Abstract
Accurate measurements are needed to target insulin resistance in CKD. Among older men with and without moderate CKD, Jia and colleagues compared insulin resistance estimated from glucose and insulin concentrations obtained while fasting or during an oral glucose tolerance test to insulin resistance measured by the gold standard hyperinsulinemic euglycemic clamp and tested associations of each with mortality. These findings move forward the study of insulin resistance in CKD and generate new questions for future work.
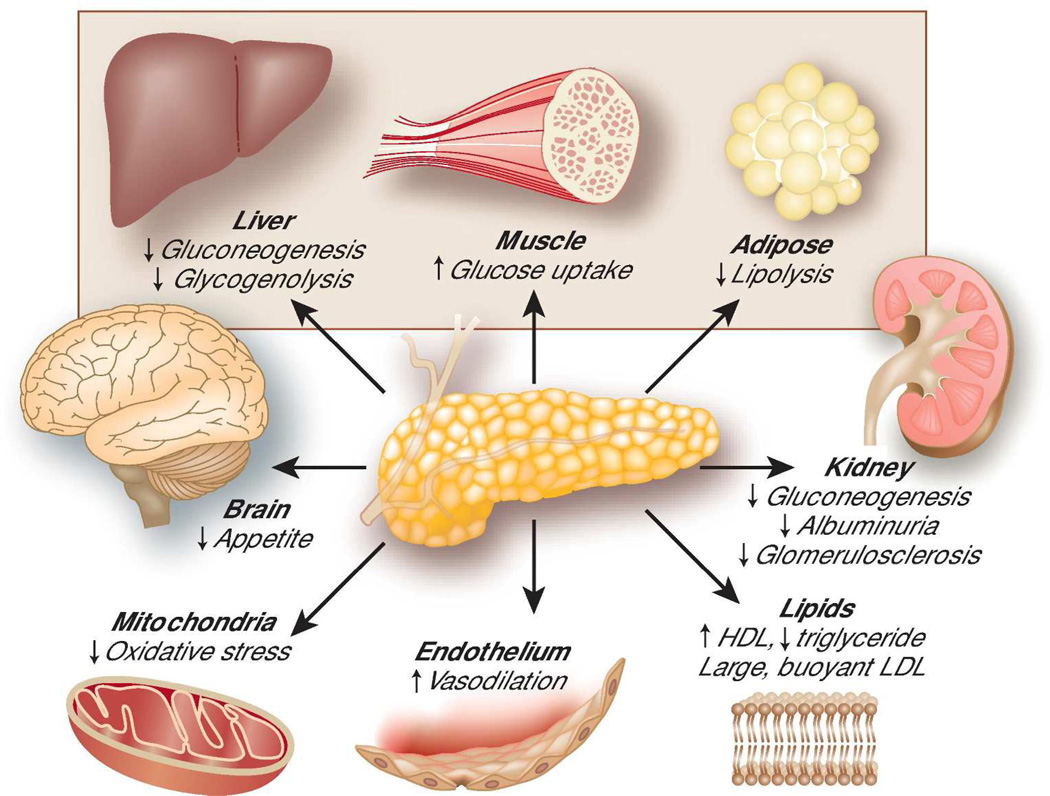
In 1931, Professor Wilhelm Falta published the theory that decreased sensitivity of target cells to the physiologic actions of insulin – in other words, insulin resistance – leads to the development of diabetes mellitus.1 Insulin is the central hormone regulating glucose homeostasis and signaling the body that it has entered the fed state. After a meal, pancreatic beta cells secrete insulin in response to increased blood nutrient concentrations. Insulin then suppresses hepatic glycolysis and gluconeogenesis, inhibits lipolysis in adipose tissue, and stimulates skeletal muscle to take up glucose, all actions that shift nutrients from the circulation into cells (Figure). When the liver, adipose tissue, and muscle are resistant to these effects, the pancreas may secrete more insulin, thereby maintaining euglycemia at the expense of hyperinsulinemia. The failure of pancreatic beta cells to compensate for insulin resistance leads to overt type 2 diabetes. As decreased physical activity and obesity have led to widespread insulin resistance in developed countries, Falta’s theory has proved correct, and an epidemic of diabetes has ensued.
Figure. Selected actions of insulin.
Insulin is a key regulator of glucose homeostasis through effects on liver, muscle, and adipose tissue (shaded area). Insulin also affects other organs and tissues. Decreased sensitivity of target organs to insulin – or insulin resistance – leads to impaired glucose homeostasis and dysfunction of other insulin-sensitivity organs. HDL = high density lipoprotein; LDL = low density lipoprotein.
Over time, it has become clear that insulin also has important effects beyond regulation of energy homeostasis. In particular, insulin resistance is closely coupled with mitochondrial dysfunction, generation of reactive oxygen species, endothelial dysfunction, and other cardiovascular risk factors (Figure). Though causal relationships among these processes are difficult to disentangle, insulin resistance may contribute to these harmful processes. Human studies suggest that these effects may be clinically relevant, with multiple observational cohort studies reporting associations of insulin resistance with increased risks of cardiovascular events and death.2,3 Of additional interest to nephrologists, insulin has direct effects on multiple cells in the kidney. Induction of insulin resistance in podocytes leads to glomerulosclerosis in animal models, and insulin resistance is associated with albuminuria and the development of CKD in observational human studies.4
Building on work performed in the 1960’s, DeFronzo and colleagues published a series of manuscripts from 1978–1983 documenting severe insulin resistance in hemodialysis patients, specifically those without overt diabetes.5 These studies used a technique called the hyperinsulinemic euglycemic clamp to directly measure whole body glucose uptake in response to an insulin stimulus. Using the euglycemic clamp and related techniques, DeFronzo and others demonstrated that ESRD patients are very insulin resistant, that the site of insulin resistance is localized to skeletal muscle, and that insulin resistance partially improves with dialysis.5 Recent studies have identified specific uremic toxins that may mediate this effect, including gut-derived molecules such as p-cresyl sulfate.6 These observations, coupled with the epidemiology of insulin resistance and cardiovascular disease described above, suggest that insulin resistant may explain, in part, the accelerated cardiovascular disease observed in patients with CKD. Accurate, valid measures of insulin resistance are needed to determine the extent to which insulin resistance is a therapeutic target and, if so, to develop interventions to improve insulin sensitivity.
The euglycemic clamp is still the gold standard for measuring insulin resistance. To perform this procedure, a constant infusion of insulin is administered and a dextrose infusion is titrated to maintain euglycemia. At steady state (constant blood insulin and glucose concentrations), the rate of dextrose administration is equal to the rate of glucose uptake into cells. This rate of dextrose administration represents a direct measure of whole body insulin sensitivity.
Because the euglycemic clamp is time-consuming, invasive, and expensive, most recent studies of the prevalence and health consequences of insulin resistance have relied on simpler estimates of insulin resistance. Estimates can assess insulin resistance in the static (fasting) state, in which fasting glucose and insulin concentrations largely reflect hepatic insulin resistance, or in the dynamic state (challenged with glucose or insulin, as with the euglycemic clamp), in which skeletal muscle plays a larger role.2 The homeostatic model assessment of insulin resistance (HOMA-IR), based on a mathematical equation describing glucose regulation by insulin as a feedback loop, is a common static estimate. Dynamic estimates are commonly based on a glucose challenge during an oral glucose tolerance test (OGTT). In the general population, either type of estimate correlates well with insulin resistance measured using the euglycemic clamp (correlation coefficients generally 0.7–0.9).
However, thus far, estimates of insulin resistance have not been well validated in CKD. In health, the kidney catabolizes approximately half of insulin in the systemic circulation.7 The insulin concentrations used to estimate insulin resistance could therefore also reflect renal insulin clearance. Moreover, fasting glucose concentrations are determined largely by hepatic glucose production and may not reflect insulin resistance in skeletal muscle, the site of the most important defects in CKD. For these reasons, estimates of insulin resistance generated in the general population need to be validated in people with CKD prior to their application in the CKD population.
In this regard, the paper by Jia and colleagues in this issue of Kidney International makes an important contribution to the evaluation of insulin resistance in CKD.8 The authors analyzed data collected in 1991–1995 as part of the Uppsala Longitudinal Study of Adult Men. 1,074 Swedish men ages 70–71 years not treated for diabetes were evaluated using the hyperinsulinemic euglycemic clamp. The performances of insulin resistance estimated from fasting measurements of glucose and insulin or from glucose and insulin measured during an OGTT were compared to results from the clamp. Analyses were stratified by GFR estimated from cystatin C: <60 mL/min/1,73m2 (CKD, N=495) versus ≥60 mL/min/1.73m2 (normal estimated GFR, N=579). The evaluation of insulin resistance using the euglycemic clamp in such a large population, including a substantial proportion with CKD, is unprecedented.
Jia and colleagues report that the correlation of insulin resistance estimates based on fasting glucose and insulin performed slightly less well in participants with versus without CKD, though differences in performance by CKD status were not statistically significant. For example, the correlation of HOMA-IR with clamp insulin sensitivity was −0.67 with CKD versus −0.71 without CKD.8 (The negative sign reflects estimation of insulin resistance versus insulin sensitivity.) Insulin resistance estimates derived from the OGTT performed better. For example, among people with CKD, the correlation of the Matsuda index (a dynamic insulin sensitivity estimate derived from glucose and insulin measured during an OGTT) with clamp insulin sensitivity was 0.74. Improved performance of OGTT-derived versus fasting estimates was also observed in analyses examining predictive accuracy, receiver operating characteristic curves, and reliability. Together, these data suggest that estimates of insulin resistance based on fasting measurements perform reasonably well in the studied population. The data also support the hypothesis that estimates based on dynamic testing (the OGTT) perform better.
Surprisingly, Jia and colleagues found no significant, independent association of insulin resistance with all-cause or cardiovascular mortality over long-term follow-up, whether insulin resistance was estimated or measured by clamp. Expected associations of insulin resistance with increased mortality risk were evident in unadjusted analyses. With adjustment for traditional cardiovascular risk factors, these associations weakened and became insignificant. However, a relatively small number of events was observed, particularly after dividing into groups with and without CKD, and confidence intervals still included associations with high clinical relevance. In addition, the study consisted of older Scandinavian men, and results may not generalize to younger ages, other races and ethnicities, or women. Therefore, insulin resistance should not be ruled out as an important risk factor for cardiovascular disease and death based on these results, particularly given prior studies supporting this link.
Of course, more remains to be learned about the measurement of insulin resistance in CKD. While insulin resistance estimates correlated reasonably well clamp data, correlations were not perfect. What are the determinants of estimated insulin resistance other than insulin resistance itself? Do these determinants vary by CKD status? Could these determinants bias associations of insulin resistance with health outcomes? For example, Goodarzi and colleagues recently demonstrated in a non-CKD population using the euglycemic clamp that fasting insulin concentration, the main determinant of static estimates of insulin resistance, was determined more by insulin clearance than by insulin resistance itself.9 Insulin clearance is likely to vary by GFR and may bias the relationship of HOMA-IR with true insulin resistance, at least at low GFR. It is possible that novel estimates using tailored combinations of fasting and post-challenge glucose and insulin may better reflect insulin resistance in CKD. Alternatively, other biomarkers such as adiponectin and leptin may enhance the estimation of insulin resistance.10
Moreover, the measurement and health implications of insulin resistance should be evaluated over a broader range of GFR. In the study by Jia and colleagues, participants with CKD had a median estimated GFR of 46 mL/min/1.73m2 and only 10% had an estimated GFR <39 mL/min/1.73m2. Estimates of insulin resistance still have not been adequately validated in more severe CKD or in ESRD. These are the CKD stages in which performance of insulin resistance estimating equations may suffer most and the population in which the cardiovascular implications of insulin resistance may be greatest. Studies of patients with more severe CKD can start by modeling the work presented in this issue of Kidney International.
Acknowledgements
IHdB receives grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Blood, and Lung Institute (R01DK087726, R01DK088762, and R01HL096875). RM receives grant funding from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1DK095668). The authors appreciate the input of Leila Zelnick with regard to interpretation of statistical models.
Footnotes
The authors report no conflicts of interest.
Contributor Information
Ian H. de Boer, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
Rajnish Mehrotra, Division of Nephrology and Kidney Research Institute, Department of Medicine, University of Washington, Seattle, WA.
References
- 1.Falta W, Boller R. Insularer und Insulinresistenter Diabetes. Kkinische Wochenschrift. 1931;10(10):438–443. [Google Scholar]
- 2.Pham H, Utzschneider KM, de Boer IH. Measurement of insulin resistance in chronic kidney disease. Current opinion in nephrology and hypertension. 2011 Aug 31; doi: 10.1097/MNH.0b013e32834b23c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham H, Robinson-Cohen C, Biggs ML, et al. Chronic kidney disease, insulin resistance, and incident diabetes in older adults. Clin J Am Soc Nephrol. 2012 Apr;7(4):588–594. doi: 10.2215/CJN.11861111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Cosmo S, Menzaghi C, Prudente S, Trischitta V. Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 2013 Jan;28(1):29–36. doi: 10.1093/ndt/gfs290. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981 Feb;67(2):563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppe L, Pillon NJ, Vella RE, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013 Jan;24(1):88–99. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984 Sep;27(3):351–357. doi: 10.1007/BF00304849. [DOI] [PubMed] [Google Scholar]
- 8.Jia T. Kidney Int. 2014 [Google Scholar]
- 9.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab. 2011 Aug;301(2):E402–E408. doi: 10.1152/ajpendo.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung AM, Sundell MB, Egbert P, et al. A comparison of novel and commonly-used indices of insulin sensitivity in African American chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011 Apr;6(4):767–774. doi: 10.2215/CJN.08070910. [DOI] [PMC free article] [PubMed] [Google Scholar]