Abstract
Collagen XII, largest member of the fibril-associated collagens with interrupted triple helix (FACIT) family, assembles from three identical α-chains encoded by the COL12A1 gene. The molecule consists of three threadlike N-terminal noncollagenous NC3 domains, joined by disulfide bonds and a short interrupted collagen triple helix towards the C-terminus. Splice variants differ considerably in size and properties: "small" collagen XIIB (220 kDa subunit) is similar to collagen XIV, whereas collagen XIIA (350 kDa) has a much larger NC3 domain carrying glycosaminoglycan chains. Collagen XII binds to collagen I-containing fibrils via its collagenous domain, whereas its large noncollagenous arms interact with other matrix proteins such as tenascin-X. In dense connective tissues and bone, collagen XII is thought to regulate organization and mechanical properties of collagen fibril bundles. Accordingly, recent findings show that collagen XII mutations cause Ehlers-Danlos/myopathy overlap syndrome associated with skeletal abnormalities and muscle weakness in mice and humans.
Introduction
Collagen XII was discovered in 1987 in search for novel collagenous sequences from chick tendon fibroblasts (Gordon et al., 1987). Olsen's group found a cDNA with high similarity to collagen IX, a molecule associated with collagen II fibrils in cartilage. In the same year, collagenous pepsin-resistant fragments were isolated from chick tendons, whose amino acid sequences matched the published collagen XII cDNA (Dublet and van der Rest, 1987). Later, van der Rest and colleagues characterized the intact protein as homotrimer of 220-kDa subunits, with disulfide-bonded 190-kDa noncollagenous domains linked to a short C-terminal collagen helix (Dublet et al., 1989). Single collagen XII molecules from tendon appeared cross-shaped with three 60 nm long arms and a thinner tail 75 nm in length (Dublet et al., 1989). However, the published full-length chick collagen XII cDNA predicted a subunit of 340 kDa (Yamagata et al., 1991). This discrepancy was solved when larger molecular species were purified from chick fibroblasts (Koch et al., 1992) and a human cell line (Lunstrum et al., 1992), and shown to be collagen XII variants by peptide sequencing. Accordingly, "large" collagen XII was shown to have noncollagenous arms of >300 kDa and 90 nm in length (Koch et al., 1992). "Large" (XIIA) and "small" (XIIB) collagen XII variants were shown to arise from alternative splicing (Trueb and Trueb, 1992, Gerecke et al., 1997). In parallel, a molecule similar in structure to "small" collagen XIIB, but distinct in sequence, was isolated from fetal bovine tissue (Dublet and van der Rest, 1991). This novel collagen XIV was grouped with collagens IX and XII into a protein family called FACIT (fibril-associated collagens with interrupted triple helix). Since then, the FACIT family has grown by five members, namely collagens XVI, XIX, XX, XXI, and XXII (Ricard-Blum, 2011). This article focuses on collagen XII.
Structure: "Large" (XIIA) versus "small" (XIIB) collagen XII variants
A single gene encodes collagen XII. The full-length cDNA for the collagen XII α1-chain from chick was published in 1991 (Yamagata et al., 1991), and the human sequence in 1997 (Gerecke et al., 1997). The COL12A1 gene is located on human chromosome 6q12-q13 close to two other FACIT genes, COL9A1 and COL19A1 (Gerecke et al., 1997). Full-length COL12A1 cDNA is 9.75 kb in size and codes for the largest subunit variant of 3,063 amino acids (331 kDa). The protein sequence of human collagen XIIA shares 78% identity with the chick, and the domain structures are identical. After the signal peptide, a very large N-terminal noncollagenous domain, called NC3, consists of an array of 18 fibronectin type III (FN3) repeats, into which 4 von Willebrand factor A (VWA) modules are inserted (Fig. 1A).
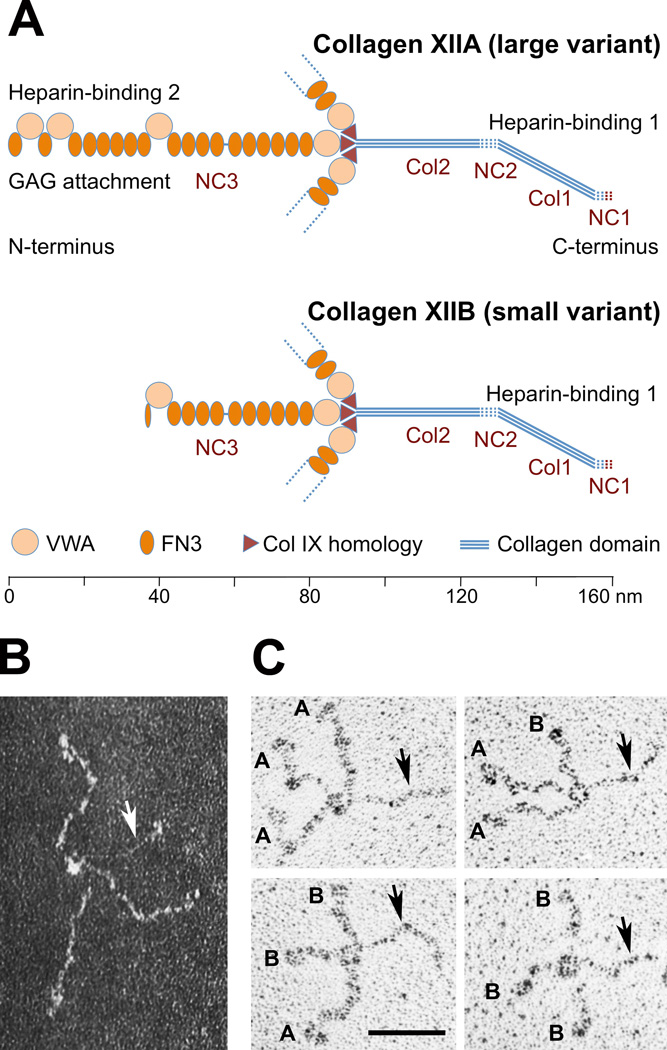
Fig. 1. Structure of collagen XII.
(A) Domain structure of "large" collagen XIIA and "small" collagen XIIB splice variants. Collagen XIIB misses the entire N-terminal half of the NC3 domain. The alternatively spliced region (8 FN3 and 2 VWA modules) contains sites for heparin-binding and glycosaminoclycan (GAG) attachment. A second heparin-binding site is present in the collagenous domain. (B) A collagen XII molecule with three XIIA subunits as seen in the electron microscope after negative staining. Note three flexible arms ≈90 nm in length corresponding to the NC3 domains, along which globular VWA modules are visible. A thin, ≈70 nm long stiff rod with a kink (arrow), the collagen triple helix, emanates from the central globe connecting NC3 domains. (C) Homo- and heterotrimeric collagen XIIA/XIIB molecules, isolated by immuno-affinity chromatography, as seen after rotary shadowing. XIIA and XIIB subunits of individual molecules were identified from the length of their NC3 domains (≈90 and ≈50 nm, respectively; Watt et al. [1992], Koch et al. [1994]). Images (from top left to bottom right) show collagen XIIA3, A2B, AB2, and B3, respectively. NC3 domains are labeled with A or B; collagen domains with arrows. Bar, 50 nm. (A, C) Modified and reproduced from Koch et al. (1994) and (B) from Koch et al. (1992).
The following region, homologous to the NC4 domain of collagen IX, links collagen XII subunits by disulfide bridges. Towards their C-terminus, three subunits intertwine in a short collagen helix that is interrupted by a small NC2 and ends in a NC1 domain. Electron micrographs of collagen XII molecules (Fig. 1B,C) fit the model predicted from the cDNA sequence. Three thicker, flexible arms correspond to the NC3 domains with their FN3 repeats. Along them, globules are located at positions predicted for VWA modules. The forth arm is a thin stiff, collagenase sensitive rod (Koch et al., 1992). It is kinked at the site of the NC2 domain and ends in the small NC1 globe. Four collagen XII subunit variants exist. One alternative splicing generates NC1 domains of either 19 or 74 amino acids (Kania et al., 1999), with little influence on structure. However, the NC3 domain comes in two very different splice variants, "large" XIIA (described above) and "small" XIIB. In the latter, the entire N-terminal half of NC3 is missing (Fig. 1A). Moreover, collagen XIIA, but not XIIB can occur as proteoglycan, with glycosaminoglycan side chain(s) attached to the alternatively spliced NC3 region. Finally, it is noteworthy that heterotrimeric collagen XII molecules assembled from XIIA and XIIB variants can be isolated (Koch et al., 1995; Fig. 1C).
Expression: Regulation by growth factors and mechanical stress
Collagen XII is widely expressed in collagen I containing mesenchymal tissues in the embryo, especially in developing bone, ligaments, tendons, fibrocartilage, smooth muscle, and skin (Walchli et al., 1994). It is also associated with the collagen II matrix of fetal articular cartilage, mainly of its superficial layers (Watt et al., 1992). After birth, collagen XII becomes restricted to certain dense connective tissues rich in collagen I/III/V fibrils, such as periodontal ligament (Karimbux and Nishimura, 1995), dermis around hair follicles (Berthod et al., 1997) or cornea of the eye (Anderson et al., 2000). Mesenchymal cells in the embryo and fibroblasts in the adult appear to be the primary source of collagen XII. Interestingly, "large" XIIA and "small" XIIB splice variants are expressed differentially, although the functional significance is unknown (Anderson et al., 2000, Koch et al., 1995). Information on the regulation of expression of collagen XII is still limited. Gene and protein are induced during fibrosis (Tzortzaki et al., 2003) and cancer progression (Karagiannis et al., 2012), but the responsible factors have not been identified. A candidate is Tgf-β1, which induced collagen XII mRNA expression in 3D tenocyte cultures (Farhat et al., 2012). In contrast to the sparse information on regulation by soluble factors, several reports showed that tensile strain acting on cells regulates collagen XII production. For example, expression was high when fibroblasts were cultured in attached (stretched) collagen gels, but dropped dramatically when gel constructs were relaxed by detachment from the substrate (Trachslin et al., 1999).
In vivo, collagen XII is induced upon chronic muscle loading (Fluck et al., 2000), and in periodontal ligament during orthodontic tooth movement (Karimbux and Nishimura, 1995). In the chick COL12A1 promoter, an enhancer region responsive to static tensile strain was identified in the first exon (Chiquet et al., 1998). Transcriptional regulators of this region have not been identified. Interestingly, a different enhancer region appears to be required for activation of the murine Col12a1 gene by cyclic strain in osteoblasts, namely an AP1 site binding c-Jun and JunD after strain (Arai et al., 2008). Thus, static versus cyclic tensile strain seem to regulate the collagen XII gene via distinct signaling pathways.
Biological function: Organization of collagen I fibrils and role in osteogenesis
There is no doubt that collagen XII is a fibril-associated collagen. First, immunogold labeling of collagen fibrils in embryonic skin showed specific association of collagen XII molecules with fibril surfaces. Second, cross-striated fibrils decorated with collagen XII could be reconstituted in vitro by co-assembly of collagen I monomers with purified collagen XII (Koch et al., 1995). Collagenase treatment of collagen XII largely abolished incorporation into collagen I fibrils, indicating that the collagenous domain is involved in co-assembly. The large collagen XII NC3 domains extending from collagen fibril surfaces appear to interact with neighboring fibrils indirectly (Fig. 2A). Collagen XII NC3 domains were shown to bind tightly to tenascin-X, an ECM component mutated in Ehlers-Danlos syndrome that interacts with collagen fibrils (Veit et al., 2006). By immuno-electron microscopy, the two proteins were found to colocalize in dermis (Fig. 2B; Veit et al., 2006). With its collagenous domain, collagen XII also binds decorin, fibromodulin (Font et al., 1996) and cartilage oligomeric matrix protein (COMP) (Agarwal et al., 2012), all of which are found on the surface of collagen fibrils. Hence, collagen XII and its binding partners could form flexible bridges between neighboring collagen fibrils that might function to absorb shear stresses upon loading (Fig. 2A). These biochemical and ultrastructural data are supported by the phenotype of Col12a1 knockout mice, which suffer from muscle weakness with decreased passive force generation suggesting increased elasticity of the muscle-tendon unit (Zou et al., 2014). Histologically, the collagen meshwork in bones of Col12a1−/− mice is heavily disorganized (Fig. 2C), and they exhibit skeletal abnormalities such as shorter/slender long bones and kyphosis of the spine (Fig. 2D; Izu et al., 2011). In cultures of Col12a1−/− osteoblasts, the formation of calcified nodules was delayed, and the expression of bone-specific proteins (osteocalcin and osteopontin) suppressed. In addition, gap junction formation and cell polarity were disturbed in Col12a1−/− osteoblasts, and osteocytes in Col12a1−/− bone did not from extensive connections via dendritic processes (Izu et al., 2011). Collagen XII is therefore indispensible for proper osteoblast/osteocyte differentiation, but it is not known yet whether this is mediated by direct interaction of collagen XII with a cellular receptor. The only candidate identified so far is integrin α1β1. However, this interaction is conserved in human and mouse but not chick (M. Koch, unpublished). Moreover, defects in integrin α1-deficient mice do not resemble those of Col12a1−/− mice, indicating that collagenXII-integrin α1 interaction does not explain the phenotype of the latter. Alternatively, collagen XII dependent changes in the structure of the pericellular matrix might affect osteoblast differentiation indirectly by altered mechanotransduction. Accordingly, collagen XII was shown to promote the contraction of collagen I gels by fibroblasts not by activating cells directly, but by changing the mobility of fibrils within the gel (Nishiyama et al., 1994).
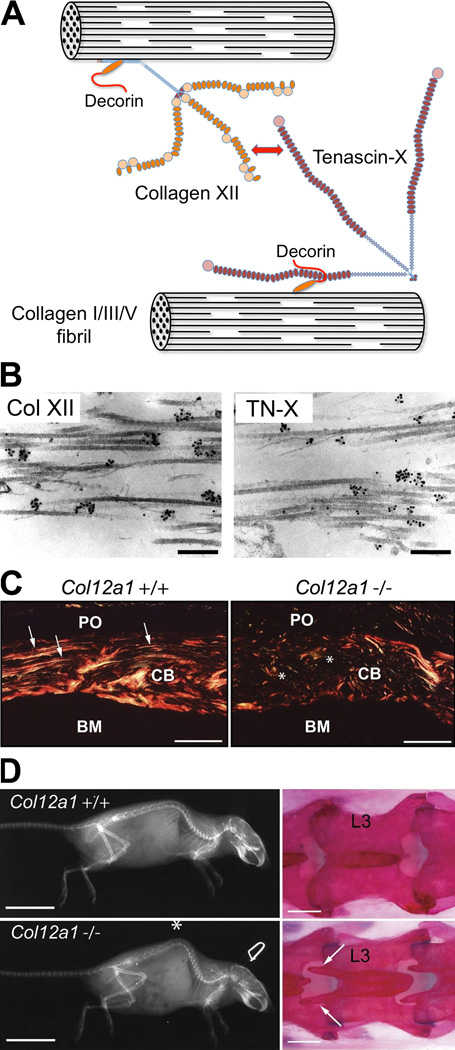
Fig. 2. Function of collagen XII.
(A) Cross-bridging of collagen I-containing fibrils by collagen XII and tenascin-X. Collagen XII interacts with the fibril surface via its collagenous domain; this might be facilitated by a ternary complex with small proteoglycan decorin. NC3 domains of collagen XII extend from the fibril surface and bind tenascin-X, a trimer of subunits with EGF repeats (blue), FN3 domains (purple), and a C-terminal fibrinogen-like globe (pink). Like collagen XII, tenascin-X binds decorin that links it to fibrils. (B) Ultrastructural colocalization of collagen XII and tenascin-X in embryonic dermis. Ultrathin sections were incubated with polyclonal antibodies to collagen XII (left) or tenascin-X (right) followed by colloidal gold-labeled secondary antibody. Note clusters of gold particles associated with cross-striated collagen fibrils in both images. Reproduced from Veit et al. (2006). (C) Disorganized collagen fiber arrangement in bone matrix of collagen XII-deficient mice. Collagen organization was analyzed in femurs of P14 Col12a1+/+ and Col12a1−/− mice under polarized light after Sirius red staining. PO, periosteum; CB, cortical bone, BM, bone marrow. (D) X-ray (left) and alizarin red staining (right) of adult Col12a1+/+ and Col12a1−/− mice. Note kyphosis of the spine (asterisk) and abnormal spinous processes of lumbar vertebrae (marked on vertebra L3 with arrows) in the Col12a1−/− mouse. (A, B) Modified and reproduced from Veit et al. (2006). (C, D) Reproduced from Izu et al. (2011). Bars: 50 nm (B); 50 µm (C); 2 cm (D, left); 1 mm (D, right).
Clinical relevance: COL12A1 mutations causing EDS/myopathy overlap syndrome
Mutations in microfibrillar collagen VI cause congenital Ullrich disease or Bethlem myopathy. These pathologies are characterized by a combination of myopathy with Ehlers-Danlos syndrome (EDS)-like connective tissue disorder, such as hypermobility of distal joints. However, certain patients with very similar symptoms have normal collagen VI genes and expression levels. Very recently, mutations in the COL12A1 gene were identified in some of these cases (Zou et al., 2014; Hicks et al., 2014). In the most affected family, two brothers are homozygous for a splice site mutation that causes nonsense-mediated decay of COL12A1 mRNA and complete absence of the protein. These patients suffer from severe hypotonia, muscle weakness and joint hypermobility, and progressive scoliosis necessitating surgical stabilization. In a second family, a de novo dominant mutation in COL12A1 was identified in a boy with a less severe, improving phenotype. In this case, a point mutation in the Ca-binding domain of the fourth VWA domain was predicted to interfere with binding to the ECM. Accordingly, a much reduced amount of collagen XII protein was detected in the ECM of cell cultures and muscle biopsies derived from this patient, although cellular mRNA and protein expression levels were close to normal (Zou et al., 2014). In two other families with five affected individuals and a Bethlem myopathy-like clinical presentation, heterozygous missense mutations led to intracellular retention of collagen XII although protein expression levels were normal (Hicks et al., 2014). One of these mutations affects the invariant glycine residue of the triple helical Gly-X-Y motif, but the full functional changes caused by them remain to be elucidated. In addition, a specific COL12A1 gene polymorphism has been associated with anterior cruciate ligament ruptures in women (Posthumus et al., 2010).
In summary, these new findings demonstrate that mutations in collagen XII are causative for human connective tissue pathologies. Moreover, they establish an informative link between the intriguing structure and molecular interactions of this FACIT and its function in maintaining extracellular matrix integrity in load-bearing connective tissues of the locomotory system.
Acknowledgements
Own work was supported by grants from the Swiss NSF (MC), NIH grant NIAMS AR044745 (DEB), intramural funds from NINDS/NIH (CGB), DFG SFB 829 grant A7 and the Köln Fortune Programme of the Medical Faculty (MK).
References
- Agarwal P, Zwolanek D, Keene DR, Schulz JN, Blumbach K, Heinegard D, Zaucke F, Paulsson M, Krieg T, Koch M, Eckes B. Collagen XII and XIV new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J Biol Chem. 2012;287:22549–22559. doi: 10.1074/jbc.M111.335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, SundarRaj S, Fite D, Wessel H, SundarRaj N. Developmentally regulated appearance of spliced variants of type XII collagen in the cornea. Invest Ophthalmol Vis Sci. 2000;41:55–63. [PubMed] [Google Scholar]
- Arai K, Nagashima Y, Takemoto T, Nishiyama T. Mechanical strain increases expression of type XII collagen in murine osteoblastic MC3T3-E1 cells. Cell Struct Funct. 2008;33:203–210. doi: 10.1247/csf.08025. [DOI] [PubMed] [Google Scholar]
- Berthod F, Germain L, Guignard R, Lethias C, Garrone R, Damour O, van der Rest M, Auger FA. Differential expression of collagens XII and XIV in human skin and in reconstructed skin. J Invest Dermatol. 1997;108:737–742. doi: 10.1111/1523-1747.ep12292122. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Mumenthaler U, Wittwer M, Jin W, Koch M. The chick and human collagen alpha1(XII) gene promoter--activity of highly conserved regions around the first exon and in the first intron. Eur J Biochem. 1998;257:362–371. doi: 10.1046/j.1432-1327.1998.2570362.x. [DOI] [PubMed] [Google Scholar]
- Dublet B, Oh S, Sugrue SP, Gordon MK, Gerecke DR, Olsen BR, van der Rest M. The structure of avian type XII collagen. Alpha 1 (XII) chains contain 190-kDa non-triple helical amino-terminal domains and form homotrimeric molecules. J Biol Chem. 1989;264:13150–13156. [PubMed] [Google Scholar]
- Dublet B, van der Rest M. Type XII collagen is expressed in embryonic chick tendons. Isolation of pepsin-derived fragments. J Biol Chem. 1987;262:17724–17727. [PubMed] [Google Scholar]
- Dublet B, van der Rest M. Type XIV collagen, a new homotrimeric molecule extracted from fetal bovine skin and tendon, with a triple helical disulfide-bonded domain homologous to type IX and type XII collagens. J Biol Chem. 1991;266:6853–6858. [PubMed] [Google Scholar]
- Farhat YM, Al-Maliki AA, Chen T, Juneja SC, Schwarz EM, O'Keefe RJ, Awad HA. Gene expression analysis of the pleiotropic effects of TGF-beta1 in an in vitro model of flexor tendon healing. PLoS One. 2012;7:e51411. doi: 10.1371/journal.pone.0051411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck M, Tunc-Civelek V, Chiquet M. Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by loading of skeletal muscle. J Cell Sci. 2000;113(Pt 20):3583–3591. doi: 10.1242/jcs.113.20.3583. [DOI] [PubMed] [Google Scholar]
- Font B, Eichenberger D, Rosenberg LM, van der Rest M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996;15:341–348. doi: 10.1016/s0945-053x(96)90137-7. [DOI] [PubMed] [Google Scholar]
- Gerecke DR, Olson PF, Koch M, Knoll JH, Taylor R, Hudson DL, Champliaud MF, Olsen BR, Burgeson RE. Complete primary structure of two splice variants of collagen XII assignment of alpha 1(XII) collagen (COL12A1), alpha 1(IX) collagen (COL9A1), and alpha 1(XIX) collagen (COL19A1) to human chromosome 6q12-q13. Genomics. 1997;41:236–242. doi: 10.1006/geno.1997.4638. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Gerecke DR, Olsen BR. Type XII collagen: distinct extracellular matrix component discovered by cDNA cloning. Proc Natl Acad Sci U S A. 1987;84:6040–6044. doi: 10.1073/pnas.84.17.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D, Farsani GT, Laval S, Collins J, Sarkozy A, Martoni E, Shah A, Zou Y, Koch M, Bonnemann CG, Roberts M, Lochmuller H, Bushby K, Straub V. Mutations in the collagen XII gene define a new form of extracellular matrix-related myopathy. Hum Mol Genet. 2014 Jan 14; doi: 10.1093/hmg/ddt637. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Izu Y, Sun M, Zwolanek D, Veit G, Williams V, Cha B, Jepsen KJ, Koch M, Birk DE. Type XII collagen regulates osteoblast polarity and communication during bone formation. J Cell Biol. 2011;193:1115–1130. doi: 10.1083/jcb.201010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania AM, Reichenberger E, Baur ST, Karimbux NY, Taylor RW, Olsen BR, Nishimura I. Structural variation of type XII collagen at its carboxyl-terminal NC1 domain generated by tissue-specific alternative splicing. J Biol Chem. 1999;274:22053–22059. doi: 10.1074/jbc.274.31.22053. [DOI] [PubMed] [Google Scholar]
- Karagiannis GS, Petraki C, Prassas I, Saraon P, Musrap N, Dimitromanolakis A, Diamandis EP. Proteomic signatures of the desmoplastic invasion front reveal collagen type XII as a marker of myofibroblastic differentiation during colorectal cancer metastasis. Oncotarget. 2012;3:267–285. doi: 10.18632/oncotarget.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimbux NY, Nishimura I. Temporal and spatial expressions of type XII collagen in the remodeling periodontal ligament during experimental tooth movement. J Dent Res. 1995;74:313–318. doi: 10.1177/00220345950740010501. [DOI] [PubMed] [Google Scholar]
- Koch M, Bernasconi C, Chiquet M. A major oligomeric fibroblast proteoglycan identified as a novel large form of type-XII collagen. Eur J Biochem. 1992;207:847–856. doi: 10.1111/j.1432-1033.1992.tb17116.x. [DOI] [PubMed] [Google Scholar]
- Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M. Large and small splice variants of collagen XII: differential expression and ligand binding. J Cell Biol. 1995;130:1005–1014. doi: 10.1083/jcb.130.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunstrum GP, McDonough AM, Marinkovich MP, Keene DR, Morris NP, Burgeson RE. Identification and partial purification of a large, variant form of type XII collagen. J Biol Chem. 1992;267:20087–20092. [PubMed] [Google Scholar]
- Nishiyama T, McDonough AM, Bruns RR, Burgeson RE. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate extracellular matrix deformability. J Biol Chem. 1994;269:28193–28199. [PubMed] [Google Scholar]
- Posthumus M, September AV, O'Cuinneagain D, van der Merwe W, Schwellnus MP, Collins M. The association between the COL12A1 gene and anterior cruciate ligament ruptures. Br J Sports Med. 2010;44:1160–1165. doi: 10.1136/bjsm.2009.060756. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachslin J, Koch M, Chiquet M. Rapid and reversible regulation of collagen XII expression by changes in tensile stress. Exp Cell Res. 1999;247:320–328. doi: 10.1006/excr.1998.4363. [DOI] [PubMed] [Google Scholar]
- Trueb J, Trueb B. The two splice variants of collagen XII share a common 5' end. Biochim Biophys Acta. 1992;1171:97–98. doi: 10.1016/0167-4781(92)90145-p. [DOI] [PubMed] [Google Scholar]
- Tzortzaki EG, Tischfield JA, Sahota A, Siafakas NM, Gordon MK, Gerecke DR. Expression of FACIT collagens XII and XIV during bleomycin-induced pulmonary fibrosis in mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1073–1080. doi: 10.1002/ar.a.10120. [DOI] [PubMed] [Google Scholar]
- Veit G, Hansen U, Keene DR, Bruckner P, Chiquet-Ehrismann R, Chiquet M, Koch M. Collagen XII interacts with avian tenascin-X through its NC3 domain. J Biol Chem. 2006;281:27461–27470. doi: 10.1074/jbc.M603147200. [DOI] [PubMed] [Google Scholar]
- Walchli C, Koch M, Chiquet M, Odermatt BF, Trueb B. Tissue-specific expression of the fibril-associated collagens XII and XIV. J Cell Sci. 1994;107(Pt 2):669–681. doi: 10.1242/jcs.107.2.669. [DOI] [PubMed] [Google Scholar]
- Watt SL, Lunstrum GP, McDonough AM, Keene DM, Burgeson RE, Morris NP. Characterization of collagen types XII and XIV from fetal bovine cartilage. J Biol Chem. 1992;267:20093–20099. [PubMed] [Google Scholar]
- Yamagata M, Yamada KM, Yamada SS, Shinomura T, Tanaka H, Nishida Y, Obara M, Kimata K. The complete primary structure of type XII collagen shows a chimeric molecule with reiterated fibronectin type III motifs, von Willebrand factor A motifs, a domain homologous to a noncollagenous region of type IX collagen, and short collagenous domains with an Arg-Gly-Asp site. J Cell Biol. 1991;115:209–221. doi: 10.1083/jcb.115.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Zwolanek D, Izu Y, Gandhy S, Schreiber G, Brockmann K, Devoto M, Tian Z, Hu Y, Veit G, Meier M, Stetefeld J, Hicks D, Straub V, Voermans NC, Birk DE, Barton ER, Koch M, Bonnemann CG. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Hum Mol Genet. 2014 Jan 20; doi: 10.1093/hmg/ddt627. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]