Abstract
BACKGROUND
Early diagnosis and endoscopic resection of adenomatous polyps is the main approach for screening and prevention of colorectal cancer (CRC). We aimed to assess polyp detection rate (PDR) and to characterize demographic, clinical, and pathological features of colorectal polyps in an Iranian population.
METHODS
We retrospectively analyzed the data from 5427 colonoscopies performed during 2007-2012 at Masoud Clinic, the main endoscopy center associated with Sasan Alborz Biomedical Research Center, in Tehran, Iran.
RESULTS
Our sample included 2928 (54%) women and 2499 (46%) men, with the mean age of 48.3 years (SD=16.1). The most common reasons for colonoscopy included screening in 25.0%, and gastrointestinal bleeding in 15.2%. Cecal intubation was successful in 86% of patients. The quality of bowel preparation was fair to excellent in 78.1% (n=4235) of colonoscopies. Overall PDR was 42.0% (95% CI: 40.6-43.3). The PDR in men (51.1%, 95% CI: 49.1-53.1) was significantly higher than women (34.2%, 95% CI: 32.4-35.9, p<0.001). Polyps were more frequently observed in patients after the 6th decade of life (F=3.2; p=0.004). CRC was detected in 2.9% (73/2499) of men and 1.9% (57/2928) of women (p=0.02). The mean age for patients with cancer was significantly higher than that for individuals with polyps, 60.9 (SD=13.4) year vs. 56.9 (SD=13.7) year, respectively (p=0.001). Almost 82.8% of the lesions were precancerous with tubular type predominance (62.3%) followed by tubulo-villous (10.3%), villous (6.6%), and serrated (3.6%). Hyperplastic/inflammatory polyps comprised 17.2% of lesions.
CONCLUSION
Distal colon was more prone to develop polyps and cancer than proximal colon in our series. These findings provide a great infrastructure for next preventive programs and have implications for colorectal cancer screening at population-level.
Keywords: Colon Cancer, Colonoscopy, Colonic Polyps
INTRODUCTION
Colorectal cancer (CRC) is the third most prevalent cancer in men and the second in women; accounting for 8% (n=608,700) of all cancer deaths worldwide 1 The highest increase in the incidence of colon cancer are in the Eastern Europe and Asia.1,2 Recent cancer statistics indicate a decreasing trend in CRC incidence in the Unites States because of the increase in timely detection and removal of precursor lesions through colonoscopy.3
Colorectal cancer is also the third most common cancer in Iranians excluding the skin cancers. It occurs at younger ages with an increasing trend similar in the Asia-Pacific countries.1,4 These increasing rates may result from the young age-structure and low rates of colon cancer in older people of these countries.2,5,6
Colon carcinomas mostly arise from adenomatous polyps and the time span for the transition process is estimated to nearly 10 years on average.7,8 Given the slow progression of colorectal adenomas into invasive adenocarcinoma,9 early detection and endoscopic resection of these precancerous lesions, have been claimed to be effective in decreasing both the incidence and mortality rate of CRC.10-12 There is a report that colonic precancerous lesions (adenomas) with a high prevalence tend to present at younger ages, therefore undergoing screening among asymptomatic adults aged 50 years for adenomas and CRC is strongly recommended.13
There is scant knowledge about the prevalence of colorectal polyps and polyp detection rate (PDR) in Iranian adult population. To the best of our knowledge, only few studies are available in the national literature that assessed colorectal polyps,14-17 but none has explicitly noted the rate of polyp detection and most of them are biased because of their small sample size. Nevertheless, our study provides comprehensive information about clinical and epidemiological features of colorectal polyps, using a relatively large sample of patients undergoing colonoscopy.
The mass screening of colorectal cancer is not yet available in Iran, therefore updating the current knowledge in the scope of colorectal polyps and CRC is essential. Hence, identifying the features of colon polyps (e.g., age of onset, changes in sub-sites distribution, location, and histology type) have great implications for developing national screening guidelines for CRC.18 The aims of the current study were to measure PDR, and to evaluate the clinical and histological characteristics of colorectal polyps in an Iranian population.
MATERIALS AND METHODS
Study design
We conducted a cross-sectional study and retrospectively assessed the colonoscopy database and pathology reports maintained by Masoud Clinic, a well-known gastrointestinal endoscopy clinic in Tehran, Iran. The Institutional Review Board of Digestive Disease Research Institute, Tehran University of Medical Sciences, approved the study protocol.
Patients, procedures and measures
We included all patients aged 15 to 90 years, who underwent their first time colonoscopy from June 2007 to March 2013. The patients with a personal history of colon cancer and polyposis were excluded from the study. Twenty two gastroenterologists certified by the Iranian National Board of Gastroenterology and Hepatology performed the procedures using two high-quality colonoscopes (OLYMPUS CV-240, and Pentax EPK-1000) under conscious sedation.
We collected the data on patients’ demographic variables, indications for colonoscopy, quality of bowel preparation, and the rate of successful cecal insertion. For all colorectal lesions, data on clinical and pathological features (i.e., number, size, site, and grade of dysplasia) were obtained.
Pathological features of colorectal lesions were determined using the World Health Organization criteria 19 as follows: hyperplastic, precancerous (serrated, tubular, tubular-villous, and villous), and cancer. The overall polyp detection rate (PDR) was defined as the proportion of procedures in which at least one polyp was detected over the total number of colonoscopies.
The following definitions were used to tabulate the proportion of polyps detected by different colonic segments. Proximal colon included transverse colon, hepatic flexure, ascending colon and cecum. Distal colon included rectum, sigmoid, descending colon, and splenic flexure.
Statistical analysis
We reviewed the endoscopic data and pathology records. Patient-level data were used for the estimates of PDR, and summary-level data for presenting pathology features and anatomic site of polyps. Histograms were developed to demonstrate polyp characteristics, i.e., size, counts, and proportion per patient. Categorical data were tested between subgroups using the Chi-square test or the Fisher exact test, where appropriate. Continuous data were presented as means (SD), and 95% confidence interval (CI). The Student t test was used for comparisons of means. For statistical significance we considered a p value of 0.05 applying 2-tailed statistical tests. All statistical analyses were performed using Stata/MP software, version 11. Plots were created in R, version 2.15.1.
RESULTS
Demographics and colonoscopy findings
All patients (n=5427) aged 15 to 90 years who underwent their first time colonoscopy from June 2007 to March 2013 were included in this study. Our sample included 2928 (54%) women and 2499 (46%) men, with the mean age of 48.3 years (SD=16.1). The most common reasons for colonoscopy included screening (i.e., asymptomatic adults aged 50 years and older, and first degree relatives of patients with CRC) in 25.0% (n=1356), and gastrointestinal bleeding in 15.2% (n=824). Other indications for colonoscopy were classified as follows: 7.5% abdominal pain (n=405), 8.5% inflammatory bowel disease (n=462), 6.3% suspected irritable bowel syndrome (n=346). In 37.5% (n=2034) of the patients, indications for colonoscopy were not noted (table 1).
Table1. Patients’ characteristics and colonoscopy findings .
| Variable | All (n=5427) | |
| Age, mean years (SD) | 48.3 (16.1) | |
| Sex, Male/Female, n (%) | 2499 (46.0)/2928 (54.0) | |
| Indication, n (%) | Screening | 1356 (25.0) |
| Bleeding | 824 (15.2) | |
| Abdominal pain | 405 (7.5) | |
| Inflammatory bowel disease | 462 (8.5) | |
| Irritable bowel syndrome | 346 (6.3) | |
| Unspecified | 2034 (37.5) | |
| Preparation quality, n (%) | Excellent-to-Fair | 4235(78.1) |
| Poor-to-Unsatisfactory | 522 (9.6) | |
| Unspecified | 670 (12.3) | |
| Cecal intubation, n (%) | Yes | 4660 (86.0) |
| No | 767 (14.0) | |
| Patients with at least 1 polyp, n (%) | 2277 (42.0) | |
| Cancer, n (%) | 130 (2.4) |
Cecal intubation was successful in 86% (n=4660) patients. The quality of bowel preparation was excellent to fair in 78.1% (n=4235) of colonoscopies vs. 9.6% (n=522) with poor to unsatisfactory preparation. However, bowel cleansing was not mentioned for 12.3% (n=670) of examinations. Approximately, 42.0% (n=2277) of patients had at least one polyp, and cancer was detected in 2.4% (n=130) of patients (table 1).
Study outcomes
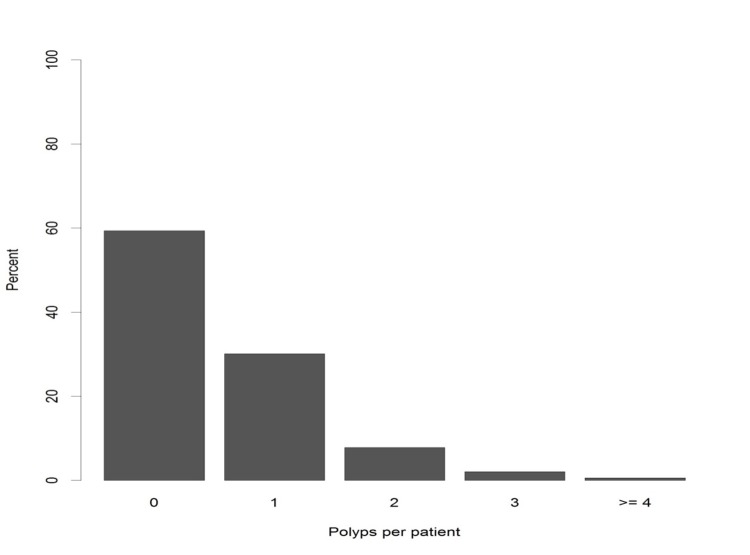
On the basis of colonoscopy reports, the overall PDR was 42.0% (95% CI: 40.6-43.3). Figure 1 depicts the overall distribution of polyps per patient, where the high proportion of patients with 1 or 2 polyps detected, is visible (figure 1). Almost 56.1% (n=1277/2277) of patients, who had at least one polyp, were men. The PDR in men (51.1%, 95% CI: 49.1-53.1) was significantly higher than that in women (34.2%, 95% CI: 32.4-35.9, P<0.001). The mean age of patients with polyp was 56.9 (SD=13.7) years. Polyps were more frequently observed in patients after the 6th decade of life (F=3.2, p=0.004, table 2).
Fig.1 .
Overall proportion of colon polyps per patien
Table 2. Polyp detection rates and cancer prevalence by age-group (n=5427) .
| <30 yrs. (n=916) | 30-39 yrs. (n=890) | 40-49 yrs. (n=1006) | 50-59 yrs. (n=1227) | 60-69 yrs. (n=908) | >=70 yrs. (n=480) |
Total
(n=5427) |
|
| Polyp, no (%) | 82 (8.9) | 179 (20.1) | 322 (32.0) | 633 (51.6) | 648 (71.4) | 413 (86.0) | 2277 (42.0) |
| Cancer, no (%) | 6 (0.7) | 9 (1.0) | 18 (1.8) | 29 (2.4) | 34 (3.7) | 34 (7.1) | 130 (2.4) |
Colorectal cancer was detected in 2.9% (73/2499) of men and 1.9% (57/2928) of women, suggesting a significantly higher prevalence among men compared with women (p=0.02). Age specific prevalence of CRC is shown in table 2, presenting a peak in the prevalence of CRC after the seventh decade of life (table 2). The mean age of patients with cancer was significantly higher than individuals with polyps, 60.9 (SD=13.4) year versus 56.9 (SD=13.7) year, respectively (p=0.001).
Characteristics of colonic lesions
A total of 3058 polyps were removed by colonoscopy. Data about the size of polyps were available for 1838 polyps; of these, 30% (n=549) were more than 10 mm. Additional information on size distribution are highlighted in figure 2, showing a higher proportion of polyps sized 10-20 mm and ≥ 20 mm in distal colon compared with the proximal colon.
Fig. 2 .
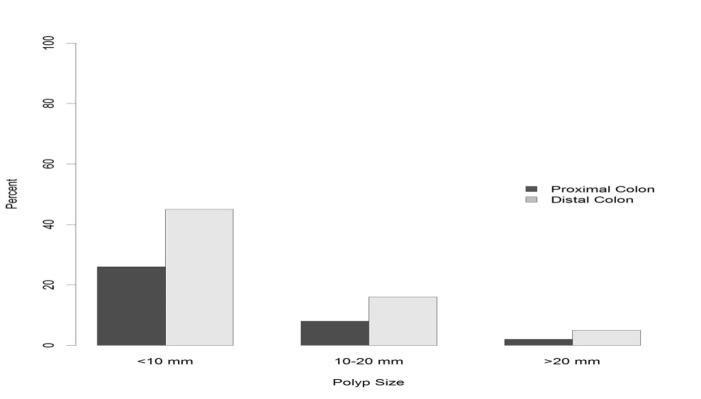
Size distribution of colon polyps per colonic segments
Table 3 shows the distribution of cancer and polyps, in different colonic segments. Overall, polyps were frequently detected in sigmoid (26.8%), rectum (19.0%), and transverse colon (15.5%). The same colonic distribution was observed for polyps’ ≥10 mm in size. Likewise, cancer was more frequently observed in sigmoid (40.0%), rectum (26.2%), and transverse colon (10.0%), (table 2). The prevalence of polyps in the distal colon was higher than that of the proximal colon (64.5% vs. 35.4%, respectively, p<0.001). Accordingly, most of the cancers were localized in the distal colon compared with the proximal colon (72.5% vs. 26.1, respectively, p<0.001).
Table 3. Distribution of polyps (count and size*) and cancer by colonic segments .
| Rectum | Sigmoid | Descending colon | Splenic flexure | Transverse colon | Ascending colon | Hepatic flexure | Cecum | ||
| Cancer (n=130) | 52(40.0) | 34 (26.2) | 8 (6.2) | 4 (3.1) | 13 (10.0) | 10 (7.7) | 2 (1.5) | 7 (5.3) | |
| Polyps* (n=3023) | 573(19.0) | 811(26.8) | 400 (13.2) | 55 (1.8) | 470 (15.5) | 339 (11.2) | 163 (5.4) | 212(7.0) | |
| Polyps**<10 mm (n=1289) | 312 (17) | 330(18.0) | 154(8.4) | 24(1.3) | 203 (11.0) | 119 (6.5) | 56 (3) | 91 (4.9) | |
| Polyps >=10 mm (n=549) | 119 (6.5) | 185(10.0) | 65(3.6) | 10(0.5) | 63(3.5) | 61(3.3) | 22(1.2) | 24(1.3) |
*Location of 35 polyps was not specified; **Size of 1220 polyps was not available.
Colonic distribution of polyps by histology was presented in table 4. Analysis of summary-level data for pathology reports indicated that 82.8% of lesions were precancerous with tubular type predominance (62.3%) followed by tubulo-villous (10.3%), villous (6.6%), and serrated (3.6%). Hyperplastic/inflammatory polyps comprised 17.2% of lesions. Precancerous lesions (i.e., adenomas and serrated polyps) with higher proportion appeared in distal colon in comparison with the proximal colon (48.2% vs. 33.6 %, respectively, table 4). High grade of dysplasia was reported among 19.5% (n=445) of resected polyps.
Table 4. Colonic* distribution of polyp count by histologic type, number (%) .
| Hyperplastic/inflammatory | Serrated | Tubular | Tubulo-villous | Villous | |
| Proximal colon (n=1184) | 157 (13.3) | 26 (2.2) | 869 (73.4) | 84 (7.1) | 48 (4.0) |
| Distal colon (n=1839) | 364 (19.8) | 85 (4.6) | 1012 (55.0) | 228 (12.4) | 150 (8.2) |
*Location of 35 polyps was not specified.
DISCUSSION
We have reported here the features of colorectal neoplasia from a referral gastroenterology clinic using a relatively large database of colonoscopy. The overall estimate for PDR in our patients was 42.0%, which would be correspondent to more than 34% rate of adenoma detection.
Older age is the most important predictor for the prevalence of adenomas, and cancer .20 In our study, the PDR and cancer prevalence reached a peak in the 6th and 8th decades of life, respectively. These data are consistent with findings reported by Bafandeh, Mirzaie, and their colleagues.14-16,21 Studies from the Middle East and the western countries also mentioned significant increase for the risk of CRC, in particular after the age of 50 years.20,22 Our patients with cancer were significantly older, 4 years on average, than patients with polyps. This relatively small level of difference in mean age is sensible, even though a difference of 10 years that is compatible with time span required for transformation of a polyp to carcinoma, were explicitly noted by other studies.15,16 Given the increased prevalence of CRC in the sixth decade of life, the age threshold to start screening for individuals with average risk is 50 years.23,24
The risk of developing polyps and cancer in colon is greater in men than in women.25,26 Our study showed significantly higher rates for both polyps and cancer among men compared with women, which is in line with current evidence that indicates male sex is an important risk factor for polyps and colon cancer.21,25-27 , Moreover, other reports from Iran support sex differences in the prevalence of colon polyps and cancer.4,14,16,28
The tubular type was the most common histological feature of adenomas in the present study, in accordance with the results of other reports.14,16,29 Distal colon was more prone to develop polyps and cancer than proximal colon in our series, comparable with results from the Asian and the Western countries.13,20,23 However, little evidence exists for the rightward shift of colonic polyps and cancer17,18,29 across Iranian population. Such an assumption was not further supported by the results of the current study and others.18,28,30 Nonetheless, because of the significance of adenomas and neoplasms present in proximal colon,18 complete colonoscopy is recommended in screening guidelines for colon cancer.23
Strengths of the current study included use of a relatively large sample of adult patients, and equal number of both sexes. The major limitation of our study was the absence of interface between our pathology reports and endoscopic database, which prevented us from estimating the detection rate of adenoma, and addressing the predictive factors for them. Finally, our sample included mostly symptomatic patients, in which the estimates may be different from screening studies with asymptomatic individuals.
In summary, data presented here may provide an infrastructure for the next preventive programs and have clinical implications for colorectal cancer screening at population-level programs. Screening-based studies, however, are required to probe the clinical and epidemiological aspects of colorectal polyps and cancer in Iran.
ACKNOWLEDGMENTS
The authors thank Mohammad Masoud Malekzadeh for data processing, and all gastroenterologists and pathologists in Masoud Clinic for their contributions in performing colonoscopies, and reviewing the pathology slides.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Delavari AR, Mardan F, Salimzadeh H, Bishehsari F, Khosravi P, Khanehzad M, Nasseri-Moghaddam S, Merat S, Ansari R, Vahedi H, Shahbazkhani B, Saberifiroozi M, Sotoudeh M, Malekzadeh R. Characteristics of Colorectal Polyps and Cancer; a Retrospective Review of Colonoscopy Data in Iran. Middle East J Dig Dis 2014;6:144-150.
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Yiu HY, Whittemore AS, Shibata A. Increasing colorectal cancer incidence rates in Japan. Int J Cancer. 2004;109:777–81. doi: 10.1002/ijc.20030. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN. et al. Annual report to the nation on the status of cancer, 1975‐2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somi MH, Mirinezhad K, Farhang S, Jazayeri E, Sani A, Seif-Farshad M. et al. Gastrointestinal cancer occurrence in East Azarbaijan: a five year study from North Western Iran. Asian Pac J Cancer Prev. 2006;7:309–12. [PubMed] [Google Scholar]
- 5.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 6.Ansari R, Mahdavinia M, Sadjadi A, Nouraie M, Kamangar F, Bishehsari F. et al. Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer lett. 2006;240:143–7. doi: 10.1016/j.canlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Ann Rev Pathol. 2009;4:343–64. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]
- 8.Levine JS, Ahnen DJ. Adenomatous polyps of the colon. N Engl J Med. 2006;355:2551–7. doi: 10.1056/NEJMcp063038. [DOI] [PubMed] [Google Scholar]
- 9.Huang CS, Farraye FA, Yang S, O'Brien MJ. The clinical significance of serrated polyps. Am J Gastroenterol. 2010;106:229–40. doi: 10.1038/ajg.2010.429. [DOI] [PubMed] [Google Scholar]
- 10.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF. et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E. et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 12.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover J. et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 13.Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C. et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA. 2011;306:1352–8. doi: 10.1001/jama.2011.1362. [DOI] [PubMed] [Google Scholar]
- 14.Mirzaie AZ, Abolhasani M, Moghaddam RM, Kabivar M. The Frequency of gastrointestinal polyps in Iranian population. Iranian Journal of Pathologhy. 2012;7:183–9. [Google Scholar]
- 15.Bafandeh Y, Khoshbaten M, Sadat ATE, Farhang S. Clinical predictors of colorectal polyps and carcinoma in a low prevalence region: results of a colonoscopy based study. World J Gastroenterol. 2008;14:1534–8. doi: 10.3748/wjg.14.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bafandeh Y, Daghestani D, Esmaili H, Aharizad S. Distribution of cancer and adenomatous polyps in the colorectum: study in an Iranian population. Asian Pac J Cancer Prev. 2006;7:65–8. [PubMed] [Google Scholar]
- 17.Khatibzadeh N, Ziaee S, Rahbar N, Molanie S, Arefian L, Fanaie S. The indirect role of site distribution in high-grade dysplasia in adenomatous colorectal polyps. J Cancer Res Ther. 2005;1:204–7. doi: 10.4103/0973-1482.19587. [DOI] [PubMed] [Google Scholar]
- 18.Eshghi MJ, Fatemi R, Hashemy A, Aldulaimi D, Khodadoostan M. A retrospective study of patients with colorectal polyps. Gastroenterol Hepatol Bed Bench. 2010;4:17–22. [PMC free article] [PubMed] [Google Scholar]
- 19. Morson BC, Sobin LH. Histological typing of intestinal tumours: World Health Organization Geneva; 1976.
- 20.Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1272–8. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 21.Bafandeh YD, Daghestani Daghestani, Heidar E. Demographic and anatomical survey of colorectal polyps in an Iranian population. Asian Pac J Cancer Prev. 2005;6:537–40. [PubMed] [Google Scholar]
- 22.Nam JH, Yang CH. Clinical characteristics and risk factors of colon polyps in Gyeongju and Pohang area. Korean J Gatroenterol. 2008;52:142–9. [PubMed] [Google Scholar]
- 23.Sung JJ, Lau JY, Young GP, Sano Y, Chiu H, Byeon J. et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut. 2008;57:1166–76. doi: 10.1136/gut.2007.146316. [DOI] [PubMed] [Google Scholar]
- 24.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS. et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi‐Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 25.Brenner H, Hoffmeister M, Arndt V, Haug U. Gender differences in colorectal cancer: implications for age at initiation of screening. Br J Cancer. 2007;96:828–31. doi: 10.1038/sj.bjc.6603628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brenner H, Hoffmeister M, Stegmaier C, Brenner G, Altenhofen L, Haug U. Risk of progression of advanced adenomas to colorectal cancer by age and sex: estimates based on 840 149 screening colonoscopies. Gut. 2007;56:1585–9. doi: 10.1136/gut.2007.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen SP, Bent S, Chen Y-H, Terdiman JP. Gender as a risk factor for advanced neoplasia and colorectal cancer: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:676–81. doi: 10.1016/j.cgh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Omranipour R, Doroudian R, Mahmoodzadeh H. Anatomical distribution of colorectal carcinoma in Iran: a retrospective 15-yr study to evaluate rightward shift. Asian Pac J Cancer Prev. 2012;13:279–82. doi: 10.7314/apjcp.2012.13.1.279. [DOI] [PubMed] [Google Scholar]
- 29.Khodadoostan M, Fatemi R, Maserat E. Clinical and pathological characteristics of colorectal polyps in Iranian population. East Afr J Public Health. 2010;7:157–9. [PubMed] [Google Scholar]
- 30.Hosseini SV, Izadpanah A, Yarmohammadi H. Epidemiological changes in colorectal cancer in Shiraz, Iran: 1980− 2000. ANZ J Surg. 2004;74:547–9. doi: 10.1111/j.1445-2197.2004.03064.x. [DOI] [PubMed] [Google Scholar]