Abstract
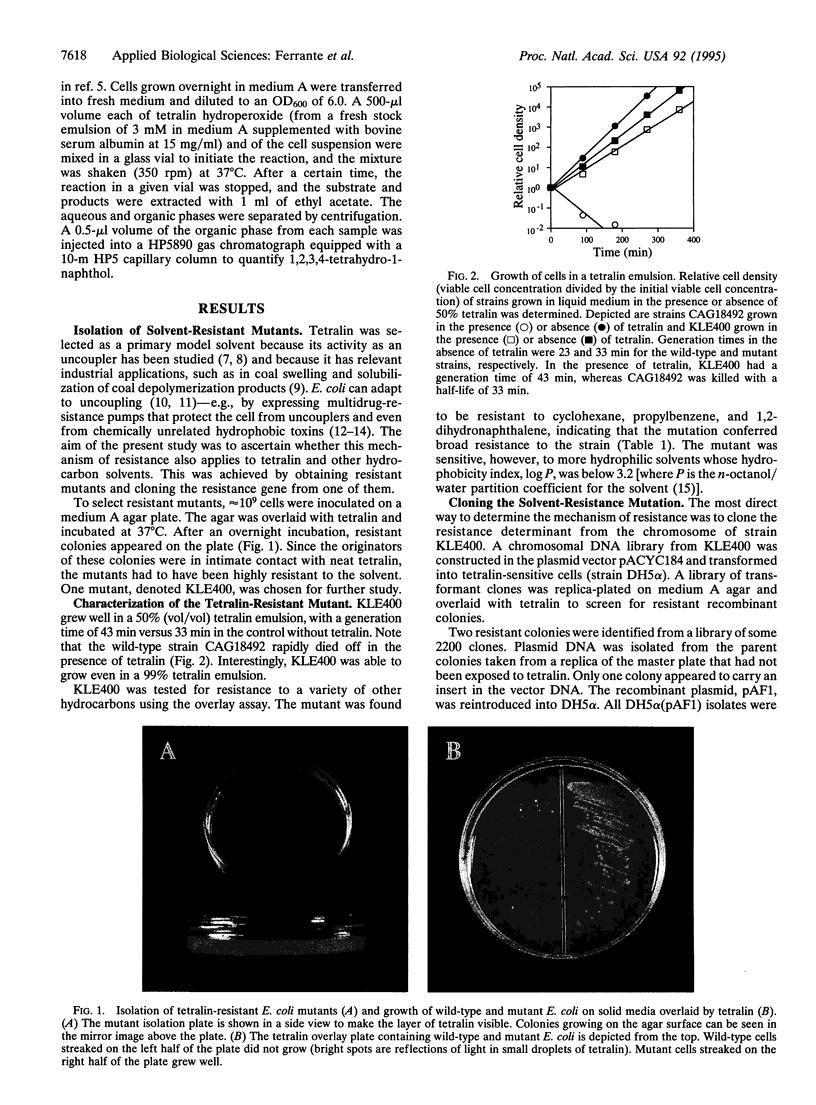
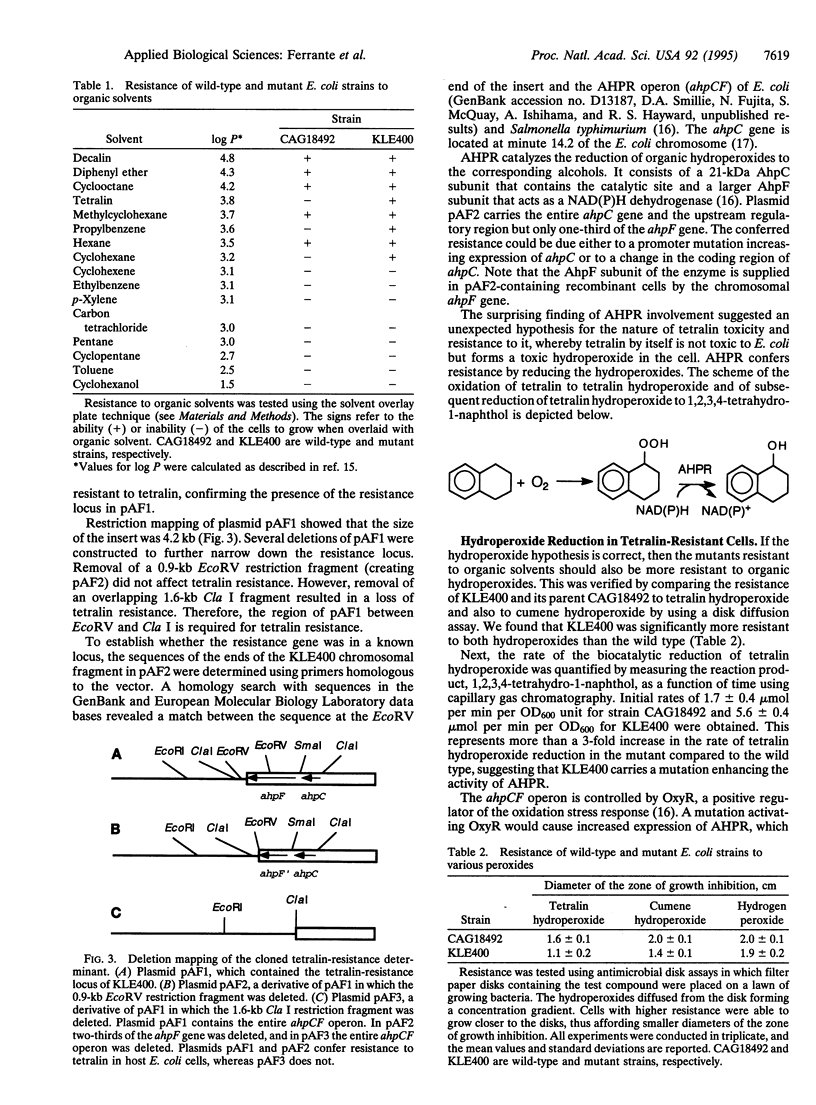
Although bacterial strain able to grow in the presence of organic solvents have been isolated, little is known about the mechanism of their resistance. In the present study, 1,2,3,4-tetrahydronaphthalene (tetralin), a solvent with potential applications in industrial biocatalysis, was used to select a resistant mutant of Escherichia coli. The resultant mutant strain was tested for resistance to a wide range of solvents of varying hydrophobicities and was found to be resistant not only to tetralin itself but also to cyclohexane, propylbenzene, and 1,2-dihydronaphthalene. A recombinant library from mutant DNA was used to clone the resistance gene. The sequence of the cloned locus was determined and found to match the sequence of the previously described alkylhydroperoxide reductase operon ahpCF. The mutation was localized to a substitution of valine for glycine at position 142 in the coding region of ahpC, which is the gene encoding the catalytic subunit of the enzyme. The ahpC mutant was found to have an activity that was three times that of the wild type in reducing tetralin hydroperoxide to 1,2,3,4-tetrahydro-1-naphthol. We conclude that the toxicity of such solvents as tetralin is caused by the formation of toxic hydroperoxides in the cell. The ahpC mutation increases the activity of the enzyme toward hydrophobic hydroperoxides, thereby conferring resistance. The ahpC mutant was sensitive to the more hydrophilic solvents xylene and toluene, suggesting that there are additional mechanisms of solvent toxicity. Mutants resistant to a mixture of xylene and tetralin were isolated from the ahpC mutant but not from the wild-type strain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chae H. Z., Robison K., Poole L. B., Church G., Storz G., Rhee S. G. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yamada M., Sato H., Matsue M., Taketani S., Nakayama K., Sugita Y., Bannai S. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J Biol Chem. 1993 Sep 5;268(25):18633–18636. [PubMed] [Google Scholar]
- Jacobson F. S., Morgan R. W., Christman M. F., Ames B. N. An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem. 1989 Jan 25;264(3):1488–1496. [PubMed] [Google Scholar]
- Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994 Mar;19(3):119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Lewis K., Naroditskaya V., Ferrante A., Fokina I. Bacterial resistance to uncouplers. J Bioenerg Biomembr. 1994 Dec;26(6):639–646. doi: 10.1007/BF00831539. [DOI] [PubMed] [Google Scholar]
- Lomovskaya O., Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D., Cook D. N., Alberti M., Pon N. G., Nikaido H., Hearst J. E. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J Bacteriol. 1993 Oct;175(19):6299–6313. doi: 10.1128/jb.175.19.6299-6313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naroditskaya V., Schlosser M. J., Fang N. Y., Lewis K. An E. coli gene emrD is involved in adaptation to low energy shock. Biochem Biophys Res Commun. 1993 Oct 29;196(2):803–809. doi: 10.1006/bbrc.1993.2320. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994 Apr 15;264(5157):382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- Sikkema J., Poolman B., Konings W. N., de Bont J. A. Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J Bacteriol. 1992 May;174(9):2986–2992. doi: 10.1128/jb.174.9.2986-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema J., de Bont J. A., Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994 Mar 18;269(11):8022–8028. [PubMed] [Google Scholar]
- Smillie D. A., Hayward R. S., Suzuki T., Fujita N., Ishihama A. Locations of genes encoding alkyl hydroperoxide reductase on the physical map of the Escherichia coli K-12 genome. J Bacteriol. 1992 Jun;174(11):3826–3827. doi: 10.1128/jb.174.11.3826-3827.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia L. A., Storz G., Brodsky M. H., Lai A., Ames B. N. Alkyl hydroperoxide reductase from Salmonella typhimurium. Sequence and homology to thioredoxin reductase and other flavoprotein disulfide oxidoreductases. J Biol Chem. 1990 Jun 25;265(18):10535–10540. [PubMed] [Google Scholar]
- Weber F. J., Isken S., de Bont J. A. Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994 Aug;140(Pt 8):2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]




