) |
Factor 1 (anxiety-like behavior) (39.7%) |
Factor 2 (locomotion) (20.6%) |
Factor 3 (exploration) (8.4%) |
|
| Time in light | 0.98 | 0.94 | |||
| % Distance in light | 0.98 | 0.96 | |||
| Latency to emerge | −0.64 | −0.63 | |||
| Light entries | 0.57 | 0.54 | |||
| Light pokes | 0.64 | 0.54 | |||
| Rearing | 0.59 | 0.50 | |||
| Dark distance | −0.57 | 0.80 | 0.86 | ||
| Total distance | 0.88 | 0.52 | 0.83 | ||
The behavioral measures loading onto factor 1 were largely similar in each age group. For both age groups, time spent in the light compartment, the percent distance traveled in the light, the latency to emerge into the light compartment, and rearing loaded onto factor 1. The latency to emerge into the light loaded negatively onto factor 1, which is consistent with the fact that shorter latencies indicate low anxiety-like behavior. The similar loading of these behaviors in each age group validates their use for the study of developmental differences in LD behavior in male rats. In contrast, the number of entries into the light compartment loaded onto factor 1 with anxiety-like behaviors in adolescents, but in adults loaded onto factor 2 with locomotor and exploratory behaviors. Light entries is an analogous measure to the number of crossings or transitions between compartments, which is often used as an anxiety-like measure in the LD test. However, this factor analysis shows that the number of light entries may not be a useful measure to investigate developmental differences in LD behavior in male rats.
Factor 2 included measures of locomotor activity and exploratory behavior in adult rats, but only measures of locomotor activity in adolescents. Total distance traveled and distance in the dark compartment loaded heavily onto factor 2 in both age groups. These are primarily locomotor measures, but they may also involve some elements of anxiety-like behavior, as shown by weaker loadings for dark distance on factor 1 in adults and total distance on factor 1 in adolescents. The number of light entries and pokes into the light compartment loaded onto factor 2 in adults only, indicating that these exploratory behaviors are more related to locomotor activity than anxiety-like behavior. Neither of these variables is strongly related to locomotion in adolescents, so light entries and light pokes may reflect different underlying behaviors in each age group. While light entries was related to anxiety-like behavior in adolescents, light pokes was not strongly correlated with either anxiety-like behavior or locomotion and loaded onto its own separate factor.
3.2. Multiple regression analysis of behavior in the light/dark test
The loading of total distance onto the same factor as anxiety-related measures in adolescents but not adults may suggest a greater association of locomotor activity with anxiety-like behavior in adolescents than adults. We further investigated this possibility by regressing age, total distance, and their interaction onto each of the other LD behavioral measures (Table 2). The regression of total distance was statistically significant with every other behavioral measure. There was a positive relationship between total distance and every behavioral measure except for latency to emerge, which was negatively related to total distance. As shown by a main effect of age, adults performed more light pokes (p < 0.001) and rearing (p < 0.001) than adolescents, and had a longer latency to emerge into the light compartment (p < 0.001).
Table 2.
Results of multiple regression of total distance and age with each LD behavioral measure. The main effects of total distance, age, and the age × total distance interaction are shown.
| Total distance | Age | Age × total distance | |
|---|---|---|---|
| Time in light | r = 0.45, p < 0.001 | n.s. | n.s. |
| % Distance in light | r = 0.47, p < 0.001 | n.s. | n.s. |
| Latency to emerge | r = −0.53, p < 0.001 | p < 0.001 | p < 0.05 |
| Light entries | r = 0.59, p < 0.001 | n.s. | n.s. |
| Light pokes | r = 0.50, p < 0.001 | p < 0.001 | p < 0.01 |
| Rearing | r = 0.61, p < 0.001 | p < 0.01 | n.s. |
| Dark distance | r = 0.53, p < 0.001 | n.s. | n.s. |
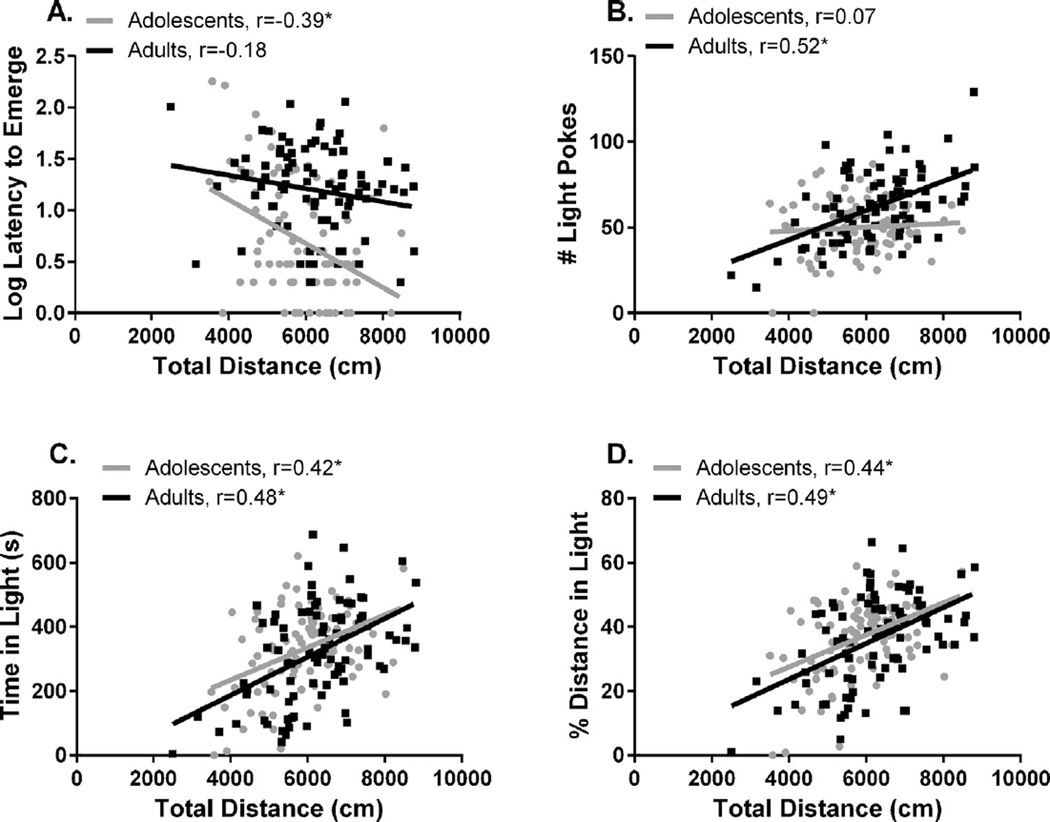
There were age differences in the relationship of total distance with the latency to emerge into the light compartment (p < 0.05) and the number of light pokes (p < 0.01), as shown by an age × total distance interaction. We then performed separate regressions for each age group to further investigate these interactions. Regression of total distance with the latency to emerge (Fig. 1A) revealed that adolescents with a high total distance score emerged into the light more quickly (r = −0.39, p < 0.001), but this relationship was not significant in adults (r = −0.18, n.s.). Adult rats with greater distance traveled made more light pokes (Fig. 1B, r = 0.52, p < 0.001), but the regression was not significant in adolescents (r = 0.07, n.s.).
Fig. 1.
Regression of total distance with (A) latency to emerge into the light compartment, (B) pokes into the light compartment, (C) time in the light compartment, and (D) percent of total distance traveled in the light compartment. The r value is listed for each regression. *Significant regression, p < 0.05.
These multiple regression data show that locomotor behavior is not more strongly associated with most anxiety-like behaviors in adolescents than in adults (Fig. 1C,D). There is a negative relationship between the latency to emerge and total distance in adolescents, but not adults. However, this should not preclude the usefulness of latency to emerge to compare developmental differences in LD behavior, as total distance accounted for only 15% of the variance of latency to emerge in adolescents (r2 = 0.15). This is consistent with the weak loading of total distance on the anxiety-like factor in adolescents in addition to its heavy loading on the locomotor factor. Factor analysis and multiple regression therefore validate the following measures for assessment of anxiety-like behavior in adult and adolescent male rats: time in light compartment, percent distance in light compartment, latency to emerge into the light, and rearing. We then used these measures to assess baseline LD behavior and the effects of the anxiogenic drugs FG-7142 and yohimbine in each age group.
3.3. Comparison of LD behavior
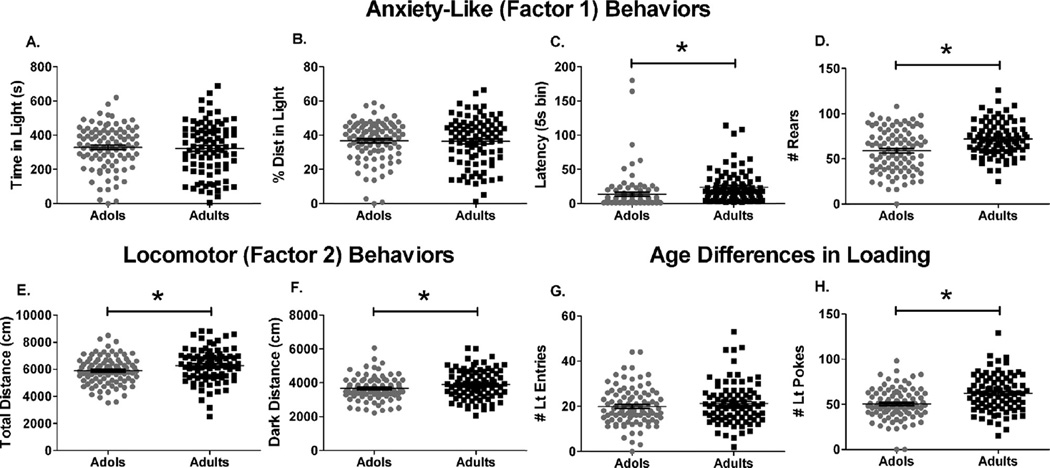
Comparison of LD behavior of control animals compiled over many experiments revealed differences in latency to emerge into the light compartment, rearing, and pokes into the light compartment that may reflect greater risk taking or novelty seeking in adolescent rodents, as well as less risk assessment. Analysis of anxiety-like behaviors that loaded onto factor 1 revealed that adult and adolescent rats exhibited similar time and percent of total distance traveled in the light compartment (Fig. 2A, B), but adolescent rats emerged into the light compartment more quickly than adults (Fig. 2C, p < 0.001). Adolescent rats also performed fewer rears than adults (Fig. 2D, p < 0.001). Adolescents engaged in less locomotor activity than adults as shown by both factor 2 behaviors, distance in the dark compartment (Fig. 2F, p < 0.05) and total distance traveled (Fig. 2E, p < 0.05). This shows that the shorter latency to emerge in adolescents was not caused by greater locomotor activity. For the behaviors with differential loading between age groups, no age differences were detected in the number of light entries (Fig. 2G), but adults made more pokes into the light compartment than adolescents (Fig. 2H, p < 0.001).
Fig. 2.
Behavior of control adults (n = 88) and adolescent (n = 93) animals in the LD test. (A) time spent in the light compartment, (B) percent of total distance traveled in the light compartment, (C) latency to emerge into the light compartment, (D) rearing across both compartments, (E) total distance traveled, (F) distance traveled in the dark compartment, (G) entries into the light compartment, (H) pokes into the light compartment. *Significant age difference, p < 0.05.
3.4. Behavioral effects of FG-7142
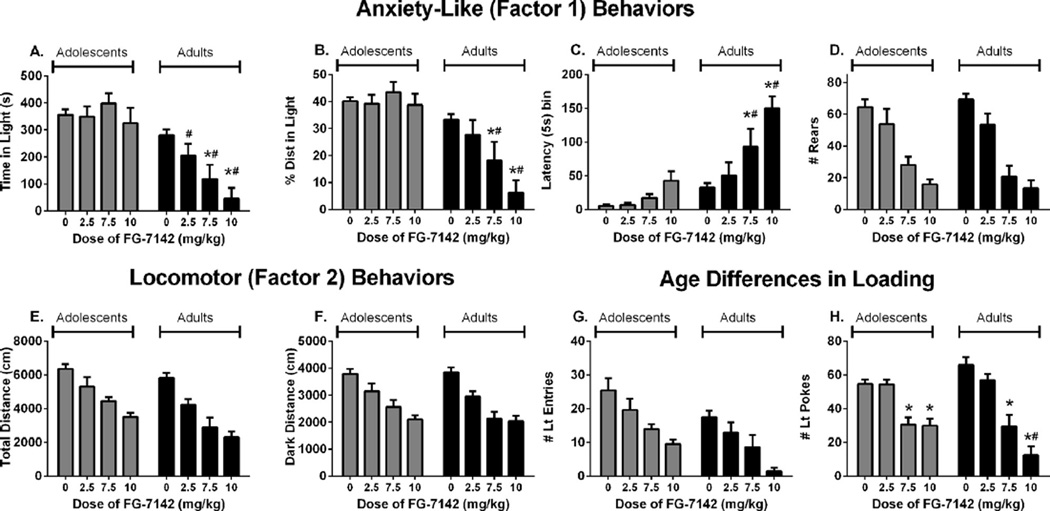
Treatment with FG-7142 produced dose dependent effects on all behaviors measured in the LD test (Fig. 3). As shown by a main effect of dose, FG-7142 reduced time spent in the light compartment (Fig. 3A, F(3,87) = 5.53, p < 0.01), the percent of total distance in the light compartment (Fig. 3B, F(3,87) = 6.80, p < 0.001), rearing (Fig. 3D, F(3,87) = 46.37, p < 0.001), total distance traveled (Fig. 3E, F(3,87) = 36.78, p < 0.001), distance traveled in the dark compartment (Fig. 3F, F(3,87) = 32.86, p < 0.001), light entries (Fig. 3G, F(3,87) = 13.37, p < 0.001), and light pokes (Fig. 3H, F(3,87) = 38.28, p < 0.001). FG-7142 also increased the latency to emerge into the light compartment (Fig. 3C, main effect of dose, F(3,87) = 18.91, p < 0.001).
Fig. 3.
The effects of treatment with FG-7142 on: (A) time spent in the light compartment, (B) percent of total distance traveled in the light compartment, (C) latency to emerge into the light compartment, (D) rearing across both compartments, (E) total distance traveled, (F) distance traveled in the dark compartment, (G) entries into the light compartment, (H) pokes into the light compartment. *Significantly different from age-matched vehicle, p < 0.05. #Significantly different from adolescents treated with same dose, p < 0.05. Vehicle, n = 20 per age group, 2.5 mg/kg, n = 8 per age group, 7.5 mg/kg, n = 8 per age group, 10 mg/kg, n = 12 adolescents, 11 adults.
FG-7142 produced greater effects in adult rats than in adolescents in three of the four anxiety-like behaviors validated by factor analysis and multiple regression (time spent in the light compartment, percent of total distance in light, and latency to emerge into the light). ANOVA revealed that FG-7142 produced greater anxiogenic effects in adults than adolescents by an age × dose interaction for time spent in the light compartment (Fig. 3A, F(3,87) = 4.41, p < 0.01), the percent of total distance in the light compartment (Fig. 3B, F(3,87) = 6.22, p < 0.01), and the latency to emerge into the light compartment (Fig. 3C, F(3,87) = 5.13, p < 0.01). Post hoc testing showed that adult rats, but not adolescents, treated with 7.5 and 10 mg/kg FG-7142 exhibited reduced time and percent distance in the light compartment and increased latency to emerge compared to vehicle treated adults. Additionally, adults treated with 7.5 and 10 mg/kg FG-7142 exhibited less time and percent distance in the light compartment and longer latency to emerge than adolescent animals treated with the same dose. No age differences were detected in the effects of FG-7142 on rearing (Fig. 3D, age × dose F(3,87) = 0.46, p > 0.70).
In contrast to the anxiety-like behaviors, FG-7142 produced similar effects in each age group on the activity-related behaviors of total distance traveled (Fig. 3E, age × dose F(3,87) = 0.84, p > 0.47) and distance in the dark compartment (Fig. 3F, age × dose F(3,87) = 0.45, p > 0.72). No age differences were detected in the reduction of light entries (Fig. 3G, age × dose F(3,87) = 0.08, p > 0.97). Pokes into the light compartment was the only non-anxiety-like behavior in which FG-7142 produced age-dependent effects. ANOVA revealed an age × dose interaction for the number of pokes into the light compartment (Fig. 3H, F(3,87) = 4.15, p < 0.01). Post hoc testing showed that 7.5 and 10 mg/kg FG-7142 reduced the number of light pokes in each age group relative to controls, and that 10 mg/kg FG-7142 produced a greater reduction of light pokes in adults than adolescents.
3.5. Behavioral effects of yohimbine
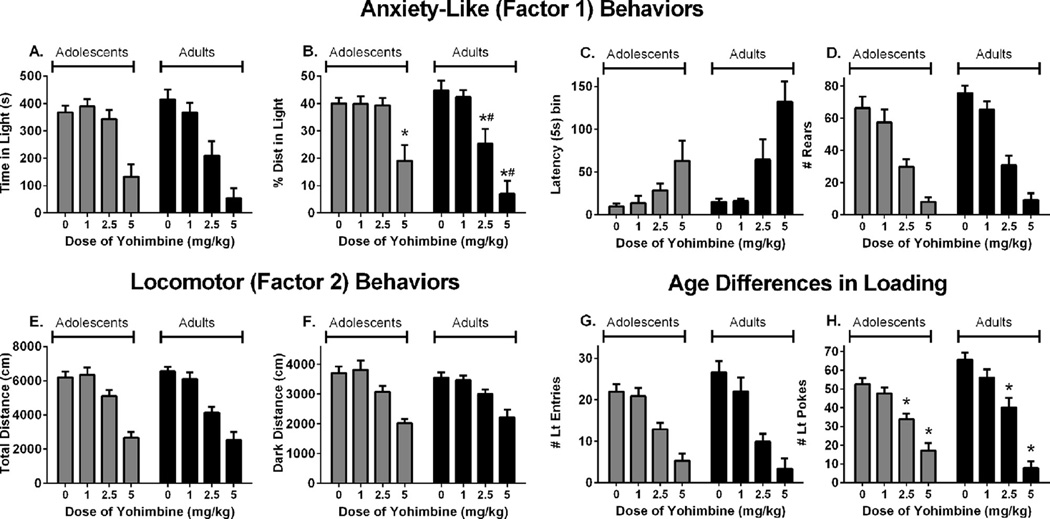
Yohimbine also produced dose-dependent effects in each age group on all behaviors measured in the LD test (Fig. 4). As shown by a main effect of dose, yohimbine reduced time spent in the light compartment (Fig. 4A, F(3,70) = 27.37, p < 0.001), the percent of total distance in the light compartment (Fig. 4B, F(3,70) = 25.45, p < 0.001), rearing (Fig. 4D, F(3,70) = 47.31, p < 0.001), total distance traveled (Fig. 4E, F(3,70) = 48.05, p < 0.001), distance traveled in the dark compartment (Fig. 4F, F(3,70) = 21.30, p < 0.001), light entries (Fig. 4G, F(3,70) = 31.20, p < 0.001), and light pokes (Fig. 4H, F(3,70) = 57.88, p < 0.001), and increased the latency to emerge into the light compartment (Fig. 4C, main effect of dose, F(3,70) = 17.86, p < 0.001).
Fig. 4.
The effects of treatment with yohimbine on: (A) time spent in the light compartment, (B) percent of total distance traveled in the light compartment, (C) latency to emerge into the light compartment, (D) rearing across both compartments, (E) total distance traveled, (F) distance traveled in the dark compartment, (G) entries into the light compartment, (H) pokes into the light compartment. *Significantly different from age-matched vehicle, p < 0.05. #Significantly different from adolescents treated with same dose, p < 0.05. Vehicle, n = 15 adolescents, 14 adults, 1 mg/kg, n = 8 per age group, 2.5 mg/kg, n = 8 per age group, 5 mg/kg n = 9 adolescents, 8 adults.
Adolescent rats were less sensitive than adults to the anxiogenic effects of yohimbine, though the age differences in drug response were not as dramatic as seen with FG-7142. Yohimbine produced greater anxiogenic effects in adult rats than adolescents on the percent of total distance in the light compartment (Fig. 4B, age × dose, F(3,70) = 3.44, p < 0.05). Post hoc testing revealed that 5 mg/kg yohimbine reduced the percent distance in the light compartment in both age groups, but that adult rats had lower percent distance than adolescents after both 2.5 and 5 mg/kg yohimbine. There were also trends for greater effects of yohimbine in adult rats on time in the light compartment (Fig. 4A, age × dose F(3,70) = 2.46, p < 0.07) and the latency to emerge into the light (Fig. 4C, age × dose F(3,70), p < 0.06). In contrast, yohimbine reduced total distance traveled (Fig. 4E, age × dose F(3,70) = 1.22, p > 0.30), distance in the dark compartment (Fig. 4F, age × dose F(3,70) = 0.47, p > 0.70), and light entries (Fig. 4G, age × dose F(3,70) = 1.24, p > 0.30) similarly in each age group. Analysis of pokes into the light compartment revealed an age × dose interaction (Fig. 4H, F(3,70) = 3.19, p < 0.05). Post hoc testing revealed that 2.5 and 5 mg/kg yohimbine reduced light pokes in each age group, but there were no age differences at any single dose.
4. Discussion
This study supports the validity of the LD test for assessing anxiety-like behavior in adult and adolescent male rats. Factor analysis and multiple regression show that for each age group, behavior in the LD test reflects generally similar components of anxiety-like behavior and locomotor/exploratory behavior. Behavioral measures commonly used to assess anxiety-like behavior, such as time in the light compartment, the percent of total distance in light, and the latency to emerge into the light, loaded similarly in factor analysis of each age group. This finding validates the use of these measures for comparison of anxiety-like behavior between adults and adolescents. In agreement with factor analysis results, the anxiogenic drugs FG-7142 and yohimbine produced greater effects on these behavioral measures in adult rats than adolescents, but similarly reduced other behavioral measures in each age group.
There were developmental differences in loading of several locomotor and exploratory measures by factor analysis. Total distance traveled and distance in the dark both loaded heavily onto factor 2 in each age group, suggesting that both measures primarily indicate locomotor activity. Light entries loaded onto factor 2 (locomotor activity) in adults, but loaded onto the anxiety factor (factor 1) in adolescents. The number of light entries is often used as a measure of anxiety-like behavior, but this analysis reveals that it may not be useful for comparing developmental differences in LD behavior in male rats. The number of light pokes also loaded on the locomotor factor in adults, while in adolescents it loaded onto its own separate factor. This was confirmed by multiple regression analysis of total distance and light pokes between age groups.
A potentially important developmental difference in the factor loading of LD behaviors was that total distance traveled loaded onto the anxiety-related factor in adolescents, but not adults. This could suggest a greater relationship of locomotor activity with anxiety-like behavior in adolescents than adults. However, multiple regression showed a similar relationship between locomotor activity and most anxiety-like behaviors in each age group. Latency to emerge had a significant negative regression with total distance traveled in adolescents but not adults, but this does not invalidate latency to emerge for developmental comparisons due to the small percent of latency variance explained by total distance (15%).
The results from factor analysis and multiple regression of LD behavior in each age group are generally consistent with those previously reported for adult animals. Factor analyses of the behavior of rats and mice in the LD test have revealed anxiety-like and locomotor factors that explain most of the variance in behavior [7,10,33,35,41]. Time and distance in the light compartment, the number of transitions between compartments, and latency measures often load onto the anxiety-like factor, while distance in the dark compartment has been reported as loading onto the locomotor factor [7,10,33,35,41]. The primary difference between the current analysis and several in the literature is that light entries loaded onto the locomotor factor in adult rats in the current analysis, while some studies show it as loading onto an anxiety-like factor [2,9,10]. However, other studies have also reported loading of entries or transitions onto a locomotor factor, so this may be affected by species, strain, or methodological differences between studies [4,7,35].
Factor analysis and multiple regression defined time spent in the light compartment, percent distance in light, latency to emerge, and rearing as valid measures of anxiety-like behavior in adolescent and adult male rats. Additionally, total distance traveled and distance in the dark provide indices of locomotor activity in both groups. Light entries appears to reflect different types of behavior in each age group, while multiple regression suggests that greater influence of locomotion on light pokes may underlie its differential loading between age groups. These analyses provide criteria by which to evaluate LD behavior and drug effects in adolescent and adult rats.
We then compared LD behavior in our large pool of control animals, and used the factor analysis to help interpret the results. There were significant age differences in two of the four anxiety-like behaviors. While both age groups had comparable time and percentage of distance in the light compartment, adolescent rats emerged into the light for the first time more quickly than adults. This may indicate greater approach behavior in adolescent rats than adults, with similar avoidance behavior between age groups. The LD test is based on an approach/avoidance conflict between exploration of and escape from the novel, aversive light compartment [1,2,9,42]. The time and percent distance traveled in the light are determined by the combination of approach and active avoidance behavior, as animals are able to freely enter and escape the compartment during the test. However, the latency to emerge into the light may be a more pure measure of approach to a novel, potentially aversive area. Adolescent rats also engaged in less rearing and made fewer pokes into the light compartment, which suggests less risk assessment in adolescents than in adults. Rearing is an investigatory behavior that correlates with other measures of anxiety-like behavior, and pokes into the light compartment may be a measure of risk assessment similar to the stretched attend posture in the EPM [3,41,43,44].
Taken together, these behavioral differences between adolescents and adults suggest a combination of greater approach behavior and lower risk assessment in a novel, aversive environment that is consistent with other models of adolescence [13,45]. It is unlikely that these behavioral differences were caused by greater arousal or locomotion in adolescents, as adolescent rats had lower distance traveled in the dark compartment and lower total distance traveled than adults. Instead, these behavioral age differences could be due to differences in anxiety, impulsivity, and/or the response to novelty. Anxiety-like behavior in unconditioned tests such as the LD is thought to involve increased behavioral inhibition, and has been suggested to reflect risk taking behavior or impulsivity [15–17,46,47]. It is therefore possible that the developmental differences in behavior we observed reflect age differences in behavioral inhibition in a novel, aversive environment. The quick emergence into the light compartment observed in adolescents may also be influenced by the novelty-seeking that peaks during this age period [27–30]. The majority of animals used for this comparison were vehicle-treated controls from multiple experiments, so injection stress could theoretically have influenced these data. However, adolescent rats were previously shown to have a greater response than adults to restraint stress, which would have predicted the opposite response [19]. This previous finding suggests that age differences in the latency to emerge are not caused by injection stress.
We tested the effects of the classic anxiogenic drugs FG-7142 and yohimbine to further investigate age differences in modulation of LD behavior. In addition to probing the maturity of neural systems that modulate LD behavior, these experiments provided a test of the results of the factor analysis. Adolescent rats were less sensitive to the anxiogenic effects of FG-7142 on three of the four behaviors validated by factor analysis, while locomotor measures were similarly suppressed in each age group. This supports the results of the factor analysis, and may reflect immature modulation of anxiety-like behavior by benzodiazepine-sensitive GABA receptors in adolescents. The similar suppression of locomotion in adolescents and adults is consistent with a previous report of similar reduction of locomotion in PN24 and adult rats after 15 mg/kg FG-7142 [48]. The expression of benzodiazepine-sensitive GABA receptor subtypes is mature by early adolescence [49–52]. However, exposure to the social interaction test for anxiety differentially affects benzodiazepine receptor function in adult and adolescent rats, suggesting developmental differences in GABAergic modulation of anxiety-like behavior [53].
The serotonergic system may also be involved in the lower adolescent sensitivity to FG-7142. FG-7142 injected into the dorsal raphe nucleus produces anxiogenic effects [54]. Systemic administration of FG-7142 increases serotonin and 5-HIAA levels in the prefrontal cortex, and the anxiogenic effects of FG-7142 can be prevented with a 5-HT2C receptor antagonist [55,56]. Activation of the serotonergic system by FG-7142 might induce a greater anxiogenic effect in adult rats, as adolescents are less sensitive to the anxiogenic effects of the indirect serotonin agonist fenfluramine [21].
Adolescents were also less sensitive than adults to the anxiogenic effects of yohimbine, though this age difference was less dramatic than with FG-7142. As with FG-7142, adolescents exhibited a different response than adults only in anxiety-like behaviors. Adolescents had a lower anxiogenic response to yohimbine than adults in the percent of total distance traveled in the light compartment, with strong trends for a lower response in time spent in the light compartment and the latency to emerge into the light. In contrast, locomotor measures were similarly suppressed in each age group, as were measures with age differences in factor loading. Lower adolescent sensitivity to yohimbine is consistent with reports of the immaturity of the noradrenergic system during early and mid-adolescence in rats [57]. Yohimbine’s anxiogenic effects could be due to antagonism of both pre- and postsynaptic α2 receptors. Yohimbine antagonism of noradrenergic α2 autoreceptors increases norepinephrine release and could thus increase anxiety-like behavior [58]. The presynaptic effects of yohimbine are critical for yohimbine’s enhancement of acoustic startle [59]. However, postsynaptic α2 receptors also appear to modulate anxiety-like behavior, as the α2 agonist clonidine exerts anxiolytic effects that are unaffected by lesioning noradrenergic terminals [60]. Norepinephrine tissue content and innervation density are lower in adolescent rats than in adults, though forebrain α2 receptor binding appears to be similar to adults [57,61]. The lower adolescent sensitivity to yohimbine may therefore be driven by immaturity of presynaptic noradrenergic function.
This study reveals developmental differences in baseline LD behavior and the response to anxiogenic drugs that may have implications for the neurobiology underlying adolescent risk taking. Adolescent rats exhibited more approach behavior and less risk assessment in a novel, aversive environment and were less sensitive to the anxiogenic effects of FG-7142 and yohimbine. In addition to the reduced anxiogenic effects of multiple drugs in adolescent rats in the LD test, adolescents are less sensitive than adults to the aversive effects of many drugs in the conditioned taste aversion paradigm [20,21,62–68,69,70]. Adolescent rats also have a deficit in expression of conditioned fear despite being able to retain the fear memory and express a normal freezing reaction in adulthood [71]. These data are consistent with the hypothesis that a combination of hyperactive approach and hypoactive avoidance contribute to the peak in risk taking and novelty seeking behavior during adolescence [13,14,72,73].
HIGHLIGHTS.
Adolescents exhibited less risk assessment and more risk taking in the light/dark test.
Factor analysis revealed similar measures of anxiety-like behavior in each age group.
Adolescent rats were less sensitive to the anxiogenic effects of FG-7142 and yohimbine.
Adolescent GABAergic and noradrenergic modulation of LD behavior may be immature.
Acknowledgements
This work was supported by National Institute on Drug Abuse grants DA019114 and 1F31DA032532. The authors thank Dr. Joseph Lucas and the Duke Biostatistics Core Facility for help with factor analysis and multiple regression.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbr.2013.05.035.
References
- 1.Crawley JN. Exploratory behavior models of anxiety in mice. Neuroscience & Biobehavioral Reviews. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- 2.Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology Biochemistry and Behavior. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 3.Crawley JN. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacology Biochemistry and Behavior. 1981;15:695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 4.Bourin M, Hascoet M. The mouse light/dark box test. European Journal of Pharmacology. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 5.Costall B, Jones BJ, Kelly ME, Naylor RJ, Tomkins DM. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacology Biochemistry and Behavior. 1989;32:777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- 6.Young R, Johnson DN. A fully automated light/dark apparatus useful for comparing anxiolytic agents. Pharmacology Biochemistry and Behavior. 1991;40:739–743. doi: 10.1016/0091-3057(91)90078-g. [DOI] [PubMed] [Google Scholar]
- 7.Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes, Brain and Behavior. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- 8.Bill DJ, Fletcher A, Glenn BD, Knight M. Behavioural studies on WAY100289, a novel 5-HT3 receptor antagonist, in two animal models of anxiety. European Journal of Pharmacology. 1992;218:327–334. doi: 10.1016/0014-2999(92)90186-8. [DOI] [PubMed] [Google Scholar]
- 9.Merlo Pich E, Samanin R. A two-compartment exploratory model to study anxiolytic/anxiogenic effects of drugs in the rat. Pharmacological Research. 1989;21:595–602. doi: 10.1016/1043-6618(89)90201-6. [DOI] [PubMed] [Google Scholar]
- 10.Chaouloff F, Durand M, Mormede P. Anxiety- and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behavioural Brain Research. 1997;85:27–35. doi: 10.1016/s0166-4328(96)00160-x. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg L. A social neuroscience perspective on adolescent risk-taking. The Development Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, et al. Youth risk behavior surveillance - United States, 2011. MMWR - MMWR Publications - Surveillance Summaries. 2012;61:1–162. [PubMed] [Google Scholar]
- 13.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasa RA, Pine DS, Thorn JM, Nelson TE, Spinelli S, Nelson E, et al. Enhanced right amygdala activity in adolescents during encoding of positively valenced pictures. Developmental Cognitive Neuroscience. 2011;1:88–99. doi: 10.1016/j.dcn.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harro J. Long-term partial 5-HT depletion: interference of anxiety and impulsivity? Psychopharmacology. 2002;164:433–434. doi: 10.1007/s00213-002-1265-1. [DOI] [PubMed] [Google Scholar]
- 16.Macri S, Adriani A, Chiarotti F, Laviola G. Risk taking during exploration of a plus-maze is greater in adolescent than in juvenile or adult mice. Animal Behavior. 2002;64:541–546. [Google Scholar]
- 17.Olausson P, Engel JA, Soderpalm B. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology (Berl) 1999;142:111–119. doi: 10.1007/s002130050869. [DOI] [PubMed] [Google Scholar]
- 18.Hascoet M, Colombel MC, Bourin M. Influence of age on behavioural response in the light/dark paradigm. Physiology & Behavior. 1999;66:567–570. doi: 10.1016/s0031-9384(98)00333-3. [DOI] [PubMed] [Google Scholar]
- 19.Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult Sprague-Dawley rats. Behavioral Neuroscience. 2005;119:1477–1483. doi: 10.1037/0735-7044.119.6.1477. [DOI] [PubMed] [Google Scholar]
- 20.Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191:867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- 21.Arrant AE, Jemal H, Kuhn CM. Adolescent male rats are less sensitive than adults to the anxiogenic and serotonin-releasing effects of fenfluramine. Neuropharmacology. 2013;65:213–222. doi: 10.1016/j.neuropharm.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Developmental Psychobiology. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- 23.Andrade MM, Tome MF, Santiago ES, Lucia-Santos A, de Andrade TG. Longitudinal study of daily variation of rats’ behavior in the elevated plus-maze. Physiology & Behavior. 2003;78:125–133. doi: 10.1016/s0031-9384(02)00941-1. [DOI] [PubMed] [Google Scholar]
- 24.Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behavioural Brain Research. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 26.Doremus TL, Varlinskaya EI, Spear LP. Age-related differences in elevated plus maze behavior between adolescent and adult rats. Annals of the New York Academy of Sciences. 2004;1021:427–430. doi: 10.1196/annals.1308.057. [DOI] [PubMed] [Google Scholar]
- 27.Adriani W, Chiarotti F, Laviola G. Elevated novelty seeking and peculiar d-amphetamine sensitization in periadolescent mice compared with adult mice. Behavioral Neuroscience. 1998;112:1152–1166. doi: 10.1037//0735-7044.112.5.1152. [DOI] [PubMed] [Google Scholar]
- 28.Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiology & Behavior. 2003;80:317–325. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Developmental Psychobiology. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- 30.Schramm-Sapyta NL, Cauley MC, Stangl DK, Glowacz S, Stepp KA, Levin ED, et al. Role of individual and developmental differences in voluntary cocaine intake in rats. Psychopharmacology (Berl) 2011;215:493–504. doi: 10.1007/s00213-011-2216-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacology Biochemistry and Behavior. 1999;64:731–738. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 32.Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2006;83:570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Ramos A, Berton O, Mormede P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behavioural Brain Research. 1997;85:57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 34.Ramos A. Animal models of anxiety: do I need multiple tests. Trends in Pharmacological Sciences. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Fraser LM, Brown RE, Hussin A, Fontana M, Whittaker A, O‘Leary TP, et al. Measuring anxiety- and locomotion-related behaviours in mice: a new way of using old tests. Psychopharmacology (Berl) 2010;211:99–112. doi: 10.1007/s00213-010-1873-0. [DOI] [PubMed] [Google Scholar]
- 36.Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacology (Berl) 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- 37.Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacology Biochemistry and Behavior. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Swearingen AE, Walker QD, Kuhn CM. Sex differences in novelty- and psychostimulant-induced behaviors of C57BL/6 mice. Psychopharmacology (Berl) 2013;225:707–718. doi: 10.1007/s00213-012-2860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.File SE, Baldwin HA. Effects of beta-carbolines in animal models of anxiety. Brain Research Bulletin. 1987;19:293–299. doi: 10.1016/0361-9230(87)90097-9. [DOI] [PubMed] [Google Scholar]
- 40.Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacology Biochemistry and Behavior. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 41.Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiology & Behavior. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 42.File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. Current Protocols in Neuroscience. 2004;8(8):3. doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- 43.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacology Biochemistry and Behavior. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 44.Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacology Biochemistry and Behavior. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- 45.Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray JA, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-hippocampal System. 2nd ed. Oxford/New York: Oxford University Press; 2000. [Google Scholar]
- 47.Handley SL, McBlane JW, Critchley MA, Njung’e K. Multiple serotonin mechanisms in animal models of anxiety: environmental, emotional and cognitive factors. Behavioural Brain Research. 1993;58:203–210. doi: 10.1016/0166-4328(93)90104-x. [DOI] [PubMed] [Google Scholar]
- 48.Jaskiw GE, Lipska BK, Weinberger DR. The anxiogenic beta-carboline FG-7142 inhibits locomotor exploration similarly in postweanling and adult rats. Neuroscience Letters. 2003;346:5–8. doi: 10.1016/s0304-3940(03)00384-7. [DOI] [PubMed] [Google Scholar]
- 49.Candy JM, Martin IL. The postnatal development of the benzodiazepine receptor in the cerebral cortex and cerebellum of the rat. Journal of Neurochemistry. 1979;32:655–658. doi: 10.1111/j.1471-4159.1979.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 50.Palacios JM, Niehoff DL, Kuhar MJ. Ontogeny of GABA and benzodiazepine receptors: effects of Triton X-100, bromide and muscimol. Brain Research. 1979;179:390–395. doi: 10.1016/0006-8993(79)90456-6. [DOI] [PubMed] [Google Scholar]
- 51.Lippa AS, Beer B, Sano MC, Vogel RA, Meyerson LR. Differential ontogeny of type 1 and type 2 benzodiazepine receptors. Life Sciences. 1981;28:2343–2347. doi: 10.1016/0024-3205(81)90498-7. [DOI] [PubMed] [Google Scholar]
- 52.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. The Journal of Neuroscience. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Primus RJ, Kellogg CK. Gonadal status and pubertal age influence the responsiveness of the benzodiazepine/GABA receptor complex to environmental challenge in male rats. Brain Research. 1991;561:299–306. doi: 10.1016/0006-8993(91)91608-4. [DOI] [PubMed] [Google Scholar]
- 54.Graeff FG, Viana MB, Mora PO. Opposed regulation by dorsal raphe nucleus 5-HT pathways of two types of fear in the elevated T-maze. Pharmacology Biochemistry and Behavior. 1996;53:171–177. doi: 10.1016/0091-3057(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 55.Evans AK, Abrams JK, Bouwknecht JA, Knight DM, Shekhar A, Lowry CA. The anxiogenic drug FG-7142 increases serotonin metabolism in the rat medial prefrontal cortex. Pharmacology Biochemistry and Behavior. 2006;84:266–274. doi: 10.1016/j.pbb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, et al. 5-Hydroxytryptamine2 C receptor contribution to m-chlorophenylpiperazine and N-methyl-beta-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. Journal of Pharmacology and Experimental Therapeutics. 2007;320:1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- 57.Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochemical Pharmacology. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- 59.Kehne JH, Davis M. Central noradrenergic involvement in yohimbine excitation of acoustic startle: effects of DSP4 and 6-OHDA. Brain Research. 1985;330:31–41. doi: 10.1016/0006-8993(85)90005-8. [DOI] [PubMed] [Google Scholar]
- 60.Fontana DJ, Commissaris RL. Anxiolytic-like effects of alpha-2-adrenoceptor agonists on conflict behavior in the rat: pre- versus postsynaptic receptor mechanisms. Pharmacology Biochemistry and Behavior. 1992;43:697–704. doi: 10.1016/0091-3057(92)90398-y. [DOI] [PubMed] [Google Scholar]
- 61.Happe HK, Coulter CL, Gerety ME, Sanders JD, O‘Rourke M, Bylund DB, et al. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacology Biochemistry and Behavior. 1979;11:31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- 63.Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacology Biochemistry and Behavior. 2006;84:344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186:201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- 65.Wilmouth CE, Spear LP. Adolescent and adult rats’ aversion to flavors previously paired with nicotine. Annals of the New York Academy of Sciences. 2004;1021:462–464. doi: 10.1196/annals.1308.065. [DOI] [PubMed] [Google Scholar]
- 66.Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcoholism: Clinical and Experimental Research. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- 67.Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcoholism: Clinical and Experimental Research. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, et al. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcoholism: Clinical and Experimental Research. 2010;34:2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6 J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcoholism: Clinical and Experimental Research. 2011;35:1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doremus TL, Brunell SC, Varlinskaya EI, Spear LP. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacology Biochemistry and Behavior. 2003;75:411–418. doi: 10.1016/s0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 71.Pattwell SS, Bath KG, Casey BJ, Ninan I, Lee FS. Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1182–1187. doi: 10.1073/pnas.1012975108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rewards Spear LP. aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]