Abstract
With 7.6 million deaths globally, cancer according to the World Health Organisation is still one of the leading causes of death worldwide. Interleukin 17 (IL-17) is a cytokine produced by Th17 cells, a T helper cell subset developed from an activated CD4+ T-cell. Whilst the importance of IL-17 in human autoimmune disease, inflammation, and pathogen defence reactions has already been established, its potential role in cancer progression still needs to be updated. Interestingly studies have demonstrated that IL-17 plays an intricate role in the pathophysiology of cancer, from tumorigenesis, proliferation, angiogenesis, and metastasis, to adapting the tumour in its ability to confer upon itself both immune, and chemotherapy resistance. This review will look into IL-17 and summarise the current information and data on its role in the pathophysiology of cancer as well as its potential application in the overall management of the disease.
1. Introduction
Cancer, according to the World Health Organisation, is still one of the leading causes of death worldwide, accounting for 7.6 million deaths globally. In fact, recent statistics from the United States of America show that one in four deaths in the USA is now caused by cancer [1]. With such numbers, it is prudent therefore to continue finding new and innovative ways in treating this disease. This review will look into interleukin 17 (IL-17) and summarise the current information and data on its role in the pathophysiology of cancer as well as its potential application in the overall management of the disease.
2. IL-17: Biology and Function
IL-17 is a cytokine produced by Th17 cells, a T helper cell subset developed from an activated CD4+ T cell [2]. Currently, six IL-17 family members have been identified, IL-17 A through to F. The prototypic family member has been identified as IL-17A, whilst IL-17F shows the highest degree of homology to IL-17A out of all the remaining IL-17 family members [3].
Studies have shown that the key factor stimulating the generation of Th17 cells from naïve T-cells in humans is transforming growth factor-β (TGF-β), which along with certain inflammatory cytokines (either TGF-β with IL-21 or TGF-β with IL-6 and IL-23) induces the transcription factor (ROR-gammat) [4, 5]. The activation of ROR-gammat leads then to the development of naïve T-cells to cells which produce IL-17. This process has already been reviewed by Miossec et al. [6].
The main role of IL-17 in humans is in host pathogen defence, in particular to extracellular bacterial and fungi infections. In bacterial infections, the release of IL-17 stimulates a massive inflammatory response, leading to neutrophil accumulation and, in certain intra-abdominal infections, may even end with the formation of abscesses [7]. When IL-17 is missing, susceptibility to extracellular bacterial and fungi infections has been shown to exist.
Studies in IL-17 deficient mice showed increased susceptibility when infected with Klebsiella pneumonia, Toxoplasmosis gondii, and Candida albicans [8–10]. When infected with Aspergillus fumigatus and Candida albicans, there was more extensive growth of these fungi [11]. In patients with hyper-IgE syndrome, a defect in signal transducer and activator of transcription-3 (STAT3) causes a failure to generate sufficient IL-17 releasing T-cells. The consequent lack of IL-17 has been linked with the high susceptibility of these patients to Staphylococcus aureus and Candida albicans infections [12, 13]. Whilst the release of IL-17 plays a key role in preventing infection and maintaining health, the deregulation of IL-17 promotes disease, in particular inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease (IBD). In rheumatoid arthritis, it has been shown that through the activation of T-cells via the IL-17/IL-23 axis, the subsequent Th17 cells become osteoclastogenic, inducing the expression of receptor activator of nuclear factor-κB ligand (RANKL) on both T-cells and osteoblasts, which in turn activates bone destruction and resorption via osteoclasts [14], with IL-17 levels even predictive of damage progression in patients [15].
Interestingly new research suggests another potential pathway for the deregulation of IL-17 expression in arthritis. Whilst previous data has shown that forkhead box protein P3 (Foxp3) expressing Treg cells is key in suppressing immune responses and prevents the development of autoimmune diseases, Komatsu et al. have recently shown that Foxp3+ T-cells can actually convert into pathogenic Th17 cells (named, in the study, as exFoxp3 TH17 cells) with arthritogenic and autoreactive properties. This, they conclude, is via the plasticity of Foxp3+ T-cells, with unstable Foxp3+ T-cells converting to the IL-17 expressing exFoxp3 TH17 cells, thus providing an almost paradoxical pathway on the association between IL-17 and disease [16, 17].
In patients with IBD, it has been consistently shown that their mucosa displays significantly higher levels of IL-17A than normal or ischaemic colitis mucosa, both in vitro and in vivo [18–21]. In addition, the severity of inflammation and clinical activity corresponded to the degree of IL-17A expression in IBD with no significant difference observed between ulcerative colitis and Crohn's disease, the two main subcategories of IBD [22]. The role of IL-17 is further supported by knockout murine studies of the IL-17 receptor, which showed a subsequent attenuation of the inflammatory effects of IBD [23]. Whilst the importance of IL-17 in human autoimmune disease, inflammation, and pathogen defence reactions has already been established, its potential role in cancer needs to be updated.
2.1. IL-17 in Cancer
2.1.1. Tumorigenesis
The role of IL-17 in cancer starts from the initial stages of tumourigenesis having already been established as having a role in the earliest formation of a tumour by its increased presence within the tumour microenvironment [24]. In terms of the protumour role, there is strong evidence stemming from IL-17's role in chronic inflammation as highlighted above. Its protumour role was highlighted in its positive association with increased malignancy of tumours [25]. This is due to the vast accumulation of IL-17 secreting Foxp3+ cells in the tumour microenvironment with a dual function of proinflammation and regulation of local T-cell function [26]. This is achieved by blocking the entry of cytotoxic CD8 T-cells and the entry of myeloid derived suppressor cells (MDSCs), thus modifying the local environment and subsequently reducing the local immune response towards the local tumour cells [27]. These MDSCs which enter the environment then respond to the secretion of heat shock protein (HSP) 72 on exosomes from the tumour cells and stimulate the MDSC immune dampening effects [26]. This increased entry of MDSCs and their subsequent function have been associated with a reduced survival in renal cell carcinoma [28]. Despite suppressing antitumour inflammation, IL-17 has also been associated with maintaining the chronic inflammation found either in the preceding condition [29] or within the tumour tissue [30]. This takes place in response to the release of lactate from the tumour cells resulting in increased release of IL-17, in particular IL-17A, via IL-23 dependent and independent pathways [31, 32]. The subsequent rise in IL-17 can go on to trigger the release of proinflammatory cytokine IL-6 and the consequent activation of the STAT3 pathway [33]. This activation of STAT3 has subsequently been linked with the activation of the NF-κB pathway [34] which is associated with the production of proinflammatory cytokines [35]. IL-17, however, has been also shown to activate NF-κB in combination with TNF-α via the IL-17B receptor [36, 37]. Alternatively, the tumour cells are also able to perpetuate the local inflammatory response through the expression of CC chemokine ligand 2 (CCL 2) which stimulates entry of IL-17 expressing monocytes which can contribute to the local inflammatory reaction [38]. This is believed to be responsible for the tumour's ability to maintain the proinflammatory reaction locally and also continue to stimulate tumour growth, particularly in prostate cancer [39]. A possible underlying mechanism for this is again through the IL-17 activation of the STAT3 and NF-κB pathways leading to expression of antiapoptotic genes such as Bcl-2, A1, and Mcl 1 [40, 41]. These antiapoptotic factors work to increase survival of stem cells within the respective tissues to promote growth of cells with protumorigenic potential [42]. Zepp and coworkers, however, found a further alternative pathway through IL-17 receptors A and C dependent Act 1 recruitment of tumour necrosis factor receptor associated factor (TRAF) 4 and subsequent activation of the MAPKs ERK5, ERK1/2, and JNK in colonic stem cells. This provides a core set of cells and the perfect environment for the growth of the tumour.
2.1.2. Tumour Proliferation
IL-17's role in malignancy is not only limited to setting the perfect environment and base for tumour formation but is also responsible for the proliferation of the tumour cells. This has stemmed from evidence showing the involvement of the NF-κB pathway leading to proliferation of synovial cells in rheumatoid arthritis via the mTOR pathway [43]. This is supported by evidence that IL-17 is associated with the increase in proportion of multiple myeloma cells, acting on haematopoietic stem cells resulting in significant expansion of myeloma cells [44]. This may be explained by IL-17's effect on human bone marrow-derived mesenchymal stem cells (hMSCs) where they are able to stimulate the production of reactive oxygen species (ROS) via the activation of Rac1 GTPase and NADPH oxidase 1 (Nox1) and subsequent activation of the MEK-ERK pathway leading to proliferation [45]. Huang et al. also found that the mechanism of activation was very similar to that found in the colonic stem cells used by Zepp et al. involving Act 1 and TRAF 4.
Despite the evidence supporting the proliferative role of IL-17 in cancer, there is counterevidence that shows that IL-17, particularly when added via immunisation, can trigger an antitumour response and cause a reduction in tumour size. This has been demonstrated by immunisation of Th17 cells into a tumour environment trigger activation of cytotoxic CD8+ T-cells [46]. The activation of this has been associated with subsequent tumour shrinkage via IL-2 and major histocompatibility complex/peptide (pMHC)-I [47]. This enables the activation of the local immunity through the stimulation of an antitumour T-cell response [48]. Endogenous T-cells have also been shown to be highly effective at reducing tumour size in an in vitro model of malignancy where mice are inoculated with malignant cells [49]. However, it is important to note that in both cases, nonautologous Th17 and colon cancer cells were used and therefore may be a less accurate representation of the clinical picture.
2.1.3. Tumour Angiogenesis/Angiostasis
All four characters of inflammation, dolor, calor, rubor, and tumor, could at least in part be accounted for by vascular changes. Karin described how NF-κB and angiogenesis are an important link driving inflammation towards cancer [50] and in the same year Folkman highlighted that the oncogene-driven transition process from inflammation to cancer also influenced the host's prognosis [51]. Angiogenesis has received a lot of attention due to its influence on the tumour grade, metastasis, and therefore patients' prognosis; over-expression of its hallmark cytokine, vascular endothelial growth factor (VEGF), is well known amongst various malignant cancers often with poorer outcomes [52–56]. It is a crucial part of the tumour microenvironment [57] and the literature so seems to map out more pathways of Th17-mediated angiogenesis [25, 27, 33, 51, 57–91] than it does for its inhibition [64, 79, 80, 92–98]. However, the potential to reduce tumour growth via the latter opens doors in the search for potential therapeutic intervention beyond the traditional direct angiogenesis inhibitors for example, bevacizumab (VEGF inhibitor) [99]. This also raises fundamental questions as to why, where, and how such variation takes place. Tumours from different cell lines producing IL-17, the signature cytokine of Th17 cells, conveyed conflicting profiles with regard to angiogenesis. Th17's variable role has also been put down to the different properties of the cytokines it produces. Ultimately, there seems to be missing links in the tumour microenvironment at different tissues that tilts the homeostatic balance of cytokines [100, 101] that push Th17 cells' function towards proangiogenic or proangiostatic outcomes.
Proangiogenesis. High densities of Th17 cells infiltrating tumours have been associated with increased angiogenicity in studies from human subjects, gastric [25], colorectal [87], hepatocellular [82], and pancreatic [86] cancers. The gastric cancer patients whose tumours had higher levels of Th17 infiltration with greater IL-17 and IL-23 mRNA expression suffered from deeper invading disease with higher incidence of lymphatic involvement [25]. Similar to gastric cancer, pancreatic cancer patients' tumours showed a more aggressive behaviour with greater IL-17 and IL-23 expression. Increased expression of VEGF correlating with IL-17 production was found to be an independent factor worsening patients' prognosis [87]. Higher microvascular density (MVD) and lymphocyte infiltration were also seen with IL-17 in hepatocellular carcinoma patients who subsequently exhibited higher mortality rate and reduced survival [82]. Animal models echoed these findings; for example, increased expression of IL-17 in mice transfected with fibrosarcoma cells was associated with tumours exhibiting higher MVD through upregulation of VEGF, keratinocyte-derived chemokine (KC or CXCL1 and CXCL 2), macrophage inflammatory protein-2 (MIP-2), prostaglandins PGE1 and PGE2, and nitric oxide (NO) production by fibroblasts and stromal cells [66]. The consequence of IL-17's interaction with VEGF production by stromal cells and fibroblasts was associated with enhanced levels of TGF-β [74], which in this case was found to promote VEGF-receptor expression on endothelial cells [65]. An important update from Chung et al. [90] revealed Th17 cells forming a paracrine network that induced IL-17-dependent angiogenesis that was also independent of VEGF. Involved in the network were cancer-associated fibroblasts that secrete G-CSF in response to IL-17 via MEK-1/2 signaling and NF-κB; the products recruit MDSC, for example, CD11+Gr1+ cells [27]. These immature myeloid-derived cells suppress the immune response targeting cancer cell destruction and also promote VEGF-independent angiogenesis [27, 90, 102]. The consequent resistance to conventional chemotherapy is discussed later.
Numasaki et al. also demonstrated IL-17's net angiogenic effects on human nonsmall cell lung cancer (NSCLC) cells in immune-compromised mice via CXCL-8 (IL-8) production and its actions on its receptor (CXCR-2) on endothelial cells via selective production of the following angiogenic cytokines: CXCL-1, CXCL-5, CXCL-6, and CXCL-8 [71]. Among these CXCL-1 is a potent chemoattractant for neutrophils whilst CXCL-5 exert a similar but lesser attraction for neutrophils [79]. It is also known that IL-17 enhances ICAM-1 expression in fibroblasts [24] thus promoting neutrophil adherence and recruitment towards the tumour site. Clinically, neutrophil recruitment has also recently been associated with higher MVD and therefore a more aggressive disease in gastric cancer [91]. IL-17-induced IL-8 release was shown with human cervical cancer [62] and in human glioblastoma cell lines [61]; interestingly the latter study also demonstrated a dose-dependent increase in IκB-α mRNA expression, whose product protein is required for NF-κB expression. This transcription factor is well characterized to produce numerous proinflammatory cytokines including IL-6 and IL-8 [103]; an IL-6-mediated promotion of angiogenesis by IL-17 is noted in another in vivo study looking at human melanoma [33] and IL-8 is known to be a potent stimulator of angiogenesis and tumour growth [78]. One interesting study also outlined an IL-6-mediated angiogenic cytokine production and tumour growth pathway linked to STAT3 in B16 melanoma and MB49 bladder carcinoma cell lines [33]. Here their findings also outline the independent production of endogenous IL-17 from the tumour cells of IL-17 (−/−) mice. They indicate that IFN-γ may have a proangiogenic function since double knockout IL-17 (−/−) and IFN-γ (−/−) mice were resistant to tumour growth like their IL-17 (−/−) mice [93].
Antiangiogenesis. As well as producing the signature cytokine IL-17A (often referred to as IL-17), Th17 cells also secrete IL-17F, IL-21, and IL-22 [101]. Starnes et al. [94] introduced IL-17F's novel role of angiogenesis by depicting a dose-response inhibition of capillary tubule formation in vitro; they hypothesised that this may be via TGF-β (which in itself is a puzzle by having both pro- and antitumour effects [102]). Subsequently IL-17F has successfully demonstrated direct inhibition of IL-6, IL-8, and VEGF expression of hepatocellular carcinoma (HCC) in vitro [97]. It was also associated with reduced vascular cell proliferation. Their immune-deficient murine models' tumours were also smaller with reduced vascularity. A group recently demonstrated reduced CD31+ cell infiltration as well as diminished VEGF production with higher levels of IL-17F in an in vivo study of colon cancer [98]. Interestingly they could not reproduce the same VEGF patterns in vitro reflecting the potential missing pieces of the tumour microenvironment in vivo. Therefore continued work is needed to fully map out the mechanism by which tumours switch the IL-17-associated equilibrium in favour of angiogenesis to confer its ability to invade and metastasise, thereby adding further molecular targets for potential pharmacological intervention. Perhaps the context-dependent function of IL-17 [104] calls for work directed towards specific types of cancer.
2.2. IL-17 and Tumour Metastasis
Again conflicting reports have arisen from IL-17's role in tumour growth and metastasis. Some IL-17 knockout models showed faster growth and a more aggressive metastatic picture [46, 96]; one of these studies [96] highlighted the potency of endogenous IL-17 in activating the innate immune cells. Here they demonstrated a significant reduction in the number of IFN-γ-positive NK, CD8+, and CD4+ cells within the tumours as well as tumour-draining lymph nodes. Since IFN-γ is a potent antitumour cytokine [24, 102], these results suggest that endogenous IL-17 could have a protective role against metastasis. However there are some important mechanisms outlined so far that point to IL-17-mediated promotion of metastasis. Angiogenesis plays an important part in tumour metastasis [24, 57, 101, 102, 105] since basic science dictates that tissues require blood supply for survival at their secondary sites. In addition, the mechanism of lymphangiogenesis is also significant since this promotes spread to lymph vessels [105] by immune cell trafficking [106–108] that includes infiltration by Th17 cells. As clarified by Lohela et al. [109], VEGF-A acting on the receptor VEGF-R2 mediates angiogenesis whilst VEGF-C and VEGF-D are the key cytokines involved in lymphangiogenesis by stimulating VEGF-R3. Intratumoural IL-17 is able to induce the production of VEGF-C and VEGF-D that correlated with increased lymphatic formation as well as worse prognosis in NSCLC patients [110]. Recently gastric cancer also exhibited similar correlation between Th17 cells and cancer metastasis and subsequent prognosis; this study focused on TGF-β's induction on IL-17 expression [91]. Chauhan et al. [111] interestingly displayed in murine cornea (an area normally free from angiogenesis/lymphangiogenesis) how IL-17 was able to induce the expression of VEGF-A and VEGF-C that relied on the presence of IL-1β in vitro. Their results showed that IL-17 could directly stimulate VEGF-D production on its own whilst IL-1β could stimulate VEGF-A and VEGF-C by itself, an effect augmented upon the addition of IL-17. Thus IL-17's positive influence on both angiogenesis and lymphangiogenesis would have contributed for the worst prognosis in patients with the various cancers studied above (in the angiogenesis section).
2.3. IL-17 and Chemoresistance
Inhibition of VEGF's proangiogenic actions or antagonising its receptor (mechanism mentioned above) has been the mainstay of adjuvant chemotherapy against a variety of cancers [112]. The effects are however temporary and cancers are known to restart further growth [113]. Suggested mechanisms of forming resistance may perhaps be viewed as similar to antimicrobials. (1) The target cells become resistant to the subject anti-VEGF agent; (2) tumours may begin to proceed with angiogenesis independent of VEGF thereby rendering the agent useless; or (3) invades surrounding tissues and incorporating preexisting blood vessels to feed the tumour [112]. The key study by Chung et al. [90] unveiled a mechanism for anti-VEGF resistance (as introduced above) that confirmed the first two suggestions. Upon treatment with anti-VEGF antibodies the subject tumours recruited higher numbers of CD4+ CD8+ T-cells (although CD4+ numbers were 10 times higher than CD8+); specifically the IL-17+-IL-22+-CD4+ (mature Th17) lymphocytic infiltration was associated with higher CD11b+Gr1+ cells and amplified expression of both G-CSF and IL-17. IL-17 levels were also raised in the peripheral circulation. Taken together the “IL-17-G-CSF” axis was associated with angiogenesis independent of anti-VEGF therapy and promotion of tumour growth with the recruit of immune-suppressive and proangiogenic CD11b+Gr1+ cells. Contributing to this may be the IL-17-dependent Bv8 secretion produced within tumours despite VEGF-blockade. They also managed to demonstrate how a simple addition of IL-17 to the tumour microenvironment conferred the new resistance, which may suggest that, as far as current knowledge is concerned, IL-17 does not need to rely on other T-cells for developing resistance. It is of note that G-CSFR deficient mice showed similar levels of tumour suppression as those deficient in IL-17RC upon treatment with anti-VEGF antibodies. It is also worth mentioning how IL-17 in this case did not significantly affect the M1 cells and therefore may not be involved in causing VEGF-resistant angiogenesis. A combination treatment involving VEGF blockade as well as invoking IL-17R deficiency reduced tumour growth by almost 80%; however it must be noted that pharmacological inhibition of IL-17A proved to be slightly less effective due to the difficulty in either maintaining or completely eradicating IL-17A signalling.
2.4. IL-17 and Tumour Immune-Resistance
2.4.1. Antitumour Effects
Tumour eradications, via a direct cytotoxic effect of Th17 cells, have been shown to exist via the expression of IFN-γ; although this effect was similar to Th1 effector cells in relying on IFN-γ in immune-resistance against tumours, the effect was significantly greater than that produced by the Th1 cells [114]. A possible indirect mechanism is via the association of IL-17 and the recruitment of tumour-infiltrating IFN-γ + effector T-cells, CD8+ T-cells, and NK cells whilst reducing the numbers of Treg cells, thus mediating tumour regression [96].
2.4.2. Protumour Effects
Cancer (besides other pathological diseases) inhibits the normal differentiation of immature myeloid-derived cells into mature cells; thus the population of the immature MDSCs expands [115]. Though copious evidence exploring Th17 cells and their potential tumour suppressing or enhancing effects has been looked at [102, 104, 105], a specific link between the production of IL-17 itself per se and effects on tumour immunity has not been clear. He et al. [27] suggested that IL-17 is necessary for the development of MDSCs when comparing IL-17R knockouts and wild type. Functional IL-17 also increased recruitment of MDSCs but reduced CD8+ T-cells; these contributed to their finding of enhanced tumour growth with IL-17R (−/−) and IFN-γR (−/−) double-knockout mice that agreed with a previous study [33]. This indicated a potential tumour-promoting role of the IL-17-IFN-γ coupling in contrast to other studies in different cancer models [46, 96]. The recently discovered paracrine network of IL-17 also saw an augmented infiltration of MDSCs [90]. The importance of MDSCs' immunosuppressive activity is growing, with higher levels of MDSCs linked with suppression of T-cell activity [115]. Furthermore, as mentioned above, its presence in the tumour microenvironment could be one of the key factors in favouring tumour growth, angiogenesis, and therefore metastasis.
2.5. IL-17 as Diagnostic Target
It is well established that early detection of cancer plays a significant part in decreasing morbidity and mortality in patients. With the intricate role IL-17 plays in cancer pathophysiology, evidence is now growing which supports the ability of IL-17 to act as a key diagnostic marker, differentiating between benign and malignant pathology as well as predicting prognosis. It could potentially be a significant tool in allowing early detection.
In lung pathology, differentiating between malignant and benign pleural effusions, caused by a variety of different underlying diseases, is a key factor affecting the management of the patient [116–118]. The diagnosis of a malignant pleural effusion is made via the detection of tumour cells in the pleural fluid. However the likelihood of finding such cells from a pleural fluid cytology (diagnostic method of choice) or needle biopsy is low, with sensitivities ranging between 30 and 60% [119]. In comparison, IL-17 was found to be significantly raised in pleural effusion cytology when detected using enzyme-linked immunosorbent assay (ELISA), [116, 120] with sensitivities as high as 76.8%, increasing to 96.4% when combined with other markers, such as carcinoembryonic antigen (CEA) [116]. In fact, even when testing the serum, patients with malignant serum effusions had significantly higher levels of IL-17 [118, 121]. This provides an alternative diagnostic test for patients with pleural effusions, especially ones of unknown origin.
Similarly, having established a strong connection between multiple myeloma and IL-17, patients suffering from multiple myeloma also display a significant rise in IL-17 levels both in the serum and in bone marrow biopsies [44, 122, 123], with myeloma marrow infiltrating lymphocytes (displaying the IL-17 phenotype) being a strong predictor of lytic bone disease, an effect of the activation of osteoclasts by IL-17 [124].
In oesophageal carcinoma, immunochemical analysis between disease free patients and patients with varying severities of the disease shows a significant strong correlation between IL-17 levels and disease progression in the tissue samples, [125, 126] with Chen et al. showing an associated increase in Th-17 cells in the serum too, again with levels also mirroring disease progression [126]. This could be a potential target for future diagnostic test or to monitor disease progression and response to treatment. However it is worth noting that IL-17 levels as a serum diagnostic marker are not appropriate for all cancer types. Results show that, despite the strong link between IL-17 and inflammatory bowel disease, there is no significant increase in IL-17 levels in patients with colorectal carcinomas [127, 128].
2.6. IL-17 as Therapeutic Target
With ever increasing evidence demonstrating the intricate role IL-17 plays in the pathophysiology of cancers, it is inevitable that attention is also now turning towards developing this knowledge into a novel therapeutic method in combating cancer.
Anti-IL-17 monoclonal antibodies are already in existence for the treatment of both inflammatory and immune-mediated conditions; they are listed in Table 1. In the immune-mediated condition psoriasis, using secukinumab (anti IL-17A monoclonal antibody), ixekizumab (anti IL-17 monoclonal antibody), and even an antibody against the receptor itself brodalumab (IL-17RA monoclonal antibody) has been shown to be effective in inducing and maintaining a remittance state in patients with moderate to severe plaques [129–131]. Secukinumab has also been shown to be effective when used to treat the inflammatory condition ankylosing spondylitis [132]. When used in psoriatic arthritis, secukinumab was shown to be both safe and effective in increasing the acute phase reactant and clinical response in patients as well as improving their quality of life [133].
Table 1.
The current clinical trials for monoclonal antibody treatments available in targeting IL-17.
| Drug name | Clinical trial | Details |
|---|---|---|
| Secukinumab | FIXTURE trial: full year investigative examination of secukinumab versus eTanercept using 2 dosing regimens to determine efficacy in psoriasis. | Phase III trial using 150 mg and 300 mg of secukinumab ClinicalTrials.gov identifier: NCT01358578 |
|
| ||
| Ixekizumab | UNCOVER-2 trial: a multicenter, randomized, double-blind, placebo-controlled study comparing the efficacy and safety of LY2439821 (ixekizumab) to etanercept and placebo in patients With moderate to severe plaque psoriasis. | Phase III trial using 80 mg of ixekizumab ClinicalTrials.gov identifier: NCT01597245 |
|
| ||
| Brodalumab | AMAGINE-2: study to evaluate the efficacy and safety of induction and maintenance regimens of brodalumab compared with placebo and ustekinumab in subjects with moderate to severe plaque psoriasis | Phase III trial using 210 mg and 140 mg of brodalumab ClinicalTrials.gov identifier: NCT01708603 |
In lung cancer, not only is IL-17 a potential diagnostic marker, but it also may act as a novel therapeutic target. A mice experimental model showed that when anti-IL-17A antibodies were applied locally to the lungs of mice with adenocarcinoma, it induced a reduction in tumour growth, promoted antitumour immunity (increased levels of IFNγ, which has been linked to inhibition of proliferation and angiogenesis of tumours), and increased overall survival in the animals [134, 135]. With the emerging roles of IL-17 in lung cancer over time, more experiment is needed to show the potential therapeutic option anti-IL-17 could play in lung cancer management [136]. This inhibition of tumour growth via the administration of an antibody to IL-17 was replicated in a mice model for lymphoma [27] and colonic cancer, [137] with even an abrogated development of metastasis reported as well in a breast cancer mice study [138]. Furthermore, IL-17A knockout mice consistently display a tumour resistance phenotype to a whole variety of cancers, including melanoma, [139] bladder carcinoma, [139], prostate adenocarcinoma, [140] and, with APC genetic predisposition, a decrease in colon tumour initiation [141]. These studies further strengthen the call for more research to ascertain whether anti-IL-17 antibodies could be a potential future cancer therapy, especially as anti-IL-17 monoclonal antibodies are already available and licenced for the use in other diseases.
An alternative way IL-17 could play in cancer treatment is not in the actual targeting of the disease itself, but to assess and monitor the effectiveness of treatment in patients. In ovarian carcinoma, a higher level of IL-17 tumour immune cell infiltration reflects a more chemosensitive tumour to platinum based therapies. At the same time, patients with persistently higher levels of IL-17 tumour immune cell infiltration also indicate the need for a longer course of chemotherapy as these dominated the significant proportion of all recurrences [142]. Similarly, in breast cancer, higher expression of IL-17 was linked with greater probability for recurrence, greater chemotherapy resistance (to docetaxel), shorter disease free survival, and poorer prognosis [143]. In patients with multiple myeloma, serum as well as bone marrow levels of IL-27 decrease whilst IL-17 increases in line with disease progression, with a high IL-27 : IL-17 ratio (i.e., high IL-27 and low IL-17) in the bone marrow associated with better prognosis [144]. These results suggest that IL-17 could potentially be another indicator predicting patient prognosis, thus allowing treatment to be tailored accordingly.
Interestingly, an inverse relationship was found in cervical cancer. A higher risk of recurrence was found in patients who had lower intratumoural levels of IL-17 postradical resection, thus potentially providing a biomarker for patients who may benefit from adjuvant chemotherapy [145]. The monoclonal antibodies in targeting IL-17 at the phase III clinical trial stage are summarised in Table 1.
3. Summary
With the prevalence of cancer increasing year to year, it is of utmost importance to find new ways to combat this disease. Here we summarised the ever expanding amount of evidence supporting the intricate role IL-17 plays in the pathophysiology of cancer, from potentially stimulating tumorigenesis, proliferation, angiogenesis, and metastasis, to adapting the tumour in its ability to confer upon itself both immune- and chemotherapy resistance; though often there were opposite views expressed in the literature.
Right from tumourigenesis, IL-17 has been shown to play a role via combination of releasing MDSCs to dampen the body's immune defence system, as well as stimulating proinflammatory cytokines systemically (via the NF-κB pathway) and locally (via CCL2) to maintain an inflammatory environment, resulting in the stimulation of tumour growth via the subsequent expression of antiapoptotic genes and the consequent increased survival of cells with protumourigenic potential. Tumour proliferation was further aided by the IL-17 induced activation of the NF-κB pathway subsequently stimulating the MEK-ERK pathway.
Studies investigating the role of IL-17 in angiogenesis show both a pro- and antiangiogenesis picture. Via the expression of VEGF, IL-17 exerts a proangiogenesis effect, leading to higher microvascular densities and lymphocytes infiltration in certain cancers. A VEGF independent pathway was also found via MEK-1/2 signaling and NF-κB, which leads to suppression of the immune response targeting cancer cells via MDSCs.
Further to its role in angiogenesis, when investigating the effects of IL-17 and tumour metastasis, IL-17 was shown to enhance metastasis via the expression of VEGF inducing both angiogenesis and lymphangiogenesis, subsequently leading to metastasis of tumours. However knockout IL-17 models have also shown a more aggressive metastatic picture, possibly via the loss of the potent antitumour cytokine IFN-γ stimulated by IL-17, thus presenting a mixed picture.
However when looking specifically at IL-17F, a subtype of the IL-17 family, an anti-angiogenesis picture was found with decreased levels of VEGF seen. Additionally, there is clear evidence linking IL-17 and the failure of chemotherapy. Via the amplified expression of both G-CSF and IL-17, tumours were able to negate the effects of anti-VEGF chemotherapy. Yet when anti-VEGF therapy was used in conjunction with knocking out IL-17R, a massive reduction in tumour growth was seen.
With regard to tumour immune-resistance, again a mixed picture is presented. A protumour environment was demonstrated by IL-17-IFN-γ coupling as well as stimulating MDSCs to suppress T-cell activation. Yet an antitumour direct cytotoxic effect was also reported via the expression of IFN-γ, thus highlighting an area requiring more research. The above findings have been summarised in Figure 1.
Figure 1.
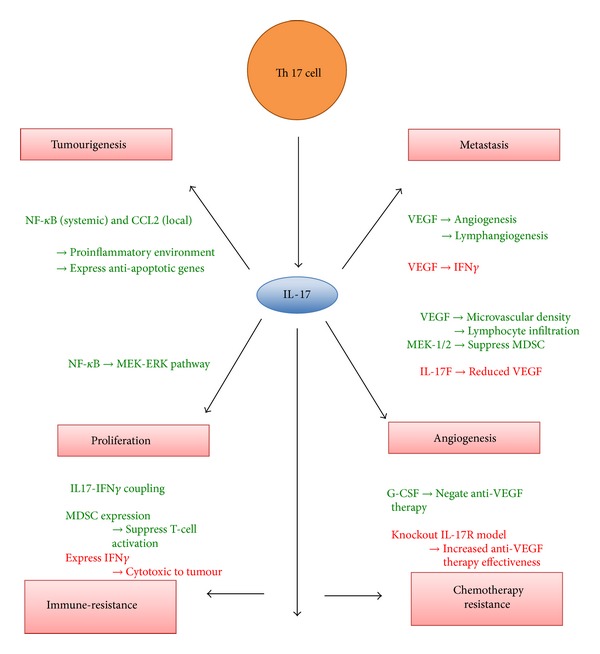
Diagram summarising the reviewed mechanisms by which IL-17 induces (green) or inhibits (red) different aspects of cancer pathophysiology. IL17: interleukin 17; VEGF: vascular endothelial growth factor; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; CCL2: chemokine (C-C motif) ligand 2; IFNγ: interferons γ; G-CSF: granulocyte colony stimulating factor.
With opinions still often divided on the actual role IL-17 plays in the pathophysiology of cancer, it clearly demonstrates a need for more research in this area. However, even with the current data, IL-17 and its role in cancer pathophysiology do present exciting new options for potential therapeutic and diagnostic targets, which in time will hopefully evolve into novel therapies in the fight against cancer.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer Journal for Clinicians. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annual Review of Immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 3.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-β are required for differentiation of human T H17 cells. Nature. 2008;454(7202):350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nature Immunology. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. The New England Journal of Medicine. 2009;361(9):888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. The Journal of Experimental Medicine. 2001;194(4):519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly MN, Kolls JK, Happel K, et al. Interteukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infection and Immunity. 2005;73(1):617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. Journal of Infectious Diseases. 2004;190(3):624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 11.Zelante T, de Luca A, Bonifazi P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. European Journal of Immunology. 2007;37(10):2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 12.Ma CS, Chew GY, Simpson N, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. The Journal of Experimental Medicine. 2008;205(7):1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. The Journal of Experimental Medicine. 2006;203(12):2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkham BW, Lassere MN, Edmonds JP, et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis and Rheumatism. 2006;54(4):1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- 16.Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nature Medicine. 2014;20(1):62–68. doi: 10.1038/nm.3432. [DOI] [PubMed] [Google Scholar]
- 17.Ray K. Experimental arthritis. Tipping the balance: conversion of Foxp3+ T cells to TH17 cells is crucial in autoimmune arthritis. Nature Reviews Rheumatology. 2014;10(2):p. 63. doi: 10.1038/nrrheum.2013.213. [DOI] [PubMed] [Google Scholar]
- 18.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rovedatti L, Kudo T, Biancheri P, et al. Differential regulation of interleukin 17 and interferon γ production in inflammatory bowel disease. Gut. 2009;58(12):1629–1636. doi: 10.1136/gut.2009.182170. [DOI] [PubMed] [Google Scholar]
- 20.Sarra M, Pallone F, MacDonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflammatory Bowel Diseases. 2010;16(10):1808–1813. doi: 10.1002/ibd.21248. [DOI] [PubMed] [Google Scholar]
- 21.Seiderer J, Elben I, Diegelmann J, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): Upregulated colonic IL-17F expression in active Crohn's disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflammatory Bowel Diseases. 2008;14(4):437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 22.Olsen T, Rismo R, Cui G, Goll R, Christiansen I, Florholmen J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine. 2011;56(3):633–640. doi: 10.1016/j.cyto.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases. 2006;12(5):382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 24.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. The Journal of Immunology. 2009;183(7):4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 25.Iida T, Iwahashi M, Katsuda M, et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncology Reports. 2011;25(5):1271–1277. doi: 10.3892/or.2011.1201. [DOI] [PubMed] [Google Scholar]
- 26.Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T Cells in the microenvironments of chronic inflammation and cancer. The Journal of Immunology. 2011;186(7):4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 27.He D, Li H, Yusuf N, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. Journal of Immunology. 2010;184(5):2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Zhang Y, Liu Y, et al. Association of myeloid-derived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. Journal of Immunotherapy. 1997;37(1):43–50. doi: 10.1097/CJI.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 29.Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes and Infection. 2009;11(5):625–630. doi: 10.1016/j.micinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase a in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65(11):904–910. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 31.Yabu M, Shime H, Hara H, et al. IL-23-dependent and -independent enhancement pathways of IL-17A production by lactic acid. International Immunology. 2011;23(1):29–41. doi: 10.1093/intimm/dxq455. [DOI] [PubMed] [Google Scholar]
- 32.Shime H, Yabu M, Akazawa T, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. The Journal of Immunology. 2008;180(11):7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. The Journal of Experimental Medicine. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. Journal of Molecular Medicine. 2004;82(7):434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 36.Huang CK, Yang CY, Jeng YM, et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway. Oncogene. 2013;33(23):2968–2977. doi: 10.1038/onc.2013.268. [DOI] [PubMed] [Google Scholar]
- 37.Straus DS. TNFα and IL-17 cooperatively stimulate glucose metabolism and growth factor production in human colorectal cancer cells. Molecular Cancer. 2013;12, article 78 doi: 10.1186/1476-4598-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizutani K, Sud S, McGregor NA, et al. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11(11):1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vykhovanets EV, MacLennan GT, Vykhovanets OV, Gupta S. IL-17 Expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patients. International Journal of Clinical and Experimental Pathology. 2011;4(6):552–565. [PMC free article] [PubMed] [Google Scholar]
- 40.Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine & Growth Factor Reviews. 2010;21(1):11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Jove R. The stats of cancer—new molecular targets come of age. Nature Reviews Cancer. 2004;4(2):97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 42.Zepp JA, Liu C, Gulen MF, et al. 296: IL-17-signaling in LGR5-positive stem cells promotes colon tumorigenesis. Cytokine. 2013;63(3):p. 313. [Google Scholar]
- 43.Saxena A, Raychaudhuri SK, Raychaudhuri SP. Interleukin-17-induced proliferation of fibroblast-like synovial cells is mTOR dependent. Arthritis and Rheumatism. 2011;63(5):1465–1466. doi: 10.1002/art.30278. [DOI] [PubMed] [Google Scholar]
- 44.Prabhala RH, Pelluru D, Fulciniti M, et al. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115(26):5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, Kim HJ, Chang E-J, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death and Differentiation. 2009;16(10):1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8+ T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunology, Immunotherapy. 2011;60(10):1473–1484. doi: 10.1007/s00262-011-1054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benchetrit F, Ciree A, Vives V, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99(6):2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 49.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114(2):357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441(7092):431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 51.Folkman J. Angiogenesis. Annual Review of Medicine. 2006;57(1):1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 52.Yao DF, Wu XH, Zhu Y, et al. Quantitative analysis of vascular endothelial growth factor, microvascular density and their clinicopathologic features in human hepatocellular carcinoma. Hepatobiliary & Pancreatic Diseases International. 2005;4(2):220–226. [PubMed] [Google Scholar]
- 53.El-Gohary YM, Silverman JF, Olson PR, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in prostatic adenocarcinoma. American Journal of Clinical Pathology. 2007;127(4):572–579. doi: 10.1309/X6NXYE57DLUE2NQ8. [DOI] [PubMed] [Google Scholar]
- 54.Iavarone M, Lampertico P, Iannuzzi F, et al. Increased expression of vascular endothelial growth factor in small hepatocellular carcinoma. Journal of Viral Hepatitis. 2007;14(2):133–139. doi: 10.1111/j.1365-2893.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- 55.Kopfstein L, Veikkola T, Djonov VG, et al. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. The American Journal of Pathology. 2007;170(4):1348–1361. doi: 10.2353/ajpath.2007.060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Möbius C, Demuth C, Aigner T, et al. Evaluation of VEGF A expression and microvascular density as prognostic factors in extrahepatic cholangiocarcinoma. European Journal of Surgical Oncology. 2007;33(8):1025–1029. doi: 10.1016/j.ejso.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 57.Qi W, Huang X, Wang J. Correlation between Th17 cells and tumor microenvironment. Cellular Immunology. 2013;285(1-2):18–22. doi: 10.1016/j.cellimm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. The Journal of Experimental Medicine. 1995;181(1):435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arenberg DA, Kunkel SL, Polverini PJ, et al. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. The Journal of Experimental Medicine. 1996;184(3):981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore BB, Arenberg DA, Addison CL, Keane MP, Polverini PJ, Strieter RM. CXC chemokines mechanism of action in regulating tumor angiogenesis. Angiogenesis. 1998;2(2):123–134. doi: 10.1023/a:1009284305061. [DOI] [PubMed] [Google Scholar]
- 61.Kehlen A, Thiele K, Riemann D, Rainov N, Langner J. Interleukin-17 stimulates the expression of IκBα mRNA and the secretion of IL-6 and IL-8 in glioblastoma cell lines. Journal of Neuroimmunology. 1999;101(1):1–6. doi: 10.1016/s0165-5728(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 62.Tartour E, Fossiez F, Joyeux I, et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Research. 1999;59(15):3698–3704. [PubMed] [Google Scholar]
- 63.Kato T, Furumoto H, Ogura T, et al. Expression of IL-17 mRNA in ovarian cancer. Biochemical and Biophysical Research Communications. 2001;282(3):735–738. doi: 10.1006/bbrc.2001.4618. [DOI] [PubMed] [Google Scholar]
- 64.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 65.Huang X, Lee C. Regulation of stromal proliferation, growth arrest, differentiation and apoptosis in benign prostatic hyperplasia by TGF-β . Frontiers in Bioscience. 2003;8:s740–s749. doi: 10.2741/1093. [DOI] [PubMed] [Google Scholar]
- 66.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 67.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-α-induced elaboration of proangiogenic factors from fibroblasts. Immunology Letters. 2004;93(1):39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends in Immunology. 2004;25(7):387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 69.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6(5):447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 70.Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Research. 2005;65(2):465–472. [PubMed] [Google Scholar]
- 71.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. Journal of Immunology. 2005;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 72.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine and Growth Factor Reviews. 2005;16(6):593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Honorati MC, Neri S, Cattini L, Facchini A. Interleukin-17, a regulator of angiogenic factor release by synovial fibroblasts. Osteoarthritis and Cartilage. 2006;14(4):345–352. doi: 10.1016/j.joca.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Jeon SH, Chae BC, Kim HA, et al. Mechanisms underlying TGF-β1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. Journal of Leukocyte Biology. 2007;81(2):557–566. doi: 10.1189/jlb.0806517. [DOI] [PubMed] [Google Scholar]
- 75.Zwaans BMM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvascular Research. 2007;74(2-3):145–158. doi: 10.1016/j.mvr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee JW, Wang P, Kattah MG, et al. Differential regulation of chemokines by IL-17 in colonic epithelial cells. Journal of Immunology. 2008;181(9):6536–6545. doi: 10.4049/jimmunol.181.9.6536. [DOI] [PubMed] [Google Scholar]
- 77.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(7):2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clinical Cancer Research. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 79.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer Cell. 2009;16(3):173–174. doi: 10.1016/j.ccr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Salibi N, Wu Y, Schweitzer ME, Regatte RR. Relaxation times of skeletal muscle metabolites at 7T. Journal of Magnetic Resonance Imaging. 2009;29(6):1457–1464. doi: 10.1002/jmri.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. Journal of Hepatology. 2009;50(5):980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 83.Pickens SR, Volin MV, Mandelin AM, 2nd Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. The Journal of Immunology. 2010;184(6):3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Silva-Santos B. Promoting angiogenesis within the tumor microenvironment: the secret life of murine lymphoid IL-17-producing γδ T cells. European Journal of Immunology. 2010;40(7):1873–1876. doi: 10.1002/eji.201040707. [DOI] [PubMed] [Google Scholar]
- 85.Wakita D, Sumida K, Iwakura Y, et al. Tumor-infiltrating IL-17-producing γδ T cells support the progression of tumor by promoting angiogenesis. European Journal of Immunology. 2010;40(7):1927–1937. doi: 10.1002/eji.200940157. [DOI] [PubMed] [Google Scholar]
- 86.He S, Fei M, Wu Y, et al. Distribution and clinical significance of TH17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. International Journal of Molecular Sciences. 2011;12(11):7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Duan Y, Cheng X, et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochemical and Biophysical Research Communications. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 88.McAllaster JD, Cohen MS. Role of the lymphatics in cancer metastasis and chemotherapy applications. Advanced Drug Delivery Reviews. 2011;63(10-11):867–875. doi: 10.1016/j.addr.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 89.Candido J, Hagemann T. Cancer-related inflammation. Journal of Clinical Immunology. 2013;33(supplement 1):S79–S84. doi: 10.1007/s10875-012-9847-0. [DOI] [PubMed] [Google Scholar]
- 90.Chung AS, Wu X, Zhuang G, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nature Medicine. 2013;19(9):1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 91.Su Z, Sun Y, Zhu H, et al. Th17 cell expansion in gastric cancer may contribute to cancer development and metastasis. Immunologic Research. 2014;58(1):118–124. doi: 10.1007/s12026-013-8483-y. [DOI] [PubMed] [Google Scholar]
- 92.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. The American Journal of Pathology. 1992;140(3):539–544. [PMC free article] [PubMed] [Google Scholar]
- 93.Beatty GL, Paterson Y. IFN-γ-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-γ . Journal of Immunology. 2001;166(4):2276–2282. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 94.Starnes T, Robertson MJ, Sledge G, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. The Journal of Immunology. 2001;167(8):4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 95.Castermans K, Tabruyn SP, Zeng R, et al. Angiostatic activity of the antitumor cytokine interleukin-21. Blood. 2008;112(13):4940–4947. doi: 10.1182/blood-2007-09-113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kryczek I, Banerjee M, Cheng P, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114(6):1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie Y, Sheng W, Xiang J, Ye Z, Yang J. Interleukin-17F suppresses hepatocarcinoma cell growth via inhibition of tumor angiogenesis. Cancer Investigation. 2010;28(6):598–607. doi: 10.3109/07357900903287030. [DOI] [PubMed] [Google Scholar]
- 98.Tong Z, Yang XO, Yan H, et al. A protective role by interleukin-17F in colon tumorigenesis. PLoS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034959.e34959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clinical Therapeutics. 2006;28(11):1779–1802. doi: 10.1016/j.clinthera.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 100.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 101.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29(42):5653–5662. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alshaker HA, Matalka KZ. IFN-gamma, IL-17 and TGF-beta involvement in shaping the tumor microenvironment: the significance of modulating such cytokines in treating malignant solid tumors. Cancer Cell International. 2011;11, article 33 doi: 10.1186/1475-2867-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. The New England Journal of Medicine. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 104.Zou W, Restifo NP. TH17 cells in tumour immunity and immunotherapy. Nature Reviews Immunology. 2010;10(4):248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: a TGF-β-dependent immunosuppressive activity? Trends in Molecular Medicine. 2012;18(12):742–749. doi: 10.1016/j.molmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 106.Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. Journal of cellular and molecular medicine. 2009;13(8):1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Massi D, Gököz Ö. The biological significance of lymphangiogenesis in human tumours. Diagnostic Histopathology. 2010;16(6):295–305. [Google Scholar]
- 108.Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 109.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Current Opinion in Cell Biology. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 110.Chen X, Wan J, Liu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 111.Chauhan SK, Jin Y, Goyal S, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118(17):4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maniati E, Hagemann T. IL-17 mediates resistance to anti-VEGF therapy. Nature Medicine. 2013;19(9):1092–1094. doi: 10.1038/nm.3333. [DOI] [PubMed] [Google Scholar]
- 113.Kieran MW, Kalluri R, Cho Y. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harbor perspectives in medicine. 2012;2(12) doi: 10.1101/cshperspect.a006593.a006593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu CH, Zhan P, Yu LK, Zhang XW. Diagnostic value of pleural interleukin 17 and carcinoembryonic antigen in lung cancer patients with malignant pleural effusions. Tumor Biology. 2014;35(2):1599–1603. doi: 10.1007/s13277-013-1220-2. [DOI] [PubMed] [Google Scholar]
- 117.Heffner JE. Diagnosis and management of malignant pleural effusions. Respirology. 2008;13(1):5–20. doi: 10.1111/j.1440-1843.2007.01154.x. [DOI] [PubMed] [Google Scholar]
- 118.Chomej P, Bauer K, Bitterlich N, et al. Differential diagnosis of pleural effusions by fuzzy-logic-based analysis of cytokines. Respiratory Medicine. 2004;98(4):308–317. doi: 10.1016/j.rmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 119.Sriram KB, Relan V, Clarke BE, et al. Diagnostic molecular biomarkers for malignant pleural effusions. Future Oncology. 2011;7(6):737–752. doi: 10.2217/fon.11.45. [DOI] [PubMed] [Google Scholar]
- 120.Klimatsidas M, Anastasiadis K, Foroulis C, et al. Elevated levels of anti inflammatory IL-10 and pro inflammatory IL-17 in malignant pleural effusions. Journal of Cardiothoracic Surgery. 2012;7(1, article 104) doi: 10.1186/1749-8090-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunology Letters. 2012;148(2):144–150. doi: 10.1016/j.imlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 122.Lemancewicz D, Bolkun L, Jablonska E, et al. The role of interleukin-17A and interleukin-17E in multiple myeloma patients. Medical Science Monitor. 2012;18(1):BR54–BR59. doi: 10.12659/MSM.882204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dhodapkar KM, Barbuto S, Matthews P, et al. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112(7):2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Noonan K, Marchionni L, Anderson J, Pardoll D, Roodman GD, Borrello I. A novel role of IL-17-producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116(18):3554–3563. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bannister Khan AL, Jr., Khan AL, Eccleston DW, Deol-Poonia RK, Hughes SF. Interleukin-17 expression in the Barrett's metaplasia-dysplasia-adenocarcinoma sequence. ISRN Inflammation. 2012;2012:6 pages. doi: 10.5402/2012/578149.578149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen D, Hu Q, Mao C, et al. Increased IL-17-producing CD4(+) T cells in patients with esophageal cancer. Cellular Immunology. 2012;272(2):166–174. doi: 10.1016/j.cellimm.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 127.Stanilov N, Miteva L, Deliysky T, Jovchev J, Stanilova S. Advanced colorectal cancer is associated with enhanced IL-23 and IL-10 serum levels. Laboratory Medicine. 2010;41(3):159–163. [Google Scholar]
- 128.Kirchberger S, Royston DJ, Boulard O, et al. Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. Journal of Experimental Medicine. 2013;210(5):917–931. doi: 10.1084/jem.20122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spuls PI, Hooft L. Brodalumab and ixekizumab, anti-interleukin-17-receptor antibodies for psoriasis: a critical appraisal. British Journal of Dermatology. 2012;167(4):710–713. doi: 10.1111/bjd.12025. [DOI] [PubMed] [Google Scholar]
- 130.Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: A randomized, double-blind, placebo-controlled phase II dose-ranging study. British Journal of Dermatology. 2013;168(2):412–421. doi: 10.1111/bjd.12110. [DOI] [PubMed] [Google Scholar]
- 131.Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. The British Journal of Dermatology. 2013;168(2):402–411. doi: 10.1111/bjd.12112. [DOI] [PubMed] [Google Scholar]
- 132.Baeten D, Baraliakos X, Braun J, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. The Lancet. 2013;382(9906):1705–1713. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 133.McInnes IB, Sieper J, Braun J, et al. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: a 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Annals of the Rheumatic Diseases. 2013;73(2):349–356. doi: 10.1136/annrheumdis-2012-202646. [DOI] [PubMed] [Google Scholar]
- 134.Reppert S, Koch S, Finotto S. IL-17A is a central regulator of lung tumor growth. Oncoimmunology. 2012;1(5):783–785. doi: 10.4161/onci.19735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reppert S, Boross I, Koslowski M, et al. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nature Communications. 2011;2(1, article 600) doi: 10.1038/ncomms1609. [DOI] [PubMed] [Google Scholar]
- 136.Neurath MF, Finotto S. The emerging role of T cell cytokines in non-small cell lung cancer. Cytokine and Growth Factor Reviews. 2012;23(6):315–322. doi: 10.1016/j.cytogfr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature Medicine. 2009;15(9):1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Das Roy L, Pathangey LB, Tinder TL, Schettini JL, Gruber HE, Mukherjee P. Breast cancer-associated metastasis is significantly increased in a model of autoimmune arthritis. Breast Cancer Research. 2009;11(4, article R56) doi: 10.1186/bcr2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L, Yi TS, Kortylewski M, Pardoll DM, Zeng DF, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. Journal of Experimental Medicine. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang QY, Liu S, Ge DX, et al. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Research. 2012;72(10):2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chae W, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell ALM. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5540–5544. doi: 10.1073/pnas.0912675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Droeser RA, Güth U, Eppenberger-Castori S, et al. High IL-17-positive tumor immune cell infiltration is indicative for chemosensitivity of ovarian carcinoma. Journal of Cancer Research and Clinical Oncology. 2013;139(8):1295–1302. doi: 10.1007/s00432-013-1441-1. [DOI] [PubMed] [Google Scholar]
- 143.Cochaud S, Giustiniani J, Thomas C, et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Scientific Reports. 2013;3, article 3456 doi: 10.1038/srep03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song XN, Yang JZ, Sun LX, et al. Expression levels of IL-27 and IL-17 in multiple myeloma patients: a higher ratio of IL-27:IL-17 in bone marrow was associated with a superior progression-free survival. Leukemia Research. 2013;37(9):1094–1099. doi: 10.1016/j.leukres.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 145.Yu Q, Lou XM, He Y. Prediction of local recurrence in cervical cancer by a Cox model comprised of lymph node status, lymph-vascular space invasion, and intratumoral Th17 cell-infiltration. Medical Oncology. 2014;31(1, article 795) doi: 10.1007/s12032-013-0795-1. [DOI] [PubMed] [Google Scholar]


