Abstract
Background
Exercise intolerance is a hallmark of heart failure (HF), but factors associated with impaired exercise capacity in HF with preserved EF (HFpEF) are unclear. We hypothesized that in HFpEF, the severity of resting ventricular and vascular dysfunction are associated with impairment in exercise tolerance as assessed by peak oxygen consumption (pVO2).
Methods and Results
Subjects with HFpEF enrolled in the PhosphodiesteRasE-5 Inhibition to Improve CLinical Status And EXercise Capacity in Diastolic Heart Failure (RELAX) clinical trial (n=216) underwent baseline Doppler echocardiography, cardiopulmonary exercise testing and cardiac magnetic resonance imaging. RELAX participants were elderly (median age 69 years) and 48% were women. EF (60%) and stroke volume (77 ml) were normal, while diastolic dysfunction (medial E/e′ 16, deceleration time 185 msec, left atrial volume 44 ml/m2) and increased arterial load (arterial elastance (Ea) 1.51 mmHg/ml) were evident. PVO2 was reduced (11.7 ml/kg/min, 1141 ml/min) and age, sex, body mass index (BMI), hemoglobin and chronotropic response collectively explained 64% of the variance in raw pVO2 (ml/min). After adjustment for these variables, LV structure (diastolic dimension (1.5%, p=0.008) and LV mass (1.6%, p=0.008)), resting stroke volume (2.0%, p=0.002), LV diastolic dysfunction (deceleration time (0.9%, p=0.03) and E/e′ (1.4%, p=0.009), and arterial function (Ea (2.1%, p=0.002) and systemic arterial compliance (1.5%, p=0.007)), each explained only a small additional portion of the variance in pVO2.
Conclusions
In HFpEF, potentially modifiable factors (obesity, anemia and chronotropic incompetence) are strongly associated with exercise capacity whereas resting measures of ventricular and vascular structure and function are not.
Clinical Trial Registration
;URL: http://www.clinicaltrials.gov. Unique identifier: NCT00763867.
Keywords: heart failure with preserved ejection fraction, diastole, exercise, cardiopulmonary exercise, oxygen consumption, arterial stiffness, aortic distensibility
Exercise intolerance is a hallmark of chronic heart failure (HF)1 and is correlated with reduced quality of life and poorer outcomes.2, 3 Exercise capacity is influenced by left ventricular (LV) filling and contractile function, vascular function, chronotropic function, oxygen carrying capacity (hemoglobin) and peripheral muscle mass and function.4-12. Impairments in resting LV and vascular function are common in patients with HF and preserved ejection fraction (HFpEF). However, it is unclear whether abnormalities in resting ventricular or vascular function are tightly correlated with impairment in the capacity to enhance ventricular and vascular function during exercise. Indeed, exercise capacity varies widely in individuals with HF and reduced EF (HFrEF) who have marked abnormalities in resting LV and vascular function.
Small, single center studies have established the presence of reduced exercise capacity in HFpEF and evaluated the association of select variables with impaired exercise capacity in HFpEF.7-9, 12 The Phosphodiesterase-5 (PDE-5) Inhibition to Improve Clinical Status and Exercise Capacity in HFpEF (RELAX) trial evaluated the effect of therapy with the PDE-5 inhibitor sildenafil on clinical status and peak oxygen consumption (pVO2) in HFpEF.13 The multi-center design, rigorous entry criteria and comprehensive phenotypic characterization of the RELAX cohort afford a unique opportunity to enhance our understanding of the pathophysiology of HFpEF by evaluating factors associated with exercise capacity in HFpEF. We hypothesized that measures of resting LV diastolic function, myocardial contractility and vascular function are associated with pVO2 in HFpEF independently of age, sex, body size, hemoglobin and chronotropic function.
Methods
The RELAX trial was a multi-center, randomized clinical trial conducted within the National Heart Lung and Blood Institute (NHLBI) sponsored HF clinical research network (HFN). The institutional review boards of the participating HFN clinical centers approved the RELAX study and all the subjects provided informed consent prior to participation in the study. The rationale and study design and the primary results of the RELAX trial have been previously published.13, 14 All participants underwent a baseline cardiopulmonary exercise test (CPXT), a six minute walk test and a 2-D and Doppler transthoracic echocardiogram. Cardiac magnetic resonance imaging (CMR) without administration of contrast was performed in those without claustrophobia, implantable cardiac device or body size limitation (body circumference too large to fit in CMR chamber). Those in atrial fibrillation did not undergo CMR due to technical challenges with ECG gating in atrial fibrillation.
The current study evaluated the baseline data obtained prior to randomization. This ancillary study was designed and approved by the HFN ancillary studies committee prior to study completion. All analyses were completed by the HFN data coordinating center.
Study subjects
The RELAX trial enrolled 216 ambulatory subjects with HFpEF. Entry criteria specified NYHA class II-IV HF symptoms, LVEF≥ 50% and objective evidence of HF (HF hospitalization or invasively documented elevation in LV filling pressures at rest or left atrial enlargement in the setting of chronic diuretic therapy for HF). Further, at study entry, patients were required to have pVO2 ≤ 60% of the age/sex predicted normal value15 and either an elevated (≥ 400 pg/ml) N terminal pro-brain natriuretic peptide (NT-proBNP) or elevated (≥ 200 pg/ml) BNP plasma level or previously documented elevated LV filling pressures (at rest or with exercise) at the time NT-proBNP or BNP was not elevated.14
Doppler echocardiography
Brachial blood pressure (BP) and heart rate (HR) were measured while the echocardiogram was being recorded. LV cavity dimension and wall thicknesses were measured from 2-D images and used to calculate EF. Reported EF preferentially used biplane Simpson's method, modified Quinones formula, single plane volumetric or visual estimate. Endocardial fractional shortening (eFS), mid wall fiber shortening (mFS) and end systolic wall stress (cESS) were measured as previously described. Contractility was assessed by indexing eFS (stress corrected; sc-eFS) or mFS (sc-mFS) to (log transformed) to cESS.16 Stroke volume (SV) was calculated from the time velocity integral of the pulsed wave Doppler signal of LV outflow tract (LVOT) flow and LVOT area. Pulmonary artery systolic pressure (PASP) was calculated from the peak tricuspid regurgitant velocity and the estimated right atrial pressure using the simplified Bernoulli equation. The early diastolic medial mitral annular tissue velocity (e') was used as a measure of LV relaxation. The early mitral inflow deceleration time (DT) is a frequently reported diastolic function measure which reflects the combined effects of relaxation, LV stiffness and filling pressures. The ratio of the early transmitral flow velocity (E) to e' (E/e′) were used as a measure of LV filling pressure.
A modification of a previously established ordinal diastolic function grading system was also utilized.17 The HFN core echocardiography laboratory (Mayo Clinic, Rochester, MN) completed all measurements according to the American Society of Echocardiography recommendations.
Pulse pressure (PP) and mean arterial pressure (MAP) were calculated using standard formulae. End-systolic pressure (ESP) was estimated as 0.9*systolic blood pressure (SBP).18 Effective arterial elastance (Ea; ESP/SV), total systemic arterial compliance (SAC; SV/PP) and systemic vascular resistance (SVR; (MAP/[cardiac output])*80) were derived as previously described.18
Cardiac magnetic resonance (CMR)
Brachial BP and HR were measured during the CMR. Aortic distensibility was measured using aortic maximal (CSAmax) and minimal cross sectional area (CSAmin) as (aortic CSAmax – aortic CSAmin)/(aortic CSAmin × (PP); (10-3 mmHg).4 The HFN core CMR laboratory (Duke University, Durham, NC) performed all CMR measurements.
Cardiopulmonary exercise test and six minute walk test
A symptom limited cardiopulmonary exercise test was performed on a cycle or treadmill using specifically designed CPXT protocols and analyzed by the HFN core CPXT laboratory (Massachusetts General Hospital, Boston, MA) using established methodologies as previously described.14 Briefly, this custom CPXT protocol incorporated an initial low work rate with linear increase in order to yield a linear oxygen uptake during exercise. The protocol consisted of a 5-min rest period with collection of gas exchange data, followed by a 3-min period of low-then a 10W/min symptom-limited incremental ramp. A cycle ergometer and two treadmill protocols (< or ≥80kg, to account for the influence of weight on treadmill work rate) were designed to match the workload at a given exercise time.14 Ventilatory anaerobic threshold was determined by the modified V-slope method.19 All personnel who performed testing were blinded to treatment allocation of the study subjects.
While published normative values for pVO2 were used to document exercise limitation for study entry,15 age, sex, body size and modality predicted pVO2 was also calculated using the Wasserman equation and the % predicted VO2 achieved was calculated.20 The Wasserman equation estimates predicated pVO2 based on age, sex, body size, exercise modality and accounts for the training effect of obesity on pVO2 in healthy persons allowing a 6 ml/min increase in predicted pVO2 for each kg of weight over ideal body weight.
The age, sex, height and weight predicted six minute walk distance (6MWD) and % predicted 6MWD were calculated.21
Age-predicted maximal HR was defined by the formula (220-age).22 Chronotropic index defined as the predicted heart rate reserve achieved during exercise23 and was calculated as ([peak HR - rest HR]/[age predicted maximal HR - rest HR]). Chronotropic incompetence was deemed present if the chronotropic index was <0.8 for patients not on beta blockers and <0.62 for those taking beta blockers.
Statistical analysis
Data are presented as median (25th, 75th percentile) or % frequency as appropriate. Non-parametric rank tests and chi square test for independence (or Fisher's exact test) were used for group comparison of continuous and categorical variables, respectively. Pearson's correlation was used for bivariate analyses. For multivariable analyses, linear regression models were constructed. To display the association of resting ventricular or vascular function parameters with pVO2 after adjustment for age, sex, body size, hemoglobin and chronotropic index, the increment in the model R2 (partial R2) and p value for each parameter were calculated.
All of the analyses were 2-tailed, and a p<0.05 was considered statistically significant. Analysis was completed by the HFN data-coordinating center (Duke Clinical Research Institute, Durham, NC) using SAS statistical software (SAS Institute Inc., Cary, NC).
Results
Clinical characteristics of the RELAX participants
Baseline characteristics of the RELAX participants (n=216) have been published previously.13 Briefly, the median age of the participants was 69 years and 48% were women. Patients had NYHA class II (47%) or III (53%) HF symptoms. Most patients were obese (median BMI was 33 kg/m2). Other comorbidities including hypertension (85%), history of atrial fibrillation (51%), diabetes mellitus (43%), coronary artery disease (39%) and chronic obstructive pulmonary disease (COPD; 19%) were common.
Baseline clinical characteristics and exercise capacity
Adequate baseline CPXT data were available on 215 of the 216 patients enrolled in RELAX. Median pVO2 was 11.70 ml/kg/min which represented 41% of the age and sex predicted normal value15 and 67% of pVO2 predicted from the Wasserman equation,20 whereas median six minute walk distance (6MWD) was 307 meters (67% of predicted).21
Baseline characteristics of subjects were assessed according to tertiles of pVO2 (Table 1) which resulted in categories of pVO2 ≥ 13.50 (upper), 10.84 to 13.49 (middle) and ≤ 10.85 ml/kg/min (lower). The median pVO2 in the three tertiles were 15.90, 11.70 and 9.40 ml/kg/min, respectively.
Table 1. Baseline subject characteristics by severity of limitation in peak exercise capacity.
| Upper pVO2 tertile 15.9 (14.4, 16.9) | Middle pVO2 tertile 11.7 (11.2, 12.4) | Lower pVO2 tertile 9.4 (8.3, 10.2) | P-Value | |
|---|---|---|---|---|
| N | 73 | 71 | 71 | |
| Age, years | 66 (59, 73) | 69 (63, 79) | 70 (65, 78) | 0.006 |
| Men, n (%) | 53 (73%) | 32 (45%) | 26 (37%) | <0.001 |
| BMI, kg/m2 | 32 (28, 37) | 32 (27, 38) | 35 (31, 41) | 0.004 |
| BSA, m2 | 2.2 (2.0, 2.3) | 2.1 (1.9, 2.3) | 2.1 (1.9, 2.3) | 0.23 |
| Comorbidities, n (%) | ||||
| Ischemic heart disease | 31 (43%) | 26 (37%) | 26 (37%) | 0.71 |
| Hypertension | 61 (84%) | 58 (82%) | 63 (89%) | 0.47 |
| Atrial fibrillation (history) | 29 (40%) | 43 (61%) | 39 (41%) | 0.03 |
| Atrial fibrillation (entry ECG) | 20 (27%) | 30 (42%) | 29 (59%) | 0.12 |
| Pacemaker or ICD | 7 (10%) | 13 (18%) | 15 (21%) | 0.13 |
| COPD | 13 (18%) | 11 (16%) | 18 (25%) | 0.31 |
| Diabetes mellitus | 24 (33%) | 28 (39%) | 40 (56%) | 0.01 |
| Medications at enrolment, n (%) | ||||
| Beta blocker | 56 (77%) | 54 (76%) | 54 (76%) | 0.99 |
| Digoxin | 6 (8%) | 8 (11%) | 8 (11%) | 0.78 |
| Calcium blocker | 17 (23%) | 20 (28%) | 28 (39%) | 0.10 |
| Loop diuretic | 46 (63%) | 56 (79%) | 63 (89%) | 0.001 |
| Furosemide- dose/day, mg | 40 (20, 80) | 45 (20, 80) | 80 (40, 120) | 0.002 |
| Clinical characteristics | ||||
| NYHA functional class, n (%) | <0.001 | |||
| II | 48 (66%) | 31 (44%) | 21 (30%) | |
| III | 25 (34%) | 40 (56%) | 50 (70%) | |
| MLWHFQ total score | 37 (27, 63) | 46 (32, 62) | 47 (32, 62) | 0.36 |
| MLWHFQ physical dimension | 21(13, 29) | 22 (14, 31) | 25 (17, 32) | 0.27 |
| Rales, n (%) | 4 (5%) | 4 (6%) | 6 (8%) | 0.78 |
| Third heart sound (S3), n (%) | 0 (0%) | 5 (7%) | 4 (6%) | 0.06 |
| JVP > 8 cmH2O, n (%) | 24 (33%) | 33 (46%) | 38 (54%) | 0.04 |
| Moderate to severe edema, n (%) | 9 (12%) | 13 (18%) | 22 (31%) | 0.02 |
| Laboratory values | ||||
| Creatinine, mg/dl | 1.1 (0.8, 1.3) | 1.1 (0.8, 1.3) | 1.3 (0.9, 1.7) | 0.02 |
| eGRF, ml/min/1.73 m2 | 71 (55, 88) | 65 (51, 83) | 52 (36, 68) | 0.0001 |
| Hemoglobin, g/dl | 13.4 (12.6, 14.5) | 12.6 (11.5, 13.8) | 12.4 (11.5, 13.3) | <0.001 |
| NT-proBNP, pg/ml | 485 (93, 876) | 621 (247, 1296) | 1279 (618, 2331) | <0.001 |
Abbreviations: BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease, ECG, electrocardiogram; JVP, jugular venous pressure; MLWHF, Minnesota living with heart failure questionnaire; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; VO2; oxygen consumption
Patients with lower pVO2 were older, more likely to be women and more obese (Table 1). There was no difference in the prevalence of hypertension, coronary artery disease, or COPD across the tertiles of pVO2. However, atrial fibrillation, diabetes mellitus, renal dysfunction (lower eGFR and higher serum creatinine) and anemia (lower hemoglobin) were more common in those with lower pVO2.
There was no difference in the frequency of use of beta blockers, calcium channel blockers, or digoxin across the tertiles of pVO2. However, patients with lower pVO2 were more likely to be on loop diuretics and required higher doses of diuretics (Table 1).
Patients with lower pVO2 had more severe symptoms (NYHA class); however, the Minnesota living with HF questionnaire (MLWHF) total score was not significantly different across the tertiles of pVO2. Patients with lower pVO2 had evidence of more severe congestion (elevated jugular venous pressure, peripheral edema) and higher NT-proBNP levels (Table 1).
Functional capacity in HFpEF
Patients with lower pVO2 had lower % predicted pVO2 (Wasserman equation) and shorter 6MWD (Table 2). Peak respiratory exchange ratio (RER) was not significantly related to pVO2, likely due to an RER of ≥1 in most subjects and the narrow range of RER achieved (median 1.09, 1.02 - 1.15). Subjects with lower pVO2 exercised for shorter duration and achieved lower heart rate, chronotropic index and blood pressure at peak exercise. Patients with lower pVO2 had lower submaximal exercise capacity (VO2 at anaerobic threshold, AT).
Table 2. Exercise capacity in HFpEF.
| Upper pVO2 tertile 15.9 (14.4, 16.9) | Middle pVO2 tertile 11.7 (11.2, 12.4) | Lower pVO2 tertile 9.4 (8.3, 10.2) | P-value | |
|---|---|---|---|---|
| N | 73 | 71 | 71 | |
| Six minute walk distance, m | 368 (293, 442) | 328 (246, 382) | 236 (160, 306) | <0.001 |
| Exercise modality | 0.017 | |||
| Cycle | 38 (52%) | 53 (75%) | 43 (61%) | |
| Treadmill | 35 (48%) | 18 (25%) | 28 (39%) | |
| Rest hemodynamics | ||||
| Systolic BP, mmHg | 123 (112, 140) | 128 (114, 140) | 120 (110, 132) | 0.37 |
| Diastolic BP, mmHg | 74 (66, 80) | 70 (64, 76) | 68 (60, 76) | 0.04 |
| Heart rate, bpm | 67 (60, 76) | 69 (60, 80) | 70 (60, 76) | 0.89 |
| Peak exercise hemodynamics | ||||
| Systolic BP, mmHg | 162 (146, 190) | 158 (134, 172) | 138 (120, 158) | <0.001 |
| Diastolic BP, mmHg | 80 (61, 90) | 72 (64, 80) | 70 (60, 73) | 0.004 |
| Heart rate, bpm | 119 (111, 134) | 106 (92, 128) | 94 (81, 109) | <0.001 |
| Chronotropic index | 0.60 (0.48, 0.72) | 0.47 (0.32, 0.67) | 0.29 (0.17, 0.52) | <0.001 |
| Chronotropic incompetence | 47 (65%) | 55 (79%) | 62 (87%) | 0.007 |
| Respiratory exchange ratio | 1.1 (1.0, 1.2) | 1.1 (1.0, 1.2) | 1.09 (1.0, 1.2) | 0.82 |
| Borg score | 8 (6, 9) | 7 (5, 9) | 7 (5, 8) | 0.049 |
| Exercise duration, min | 12 (10, 14) | 10 (8, 11) | 7 (6, 9) | <0.001 |
| VO2, ml/min | 1503 (1289, 1784) | 1066 (902, 1278) | 860 (689, 1120) | <0.001 |
| VO2, ml/kg/min | 15.9 (14.4, 16.9) | 11.7 (11.2, 12.4) | 9.4 (8.3, 10.2) | N/A |
| VO2 at AT, ml/min/kg | 9.1 (8.3, 10.3) | 7.0 (6.3, 7.9) | 6.0 (5.4, 6.6) | <0.001 |
| VO2 at AT (% of pVO2) | 59 (53, 64) | 60 (54, 66) | 67 (60, 71) | <0.001 |
| % Predicted pVO2† | 75.48 (68.16, 84.03) | 66.84 (56.65, 77.03) | 54.9 (45.99, 64.84) | <0.001 |
Abbreviations: AT, anaerobic threshold; BP, blood pressure; pVO2, peak oxygen consumption;
Wasserman Equation
Relationship of cardiovascular structure and function to exercise capacity
Subjects with lower pVO2 tended to have smaller LV diastolic diameter and had lower LV mass but not LV mass indexed to body size (Table 3). There was no difference in geometry (relative wall thickness) across the tertiles of pVO2.
Table 3. Ventricular and vascular structure and function by severity of limitation in peak exercise capacity.
| Upper pVO2 tertile 15.9 (14.4, 16.9) | Middle pVO2 tertile 11.7 (11.2, 12.4) | Lower pVO2 tertile 9.4 (8.3, 10.2) | P-value | |
|---|---|---|---|---|
| N | 73 | 71 | 71 | |
| LV structure and geometry | ||||
| LV diastolic dimension, cm | 4.8 (4.3, 5.4) | 4.6 (4.2, 5.0) | 4.5 (4.1, 5.1) | 0.10 |
| LV mass, g | 170 (142, 242) | 146 (122, 215) | 148 (121, 190) | 0.047 |
| LV mass index, g/m2 | 80 (64, 105) | 77 (59, 100) | 70 (58, 85) | 0.11 |
| Relative wall thickness | 0.41 (0.35, 0.50) | 0.41 (0.36, 0.50) | 0.42 (0.36, 0.47) | 0.83 |
| LV systolic function | ||||
| Ejection fraction, % | 61 (56, 68) | 60 (55, 65) | 61 (57, 65) | 0.61 |
| Stroke volume, ml | 80 (66, 95) | 78 (65, 87) | 74 (59, 90) | 0.29 |
| Stroke volume index, ml/m2 | 38 (30, 43) | 38 (31, 43) | 36 (30, 44) | 0.62 |
| Stroke work, gm-m/beat | 97 (82, 122) | 94 (77,109) | 86 (73, 109) | 0.11 |
| eFS, % | 38 (34,45) | 38 (33,42) | 37 (34,42) | 0.80 |
| mFS, % | 30 (27, 34) | 29 (26, 32) | 29 (27, 33) | 0.68 |
| cESS, g/cm2 | 116 (94, 145) | 110 (91, 140) | 110 (95, 131) | 0.82 |
| Stress corrected eFS* | 19 (16, 23) | 19 (16, 22) | 19 (16, 22) | 0.87 |
| Stress corrected mFS* | 14 (13, 17) | 15 (12, 16) | 14 (13, 16) | 0.86 |
| LV diastolic function | ||||
| E/A ratio | 1.1 (0.9, 1.8) | 1.6 (1.0, 2.6) | 1.7 (1.1, 2.6) | 0.03 |
| e' medial, cm/s | 6 (5, 8) | 6 (5, 8) | 5 (4,7) | 0.12 |
| E/e′ medial | 13 (10, 20) | 17 (12, 24) | 18 (15, 26) | 0.004 |
| Deceleration time, msec | 200 (156, 235) | 190 (159, 212) | 176 (147, 201) | 0.04 |
| LA volume index, ml/m2 | 42 (33, 57) | 54 (39, 62) | 43 (38, 55) | 0.06 |
| Diastolic function grade | 0.14 | |||
| Normal | 5 (13%) | 7 (14%) | 2 (3%) | |
| Abnormal relaxation | 1 (3%) | 1 (2%) | 0 (0%) | |
| Pseudo-normal | 27 (68%) | 37 (74%) | 47 (80%) | |
| Restrictive | 7 (18%) | 5 (10%) | 10 (17%) | |
| PASP, mmHg | 36 (30, 46) | 41 (32, 49) | 47 (36, 56) | 0.006 |
| Systemic arterial function | ||||
| Pulse pressure, mmHg | 52 (46, 68) | 60 (48, 72) | 55 (47, 66) | 0.16 |
| Ea, mmHg/ml | 1.51 (1.18, 1.71) | 1.53 (1.18, 1.87) | 1.52 (1.23, 1.95) | 0.46 |
| SVR, dyne.sec.cm-5 | 1336 (1159, 1584) | 1326 (1085, 1637) | 1507 (1111, 1724) | 0.42 |
| SVRi, dyne.sec.cm-5.m2 | 2873 (2447, 3376) | 2648 (2295, 3540) | 3016 (2456, 3562) | 0.60 |
| SAC, ml.mmHg-1 | 1.45 (1.12, 1.85) | 1.27 (0.96, 1.76) | 1.33 (1.06, 1.70) | 0.20 |
| SACi, ml.mmHg-1.m-2 | 0.70 (0.55, 0.88) | 0.64 (0.50, 0.78) | 0.66 (0.53, 0.79) | 0.36 |
| Aortic distensibility, mmHg-1 | 1.42 (0.74, 2.90) | 1.06 (0.67, 1.22) | 1.20 (0.63, 2.05) | 0.14 |
Abbreviations: cESS, circumferential endocardial systolic wall stress; Ea, arterial elastance; eFS, endocardial fractional shortening; LV, left ventricle; LA, left atrial; mFS, midwall fractional shortening, PASP, pulmonary artery systolic pressure; SAC, systemic arterial compliance; SVR, systemic vascular resistance;
=%/log10 (g/cm2)
Neither EF nor other indices of myocardial contractility (stress corrected eFS or mFS) were different across the tertiles of pVO2 (Table 3).
Patients with lower pVO2 had more severe diastolic dysfunction, evidenced by higher mitral inflow E/A ratio and E/e′ and shorter deceleration time although e' was not different across tertiles of pVO2. Using an ordinal diastolic dysfunction severity scale, those with lower pVO2 had more advanced diastolic dysfunction. However, this difference was not statistically significant as the majority of patients enrolled in RELAX had a pseudonormal or restrictive pattern (Table 3).
Patients with lower pVO2 had higher PASP (Table 3) but there were no differences in systemic vascular function indices across tertiles of pVO2 (Table 3).
Age, sex, body size, hemoglobin and chronotropic reserve as predictors of peak VO2 (ml/min)
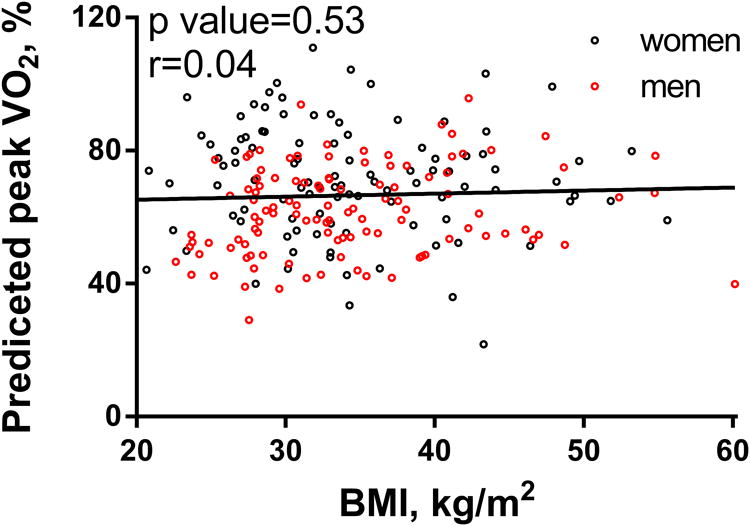
As previously established in normal persons and in persons with cardiovascular disease,24 age, sex, BMI, hemoglobin and chronotropic reserve were associated with pVO2 in HFpEF (Figure 1) and these variables explained 64% of the variability in pVO2 (ml/min) and 49% of weight-indexed pVO2 in the RELAX study population (Table 4). As expected, body mass index (BMI) was negatively associated with weight-indexed pVO2 (ml/kg/min, Table 1) but positively associated with non-indexed pVO2 (ml/min) (Figure 1, Table 4). The % predicted pVO2 (Wasserman equation) was not associated with BMI (Figure 2).
Figure 1. Correlations between pVO2 (ml/min) and age, BMI, hemoglobin and chronotropic index in men and women.
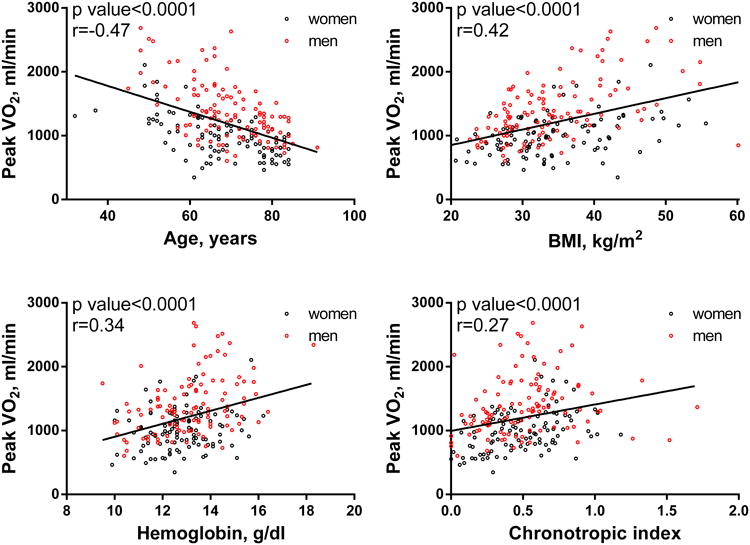
Table 4. Age, sex, BMI, hemoglobin and chronotropic index association with pVO2 Regression coefficients with 95% confidence intervals are shown.
| Peak VO2 indexed to weight (ml/kg/min) N=212; Model R2 = 0.49 | |||
|---|---|---|---|
| Regression Coefficient (ml/kg/min) | Confidence interval | P-value | |
| Age (per year) | -0.11 | -0.15, -0.08 | <0.001 |
| Sex (women vs men) | -1.88 | -2.50, -1.27 | <0.001 |
| BMI (per kg/m2) | -0.11 | -0.15, -0.06 | <0.001 |
| Hemoglobin (per g/dl) | 0.58 | 0.37, 0.79 | <0.001 |
| Chronotropic index (per 0.1) | 0.36 | 0.25, 0.47 | <0.001 |
| Peak VO2 (ml/min) N=212; Model R2 = 0.64 | |||
| Regression Coefficient (ml/min) | Confidence interval | P-value | |
| Age (per year) | -12.73 | -16.81, -8.66 | <0.0001 |
| Sex (women vs men) | -391.87 | -465.58, -318.16 | <0.0001 |
| BMI (per kg/m2) | 20.22 | 14.59, 25.85 | <0.0001 |
| Hemoglobin (per g/dl) | 68.72 | 43.67, 93.78 | <0.0001 |
| Chronotropic index (per 0.1) | 40.47 | 27.11, 53.83 | <0.0001 |
Figure 2. Correlations between BMI and % predicted peak VO2 in men and women.
In RELAX, CPXT was performed on a cycle in 81(38%) and on a treadmill in 134 (62%). Adjusting for these variables, there was no association between exercise modality and pVO2.
Relationship of cardiovascular structure and function to peak VO2 (ml/min) adjusting for age, sex, BMI, hemoglobin and chronotropic index
LV diastolic dimension (partial R21.5, p=0.008) and LV mass (partial R21.6, p=0.008) were associated with pVO2 (Table 5), whereas RWT had no association with pVO2. Stroke volume was positively associated with pVO2 explaining an additional 2.0% of the variability in pVO2 in HFpEF patients. There was no association between other resting indices of systolic function and pVO2 (Table 5).
Table 5.
Age, Sex, BMI, hemoglobin and chronotropic index adjusted association of cardiovascular structure and function with peak VO2 (ml/min): LV diastolic dimension, LV mass, stroke volume, e', SAC and mitral deceleration time were positively associated with pVO2, whereas mitral E/e′ ratio, Ea and NTproBNP were negatively associated with pVO2.
| Associated variable | Model N | Model R2 | Partial R2 for variable | P-value |
|---|---|---|---|---|
| LV structure and geometry | ||||
| LV diastolic dimension, mm | 163 | 0.6658 | 0.0153 | 0.008 |
| LV mass, gram | 157 | 0.6638 | 0.0162 | 0.008 |
| Relative wall thickness | 157 | 0.6480 | 0.0004 | 0.68 |
| LV systolic function | ||||
| Ejection fraction, % | 209 | 0.6428 | 0.0007 | 0.54 |
| Stroke volume, ml | 184 | 0.6471 | 0.0196 | 0.002 |
| eFS, % | 140 | 0.6468 | 0.0022 | 0.36 |
| mFS, % | 139 | 0.6465 | 0.023 | 0.36 |
| cESS, g/cm2 | 135 | 0.6478 | 0.0002 | 0.79 |
| Stress corrected eFS | 135 | 0.6502 | 0.0026 | 0.33 |
| Stress corrected mFS | 135 | 0.6500 | 0.0024 | 0.35 |
| LV diastolic function | ||||
| E/A ratio | 140 | 0.6601 | 0.0061 | 0.12 |
| Medial e', cm/s | 195 | 0.6328 | 0.0077 | 0.048 |
| Medial E/e′ | 187 | 0.6364 | 0.0140 | 0.009 |
| Deceleration time, msec | 148 | 0.6213 | 0.0019 | 0.03 |
| LA volume, ml | 191 | 0.6362 | 0.0091 | 0.41 |
| Diastolic function grade | 136 | 0.5232 | 0.0106 | 0.09 |
| PASP, mmHg | 148 | 0.5643 | 0.0140 | 0.22 |
| Systemic arterial function | ||||
| Pulse pressure, mmHg | 203 | 0.6458 | 0.0002 | 0.74 |
| Systolic BP, mmHg | 203 | 0.6476 | 0.0019 | 0.30 |
| Ea, mmHg/ml | 177 | 0.6510 | 0.0207 | 0.002 |
| SVR, dyne.sec.cm-5 | 174 | 0.6443 | 0.0059 | 0.10 |
| SAC, ml/mmHg | 177 | 0.6456 | 0.0154 | 0.007 |
| Aortic distensibility, mmHg-1 | 84 | 0.7054 | 0.0040 | 0.31 |
| NT ProBNP, pg/ml | 209 | 0.6623 | 0.0189 | 0.0009 |
Abbreviations: as in Table 3
Indexes of diastolic dysfunction (higher E/e′, and shorter DT) were associated with lower pVO2 with each variable explaining an additional 1% of the variability in pVO2 in HFpEF. However, the ordinal diastolic dysfunction grade, E/A ratio and LA volume were not associated with pVO2 (Table 5). PASP showed a strong trend towards association with pVO2, but this was not statistically significant (p=0.09), Table 5.
Higher total systemic arterial load (Ea) and lower SAC were each associated with lower pVO2 explaining an additional 2.1% (Ea) or 1.5% (SAC) of the variability of pVO2 in HFpEF. SVR and aortic distensibility were not significantly associated with pVO2 (Table 5).
Discussion
In this prospectively identified and rigorously characterized HFpEF cohort, age, sex, body size, hemoglobin and chronotropic reserve collectively explained 64% of the variability in pVO2. After accounting for these variables, LV size and mass, parameters reflecting the severity of diastolic dysfunction and elevated LV filling pressures, SV, and systemic arterial function were each only modestly, albeit significantly associated with pVO2. These findings suggest that potentially modifiable factors (obesity, anemia and chronotropic incompetence) potently contribute to exercise intolerance in HFpEF. The relatively weak or absent association of resting measures of LV systolic, LV diastolic or vascular function with exercise capacity suggests that the cardiovascular response to exercise is poorly correlated with resting measures in HFpEF.
Exercise capacity in RELAX
Despite striking differences in ventricular-vascular properties, studies have shown that the degree of exercise intolerance is similar in HFpEF and HFrEF.2, 3, 25, 26 Notably, pVO2 in the HFpEF patients enrolled in RELAX was lower than previously described in HFpEF cohorts with NYHA class II/III symptoms,2, 25, 27, 28 or in HFrEF with LVEF<35% and similar NYHA class.29 The severity of exercise intolerance as measured by pVO2 or 6MWD in the RELAX HFpEF cohort13 reflects not only the demographics of the HFpEF population as older persons, women and obese persons have lower reference ranges for weight-indexed pVO2 and 6MWD, but also the RELAX entry criteria which mandated a pVO2 ≤60% of predicted for age and sex.15 In comparison, a recent multicenter trial investigating the effect of spironolactone on exercise capacity in HFpEF used a more liberal entry criteria (pVO2 ≤25 ml/mg/min regardless of age and sex)28 and therefore enrolled a cohort with higher median pVO2 (16.4 ml/kg/min), milder symptoms, better diastolic function and fewer comorbidities than observed in RELAX. These differences in prospectively enrolled HFpEF cohorts underscore the spectrum of debility in HFpEF and confirm the validity of pVO2 as a marker of disease severity in HFpEF.
Association of systolic function with exercise capacity in HFpEF
Contrary to our hypothesis and consistent with small studies in HFrEF,30 neither resting LV chamber (EF and eFS) nor myocardial (stress corrected mFS) systolic performance were significantly related to exercise capacity in HFpEF although resting SV was.
In a large randomized clinical trial of exercise training in HFrEF, resting EF was positively associated with pVO2 in HFrEF,29 but explained only one percent of the variance in pVO2.29
The association between resting SV and exercise capacity observed here is consistent with a previous study in HFpEF8 where resting and peak SV were lower in HFpEF than controls and each associated with pVO2. Despite normal EF, patients with HFpEF have subtle reduction in measures of myocardial contractility,31 blunted increases in EF and SV in response to exercise 7, 9, 32, 33 and modest but significant reduction in EF over time. While lower resting SV may identify patients with more limited ability to enhance stroke volume with exercise, this association was not strong with resting SV explaining only two percent of the variance in pVO2.
Association of diastolic function with exercise capacity in HF
Diastolic function indexes were the most significant correlates of exercise capacity in previous studies in HFrEF,29, 34 where higher E/A ratio was associated with lower pVO2 after adjustment for pertinent covariates, explaining an additional 6% of the variance in pVO2.29
In some previous HFpEF studies, the severity of resting diastolic dysfunction assessed with novel indices was associated with more impaired exercise capacity7 while larger studies did not find an association between resting conventional Doppler diastolic function indices and exercise capacity.12, 35 In this fairly advanced HFpEF cohort, resting Doppler indices known to be associated with LV diastolic dysfunction and elevation of LV filling pressures were only modestly associated with impairment in pVO2 after adjustment for pertinent covariates. This may reflect the variability and/or insensitivity of the diastolic indices to elevation of LV filling pressures. Alternatively, the severity of exercise-induced diastolic dysfunction may be variably related to resting diastolic function. Studies in patients with NYHA class II HF symptoms and normal EF demonstrated marked elevations in pulmonary capillary wedge pressure with leg elevation or exercise despite normal resting wedge pressure.36, 37
Association of arterial function with exercise capacity in HF
Arterial and ventricular stiffness increase in parallel with advancing age and particularly in hypertensive heart disease and HFpEF.38, 39 Arterial stiffness was inversely associated with exercise capacity in healthy adults.40 Arterial stiffness and/or blunted reduction in Ea or systemic vascular resistance with exercise (impaired vascular reserve) were associated with severity of exercise intolerance in HFpEF.6, 9, 33, 41 In RELAX, Ea (a combined index of pulsatile and resistive load) had a significant negative correlation with exercise tolerance (pVO2) after adjustment for pertinent covariates. This association was driven by the pulsatile (aortic compliance), rather than the resistive component of Ea (SVR). Aortic distensibility measured by CMR is another (inverse) index of arterial stiffening and was directly correlated with pVO2 in a study of HFpEF patients and healthy controls.4 In RELAX, where associations were tested only in subjects with HFpEF, aortic distensibility was not associated with pVO2. Measures of vascular reserve were not assessed in RELAX. Measures of arterial stiffness were lower in senior athletes than age matched healthy but sedentary controls suggesting that exercise may prevent or reverse arterial stiffening.40 However, a trial of exercise training in HFpEF showed that training improved exercise capacity without improving arterial stiffness.41
Comorbid conditions and exercise capacity in HFpEF
This study confirms that non-cardiovascular (obesity and anemia) and cardiovascular (chronotropic incompetence) comorbidities are potently associated with impaired exercise tolerance in HFpEF. Each of these conditions is potentially modifiable and may represent therapeutic targets in HFpEF.42
Obesity is common in HFpEF and is paradoxically associated with better survival except at extreme levels of obesity.43 Obesity is associated with mobility limitation in the general population,44 where intentional weight loss was associated with improvement in mobility45. Guidelines specify indexing pVO2 to total body mass (weight) for prognostic and diagnostic use. Weight-indexed pVO2 is inversely related to BMI while non-indexed pVO2 increases with BMI in healthy persons. Cardiovascular capacity is best reflected by pVO2 indexed to lean body mass or as expressed as a percent of appropriately predicted pVO2 (accounting for the training effect of obesity) whereas work capacity is related to ambulatory activity and best expressed as peak VO2 indexed to body weight.46 Indeed, HFpEF patients have lower weight-indexed and lean body mass-indexed pVO2 than controls confirming reduction in both cardiovascular and work capacity in HFpEF.12
Here, peak VO2 was only 71% of that predicted by the Wasserman equation confirming reduced cardiovascular capacity in the RELAX HFpEF cohort. While patients with lower weight-indexed VO2 were more obese, they also had lower % predicted peak VO2 and % predicted peak VO2 was not related to BMI, suggesting that reduced cardiovascular capacity in HFpEF is not merely related to obesity. While cardiovascular capacity (% predicted VO2) did not correlate with BMI, the inverse relationship between work capacity (pVO2/kg) and BMI lends support to investigating the impact of weight reduction on functional status in HFpEF. Unfortunately, no assessment of lean body mass or peripheral muscle strength was obtained in RELAX and thus we cannot comment on whether HFpEF patients had sarcopenic obesity contributing to their exercise intolerance.11, 12
As oxygen carrying capacity is strongly correlated with exercise capacity, treatment of anemia with erythropoietin has been studied in HFrEF and HFpEF with no benefit based on 6 minute walk tests. However, in a subgroup of patients that was able to complete a cardiopulmonary exercise test, those randomized to Epoetin Alfa had significant improvement in pVO2 compared with placebo. Although these findings are inconclusive, treatment of anemia may be of benefit in some HFpEF patients.47
The impact of treatment of chronotropic incompetence by withdrawal of negative chronotropic agents and/or rate adaptive pacing has not yet been characterized in HFpEF.
Additional factors associated with exercise intolerance in HFpEF
The contribution of skeletal muscle properties and oxygen extraction to variance in pVO2 was not addressed in this analysis. Studies have varied as to whether oxygen extraction is altered in HFpEF with studies showing normal to enhanced8, 32 or reduced48, 49 extraction with exercise in HFpEF. Deconditioning impairs pVO2, whereas exercise training improved exercise capacity in small studies in HFpEF.50
Limitations
Because of the cross sectional nature of the study, we cannot prove that factors associated with lower peak VO2 are causally related to exercise intolerance. The lack of an association of most resting indices with exercise capacity in RELAX may have been influenced by the entry criteria which restricted entry to those with severe exercise intolerance and resulted in a narrower range of pVO2. Measures of ventricular and vascular function were assessed non-invasively and brachial BP was used as surrogate for central arterial pressure. We have not measured ventricular - vascular function during exercise, we therefore cannot determine the influence of these measures on exercise capacity. The insignificant association between the Minnesota living with heart failure questionnaire and peak VO2 may indicate a limitation of disease specific questionnaires; incorporation of non-disease specific questionnaires such as the short form health survey (SF-36) may overcome this limitation.
Conclusion
In this well-characterized HFpEF cohort, in addition to age and sex, several potentially modifiable factors (obesity, anemia and chronotropic incompetence) are strongly associated with the severity of exercise intolerance in HFpEF. The relatively weak or absent association of resting measures of LV systolic, LV diastolic or vascular function with exercise capacity suggests that cardiovascular response to exercise is poorly correlated with resting measures in HFpEF.
Supplementary Material
Acknowledgments
Sources of Funding: This study is supported by the National Institutes of Health grants U10HL084904 (the data coordinating center), U10HL084907 and PO1HL 76611 (MMR), T32-HL007111, Mayo clinic cardiovascular division and Mayo graduate school (SFM). Support for mentoring SFM and RZ as HF research skills development fellows is provided by HL084907 and UL1 RR024150. This study was also supported by UL1 TR000135 from the National Center for Advancing Translational Sciences.
Footnotes
Disclosures: None.
References
- 1.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;38:1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 3.Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46:1883–1890. doi: 10.1016/j.jacc.2005.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 7.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: Exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56:855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth MJ, Miller MS, VanBuren P, Bedrin NG, LeWinter MM, Ades PA, Palmer BM. Resistance training alters skeletal muscle structure and function in human heart failure: Effects at the tissue, cellular and molecular levels. J Physiol. 2012;590:1243–1259. doi: 10.1113/jphysiol.2011.219659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: Definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: Role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68:968–975. doi: 10.1093/gerona/glt011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, Lewinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013:1–10. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, Lewinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. Phosphdiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (relax) trial: Rationale and design. Circ Heart Fail. 2012;5:653–659. doi: 10.1161/CIRCHEARTFAILURE.112.969071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher GF, Balady G, Froelicher VF, Hartley LH, Haskell WL, Pollock ML. Exercise standards. A statement for healthcare professionals from the american heart association. Writing group. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 16.Schussheim AE, Diamond JA, Jhang JS, Phillips RA. Midwall fractional shortening is an independent predictor of left ventricular diastolic dysfunction in asymptomatic patients with systemic hypertension. Am J Cardiol. 1998;82:1056–1059. doi: 10.1016/s0002-9149(98)00558-x. [DOI] [PubMed] [Google Scholar]
- 17.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 18.Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: A community based study. Circ Heart Fail. 2012;5:710–9. doi: 10.1161/CIRCHEARTFAILURE.112.968594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sue DY, Wasserman K, Moricca RB, Casaburi R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. Use of the v-slope method for anaerobic threshold determination. Chest. 1988;94:931–938. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- 20.Arena R, Myers J, Abella J, Pinkstaff S, Brubaker P, Moore B, Kitzman D, Peberdy MA, Bensimhon D, Chase P, Forman D, West E, Guazzi M. Determining the preferred percent-predicted equation for peak oxygen consumption in patients with heart failure. Circ Heart Fail. 2009;2:113–120. doi: 10.1161/CIRCHEARTFAILURE.108.834168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 22.Robergs RA, Landwehr R. The surprising history of the “hrmax=220-age” equation. J Exerc Physiol. 2002;5:10. [Google Scholar]
- 23.Azarbal B, Hayes SW, Lewin HC, Hachamovitch R, Cohen I, Berman DS. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality: Superiority over 85% of maximal age-predicted heart rate. J Am Coll Cardiol. 2004;44:423–430. doi: 10.1016/j.jacc.2004.02.060. [DOI] [PubMed] [Google Scholar]
- 24.Fleg JL, Morrell CH, Bos AG, Brant LJ, Talbot LA, Wright JG, Lakatta EG. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 25.Farr MJ, Lang CC, Lamanca JJ, Zile MR, Francis G, Tavazzi L, Gaasch WH, St John Sutton M, Itoh H, Mancini D. Cardiopulmonary exercise variables in diastolic versus systolic heart failure. Am J Cardiol. 2008;102:203–206. doi: 10.1016/j.amjcard.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 26.Zile MR, Kjellstrom B, Bennett T, Cho Y, Baicu CF, Aaron MF, Abraham WT, Bourge RC, Kueffer FJ. Effects of exercise on left ventricular systolic and diastolic properties in patients with heart failure and a preserved ejection fraction versus heart failure and a reduced ejection fraction. Circ Heart Fail. 2013;6:508–16. doi: 10.1161/CIRCHEARTFAILURE.112.000216. [DOI] [PubMed] [Google Scholar]
- 27.Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, Little WC. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: Effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Loffler M, Dungen HD, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske B. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 29.Gardin JM, Leifer ES, Fleg JL, Whellan D, Kokkinos P, Leblanc MH, Wolfel E, Kitzman DW. Relationship of doppler-echocardiographic left ventricular diastolic function to exercise performance in systolic heart failure: The hf-action study. Am Heart J. 2009;158:S45–52. doi: 10.1016/j.ahj.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francis GS, Goldsmith SR, Cohn JN. Relationship of exercise capacity to resting left ventricular performance and basal plasma norepinephrine levels in patients with congestive heart failure. Am Heart J. 1982;104:725–731. doi: 10.1016/0002-8703(82)90003-5. [DOI] [PubMed] [Google Scholar]
- 31.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, Borlaug BA. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–85. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14:475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Terzi S, Sayar N, Bilsel T, Enc Y, Yildirim A, Ciloglu F, Yesilcimen K. Tissue doppler imaging adds incremental value in predicting exercise capacity in patients with congestive heart failure. Heart Vessels. 2007;22:237–244. doi: 10.1007/s00380-006-0961-x. [DOI] [PubMed] [Google Scholar]
- 35.Edelmann F, Gelbrich G, Duvinage A, Stahrenberg R, Behrens A, Prettin C, Kraigher-Krainer E, Schmidt AG, Dungen HD, Kamke W, Tschope C, Herrmann-Lingen C, Halle M, Hasenfuss G, Wachter R, Pieske B. Differential interaction of clinical characteristics with key functional parameters in heart failure with preserved ejection fraction - results of the aldo-DHF trial. Int J Cardiol. 2013;169:408–417. doi: 10.1016/j.ijcard.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tolle JJ, Waxman AB, Van Horn TL, Pappagianopoulos PP, Systrom DM. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: Implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol. 1998;32:1221–1227. doi: 10.1016/s0735-1097(98)00374-x. [DOI] [PubMed] [Google Scholar]
- 39.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: A community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 40.Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FCP, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 41.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–92. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: The cardiovascular health study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 43.Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: Results from the irbesartan in heart failure with preserved ejection fraction (i-preserve) trial. Circ Heart Fail. 2011;4:324–331. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houston DK, Ding J, Nicklas BJ, Harris TB, Lee JS, Nevitt MC, Rubin SM, Tylavsky FA, Kritchevsky SB. Overweight and obesity over the adult life course and incident mobility limitation in older adults: The health, aging and body composition study. Am J Epidemiol. 2009;169:927–936. doi: 10.1093/aje/kwp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beavers KM, Miller ME, Rejeski WJ, Nicklas BJ, Krichevsky SB. Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:80–86. doi: 10.1093/gerona/gls092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sietsema KE. Clinical exercise testing. Exercsie responses in systemic conditions Obesity, Diabetes, Thyroid Disorders, and Chronic Fatigue Syndrome. 2002;32:264–272. [Google Scholar]
- 47.Maurer MS, Teruya S, Chakraborty B, Helmke S, Mancini D. Treating anemia in older adults with heart failure with a preserved ejection fraction with epoetin alfa: Single-blind randomized clinical trial of safety and efficacy. Circ Heart Fail. 2013;6:254–263. doi: 10.1161/CIRCHEARTFAILURE.112.969717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA. Effects of exercise training for heart failure with preserved ejection fraction: A systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. doi: 10.1016/j.ijcard.2012.05.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


