Abstract
Chemoattractant receptors are a family of seven transmembrane G protein coupled receptors (GPCRs) initially found to mediate the chemotaxis and activation of immune cells. During the past decades, the functions of these GPCRs have been discovered to not only regulate leukocyte trafficking and promote immune responses, but also play important roles in homeostasis, development, angiogenesis, and tumor progression. Accumulating evidence indicates that chemoattractant GPCRs and their ligands promote the progression of malignant tumors based on their capacity to orchestrate the infiltration of the tumor microenvironment by immune cells, endothelial cells, fibroblasts, and mesenchymal cells. This facilitates the interaction of tumor cells with host cells, tumor cells with tumor cells, and host cells with host cells to provide a basis for the expansion of established tumors and development of distant metastasis. In addition, many malignant tumors of the nonhematopoietic origin express multiple chemoattractant GPCRs that increase the invasiveness and metastasis of tumor cells. Therefore, GPCRs and their ligands constitute targets for the development of novel antitumor therapeutics.
1. Introduction
Chemoattractant receptors are a family of G protein coupled seven transmembrane cell surface receptors (GPCRs). According to their source of ligands and expression patterns, the family members are categorized into classical GPCRs and chemokine GPCRs. The former include formyl peptide receptor and its variants (FPR1, FPR2, and FPR3), platelet activating factor receptor (PAFR), activated complement component 5a receptor (C5aR), and leukotriene B4 receptor and its variants (BLT1 and BLT2). Chemokine GPCRs are composed of four subfamilies based on the conserved N-terminal cysteine residues in the mature proteins of the ligands, CC-, CXC-, CX3C-, and C-, and thus are termed CCR, CXCR, CX3CR, and XCR, respectively. So far, approximately 50 chemokines and at least 18 chemokine GPCRs have been identified [1] (Table 1). Promiscuity is a characteristic of GPCRs and their ligands. Some chemoattractants bind to more than one GPCR. Conversely, some GPCRs display overlapping ligand specificities with variable affinity and functions [2]. Although chemoattractant GPCRs are mainly expressed by leukocytes and their major function has been considered as mediators of leukocyte trafficking and homing, over the past two decades, the role of GPCRs and their ligands in tumor progression began to be increasingly recognized. The expression of some GPCRs or ligands in tumor tissues has been shown to be correlated with the therapeutic outcome of tumor patients [3–10]. It is undeniable that tumor cells are one of the major sources of chemoattractants in tumor tissues and many tumor cells express one or more chemoattractant GPCRs to their advantage [11]. In addition, tumor-derived chemoattractants are mediators of leukocyte, in particular macrophage (tumor-associated macrophages, TAMs), infiltration that may result in the persistence of chronic inflammation in the tumor microenvironment together with a vigorous angiogenesis. Therefore, chemoattractant GPCRs are believed to play a crucial role in tumor progression via signaling based on dissociation of trimeric G proteins in response to ligands binding culminating in cell chemotaxis, invasion, production of mediators promoting angiogenesis, transactivation of growth factor receptors, such as epidermal growth factor receptor (EGFR), and tumor cell metastasis. (Figure 1 shows the signaling.)
Table 1.
Chemoattractant GPCRs and ligands.
| Expression | Ligands | Functions | References | |
|---|---|---|---|---|
| “Classical” | ||||
| FPR | ||||
| FPR1 | Myeloid cells, lymphocytes Tumor cells |
Bacteria and host derived peptides | Chemotaxis and activation Tumor growth, invasion, angiogenesis |
[12, 13] |
| FPR2 | Myeloid cells Tumor cells |
Bacteria and host derived peptides | Chemotaxis and activation Antitumor defense, tumor invasion |
[13] |
| FPR3 | Monocytes, dendritic cells Tumor cells |
Synthetic and host derived peptides | Chemotaxis and activation Tumor invasion |
[13] |
| PAFR | Macrophages, polymorphonuclear leucocytes, and various tissue cells Tumor cells |
PAF | Chemotaxis and activation Tumor growth and metastasis; inhibiting tumor angiogenesis |
[14, 15] |
| C5aR | Neutrophils, monocytes, eosinophils, basophils, dendritic cells, mast cells, and various nonimmune cells Tumor cells |
C5a | Chemotaxis and activation Tumor metastasis; opposite function in angiogenesis |
[16–18] |
| LTB4R | ||||
| BLT1 | Neutrophils, macrophages, T lymphocytes Tumor cells |
LTB4 | Chemotaxis and activation Tumor growth |
[19] |
| BLT2 | Most human tissues cells and leukocytes Tumor cells |
LTB4 | Chemotaxis and activation Tumor growth, metastasis |
[19] |
| “Chemokine” | ||||
| CCR | ||||
| CCR1 | Monocytes, neutrophils, T lymphocytes, dendritic cells Tumor cells |
CCL3/4/6/7/8/9/10/14/15/16/23 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[20] |
| CCR2 | Monocytes, basophils, T lymphocytes, dendritic cells, NK cells, endothelial cells Tumor cells |
CCL2/7/8/11/13/16 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[21, 22] |
| CCR3 | Eosinophils, basophils, Th2 lymphocytes, mast cells Tumor cells |
CCL7/11/13/15/24/26/28 | Chemotaxis and activation Tumor growth, metastasis |
[23] |
| CCR4 | Macrophages, monocytes, basophils, T and B lymphocytes, dendritic cells, NK cells, mast cells, platelets Tumor cells |
CCL2/4/5/17/22 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[24] |
| CCR5 | Macrophages, T lymphocytes, dendritic cells, NK cells Tumor cells |
CCL3/4/5/7/11/13/16 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[25, 26] |
| CCR6 | Neutrophils, T and B lymphocytes, dendritic cells, epithelial cells of some tissues Tumor cells |
CCL20 | Chemotaxis and activation Tumor growth, metastasis |
[27, 28] |
| CCR7 | T and B lymphocytes, dendritic cells Tumor cells |
CCL19/21 | Lymphoid tissue chemotaxis and activation Tumor growth, metastasis |
[29, 30] |
| CCR8 | Macrophages, Th2 lymphocytes, endothelial cells Tumor cells |
CCL1/16 | Chemotaxis and activation Tumor metastasis |
[31, 32] |
| CCR9 | T lymphocytes Tumor cells |
CCL25 | Small intestinal specific chemotaxis and activation Tumor growth, metastasis; inhibiting tumor metastasis in some tumors |
[33, 34] |
| CCR10 | T lymphocytes Tumor cells |
CCL27/28 | Skin-specific chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[35, 36] |
| CXCR | ||||
| CXCR1 | Neutrophils, polymorphonuclear leukocytes, endothelial cells Tumor cells |
CXCL6/8 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[37–39] |
| CXCR2 | Neutrophils, basophils, T lymphocytes, oligodendrocytes, endothelial cells Tumor cells |
CXCL1/2/3/5/6/8 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis |
[40, 41] |
| CXCR3 | Macrophages, T lymphocytes, NK cells, NKT cells, endothelial cells Tumor cells |
CXCL4/9/10/11 | Chemotaxis and activation Two variants CXCR3-A and CXCR3-B have opposite function in tumor progression |
[42, 43] |
| CXCR4 | Numerous cell types: hematopoietic cells and stem cells Tumor cells |
CXCL12 | Chemotaxis and activation Maintenance of stem phenotype Tumor growth, metastasis, angiogenesis |
[1, 44] |
| CXCR5 | T and B lymphocytes Tumor cells |
CXCL13 | Chemotaxis and activation Tumor growth, metastasis; inhibiting tumor metastasis in some tumors |
[45, 46] |
| CXCR6 | T and B lymphocytes, NK cells, NKT cells, plasma cells Tumor cells |
CXCL16 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis; inhibiting tumor migration in some tumors |
[47] |
| CXCR7 | T and B lymphocytes, dendritic cells, endothelial cells, fetal hepatocytes Tumor cells |
CXCL11/12 | Chemotaxis and activation Tumor growth, metastasis, angiogenesis; assisting with CXCR4 to regulate tumor progression |
[48–50] |
| CX3CR | ||||
| CX3CR1 | Monocytes, T and B lymphocytes, mast cells, dendritic cells, NK cells Tumor cells |
CX3CL1 | Chemotaxis and activation Tumor growth, metastasis; inhibiting tumor invasion in some tumors |
[51, 52] |
| XCR | ||||
| XCR1 | Neutrophils, T lymphocytes, dendritic cells Tumor cells |
XCL1/2 | Chemotaxis and activation Tumor cell growth, metastasis |
[53] |
Figure 1.
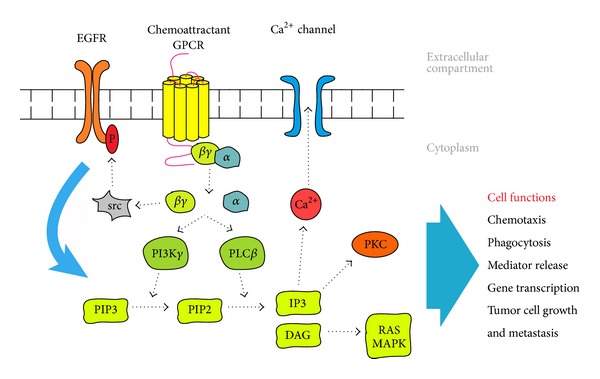
The signaling pathway of chemoattractant GPCRs. Chemoattractant GPCRs activated by ligands elicit a cascade of signal transduction pathways involving G proteins, phospholipase C (PLC), phosphoinositide (PI) 3 kinases, protein kinase C (PKC), Ca2+, RAS, and MAPKs to mediate leukocyte migration and activation. Chemoattractant GPCRs also play a crucial role in tumor progression upon activation by their ligands culminating in cell chemotaxis, invasion, production of mediators promoting angiogenesis, and transactivation of EGFR.
A tumor has been recognized as a complicated “organ,” other than a simple collection of relatively homogeneous cancer cells, whose entire biology could be understood by elucidating the autonomous properties of these cells. In contrast, various types of host cells are known to contribute in important ways to the biology of tumors, including endothelial cells (ECs), pericytes, immune cells, cancer-associated fibroblasts (CAFs), and stem and progenitor cells of the tumor stroma [54]. The interaction between these cells and their secreting factors results in an environment which markedly affects tumor progression. (Figure 2 shows the tumor.) Therefore, understanding the contribution of GPCRs and their ligands to the complexity of the tumor microenvironment is critical for the identification of novel therapeutic targets.
Figure 2.
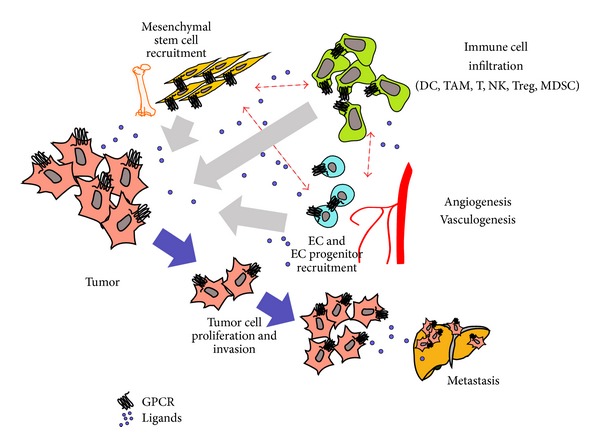
Chemoattractant GPCRs in tumor microenvironment. A tumor has been recognized as a complicated “organ.” Various types of tumor and host cells, including immune cells, fibroblasts, endothelial cells, and progenitor cells of the tumor stroma, contribute to the tumor development, growth, metastasis, immune escape, and neovascularization.
2. GPCRs in Recruiting Tumor-Associated Immune Cells
The infiltration of immune cells is a characteristic of the tumor microenvironment, which is the basis for the presence of chronic inflammation. Chemoattractants are characterized by their ability to induce directional migration and activation of leukocytes by stimulating specific GPCRs [2]. (Figure 1 shows the signaling.) The infiltrating immune cells play an important role in shaping a tumor-promoting or tumor-suppressive microenvironment [55, 56].
2.1. Tumor Infiltrating Tumor Suppressive Immune Cells
In general, infiltration of antigen presenting dendritic cells (DCs) into the tumor represents an early tumor-triggered host immune response. In hepatocellular carcinoma (HCC), tumor infiltrating DCs express the chemokine GPCRs, CCR1 and CCR5. Tumor cell apoptosis induced by suicide genes increases the number of DCs migrating into the draining lymph nodes to generate a specific cytotoxic cell population against HCC cells [57], although apoptotic tumor cells are also believed to generate tolerogenic DCs. In addition to CCR1 and CCR5, CCR6 is also commonly expressed by circulating immature DCs (iDCs). In melanoma, the profiling of GPCRs expressed by plasmacytoid DCs (pDCs) showed that the only significantly elevated GPCR is CCR6, which mediates the recruitment of pDCs from blood by the chemokine ligand CCL20 produced by melanoma cells [58]. Other immune cells in tumor microenvironment may also promote DC recruitment, such as Th9 cells, which increase DC infiltration of the tumor mediated by CCR6/CCL20 interaction that generates CD8(+) cytotoxic T lymphocyte (CTLs) responses and inhibit tumor growth [59]. After capturing antigens, DCs undergo maturation and express high levels of the chemokine GPCR CCR7 that enables DCs to migrate to T cell zones in the draining lymph nodes that produce the CCR7 ligands, CCL19 and CCL21. However, the results of interaction between DCs and tumor cells could be multifaceted based on CCR7/CCL19 or CCR7/CCL21 interaction [60, 61]. These chemokines may decide the distribution of immature or mature DCs within tumor tissues and generate opposing immunological consequences. For example, in renal cell carcinoma, tumor cells secrete CCL20 to recruit CCR6(+) immature DCs that mostly elicit tolerance, while, in the tumor invasion margin, only CCL19 and CCL21 are detected and they recruit CCR7(+) mature DCs as well as CCR7(+) T cells to form clusters that provide local foci of antitumor immune responses [62].
In addition to T cells, DCs may also cooperate with other immune competent cells, such as nature killer (NK) cells, to enhance antitumor effect. TLR9-activated pDCs could induce CTLs cross primed against multiple B16 tumor antigens, which is completely dependent on early recruitment and activation of NK cells. CCR5 expressing NK cells are recruited by CCL3, CCL4, and CCL5 secreted by pDCs, while IFN-γ was produced by NK cells stimulated by OX40L expressed on pDCs [63]. Conversely, IL-18-primed NK cells produce high levels of the iDC-attracting chemokines CCL3 and CCL4 to recruit iDCs in a CCR5-dependent manner and induce the production of CXCR3 and CCR5 ligands, CXCL9, CXCL10, and CCL5, by iDCs to facilitate the subsequent recruitment of CD8(+) T cells [64]. In breast cancer, NK cells take advantage of their own production of IFN-γ to enhance the secretion of chemokines CXCL9, CXCL10, and CXCL11 by tumor cells, which in turn accelerate the infiltration of CXCR3 expressing NK cells into the tumor site [65]. Hence, a positive feedback of DCs, NK cells, and tumor cells may result in the enhancement of antitumor immune responses. In addition, CCR5 and CXCR3 expressing CD8(+) T cells recruited by DCs are predominantly of the Th1 type that executes antitumor effect and colocalizes with macrophages and neutrophils to amplify the cell-mediated immune responses [56].
2.2. Tumor Infiltrating Immune Suppressive Cells
Immune suppressive cells recruited into tumor microenvironment subvert the host defense and create a microenvironment favoring tumor escape. These cells include myeloid-derived suppressor cells (MDSCs), TAMs, and regulatory CD4(+) T cells (Tregs). For example, in a melanoma model, when CTLs are injected intravenously into tumor-bearing mice, the cells are detected in the tumor as early as on day 1, peaking on day 3, and inhibit tumor growth. However, the antitumor effect is soon diminished with accumulation of MDSCs in the tumor, which outnumber CTLs by day 5. MDSCs produce nitric oxide, arginase I, and reactive oxygen species that inhibit the proliferation of antigen-specific CD8(+) T cells and reduce tumor cell killing. In CCR2−/− mice, the accumulation of MDSCs is significantly reduced, indicating that MDSC infiltration in the tumor is dependent on the chemokine GPCR CCR2 and its ligands, mainly CCL2 produced in the tumor [66].
Different T cell types appear in tumors at different stages of progression. In human ovarian cancer, recruitment of high numbers of Th1 cells was observed in stage II tumors, whereas activated Tregs along with high numbers of monocytes/macrophages and myeloid DCs (mDCs) were observed in disseminated tumors (stages III-IV). All tumor cells, monocytes/macrophages, and mDCs produce CCL22 to recruit Tregs via the GPCR CCR4. The specific recruitment of Tregs results in immune suppression in the advanced stages of ovarian cancer [67]. The paradox that early stage tumors are inhibited by infiltrating antitumor immune cells which is reversed by suppressive Tregs through CCR4/CCL22 interaction is also observed in myeloma [68]. Thus, chemokines and GPCRs play a crucial role in regulating pro- and antitumor responses by recruiting different types of immune cells (Table 2).
Table 2.
Chemoattractant GPCRs associated with stromal cell infiltration.
| GPCRs | Tumor types | |
|---|---|---|
| Immune cells | ||
|
| ||
| Dendritic cells | CCR1 | Hepatocellular carcinoma [57] |
| CCR5 | Hepatocellular carcinoma [57], ovarian cancer [69] | |
| CCR6 | Breast cancer [70, 71], colorectal cancer and lung cancer [72], lymphoma [73], melanoma [58, 72], lymphocyte-rich gastric cancer [74], renal cell carcinoma [62], thyroid cancer [75] | |
| CCR7 | Breast cancer [76], renal cell carcinoma [62] | |
| CXCR1/2 | Colorectal cancer [77–79], gastric cancer [79], hepatocellular carcinoma [78], pancreatic cancer [78] | |
|
| ||
| Myeloid-derived suppressor cells | CCR2 | Basal cell carcinomas [80], melanoma [66] |
| CXCR2 | Colitis-associated cancer [81] | |
| CXCR4 | Gastric cancer [82], ovarian cancer [83] | |
|
| ||
| Tumor-associated macrophages | PAFR | Melanoma [84] |
| CCR2 | Breast cancer [85], cervical cancer [86], colitis-associated cancer [87], lymphoma [88], nasopharyngeal carcinoma [89], oral cancer [90], prostate cancer [91], pancreatic cancer [92], thyroid cancer [93] | |
| CCR4 | Lung cancer [94] | |
| CCR5 | Hepatocellular carcinoma [95], nasopharyngeal carcinoma [89] | |
| CXCR3 | Breast cancer [42] | |
| CX3CR1 | Breast cancer [96], glioma [97] | |
|
| ||
| Regulatory T cells | CCR4 | Breast cancer [98], cervical cancer [99], Hodgkin lymphoma [100], gastric cancer [101], glioma [102], melanoma [103] |
| CCR5 | Colorectal cancer [104], lymphoma [105], pancreatic cancer [106], renal cell carcinoma [107] | |
| CCR6 | Breast cancer [108], colorectal cancer [109], hepatocellular carcinoma [110], Hodgkin lymphoma [111], renal cell carcinoma [107] | |
| CCR7 | Melanoma [112], ovarian cancer [113] | |
| CCR10 | Ovarian cancer [35] | |
| CXCR1 | Lung cancer, mesothelioma, melanoma [114] | |
| CXCR3 | Renal cell carcinoma [107] | |
| CXCR4 | Breast cancer [115], B cell lymphoma [116], hepatocellular carcinoma [117], lung cancer [118], glioma [119], ovarian cancer [120, 121] | |
| CXCR6 | Nasopharyngeal carcinoma [122], renal cell carcinoma [107] | |
|
| ||
| Stromal cells | ||
|
| ||
| Mesenchymal stem cells | FPR2 | Ovarian tumor [123] |
| CCR2 | Breast cancer [124], glioma [125], lymphoma [88] | |
| CXCR1 | Glioma [126, 127] | |
| CXCR2 | Kidney cancer [128], glioma [127] | |
| CXCR4 | Breast cancer [129], gastric cancer [130], glioma [125, 131, 132] | |
| CXCR6 | Glioma [132], prostate cancer [133] | |
| CX3CR1 | Colorectal cancer [134] | |
2.2.1. Tregs
Treg is a CD4(+)CD25(+)FoxP3(+) T cell subtype. Treg expresses chemokine GPCR CCR4 and responds to the ligands CCL1 and CCL22 to accumulate in tumors. The degree of Treg infiltration is correlated with the prognosis of tumor patients [108, 110, 135]. A similar prognostic value was also obtained by the ratio of CD8(+) T cell/CCR4(+) Treg [136]. In melanoma, deletion of CD45RA(−)FoxP3(hi)CD4(+) Tregs (effector Tregs) using anti-CCR4 antibody significantly augmented CD8(+) T cell infiltration in the tumor and unmasked a nascent antitumor host response [137]. The recruitment of Tregs into the tumor microenvironment depended on the presence of CD8(+) T cells that produce ligands for CCR4 [138]. Therefore, the balance of infiltrating CCR4(+) Tregs and CD8(+) T cells in tumor tends to be a seesaw. Tregs can also interact with other cells in the tumor microenvironment. For instance, in a highly metastatic breast cancer model, only a proportion of CCR4(+) tumor cells in the primary tumor establish lung metastasis. Implanted orthotopic primary tumors “remotely” stimulate the expression of CCL17 and CCL22 in the lungs, which attract both CCR4(+) Tregs and tumor cells. CCR4(+) Tregs protect CCR4(+) tumor cells from being attacked by antitumor host immune cells. In fact, in the absence of CCR4(+) Tregs, CCR4(+) tumor cells disseminated into the lung are efficiently eliminated by NK cells, because CCR4(+) Tregs directly kill NK cells using beta-galactoside-binding protein [139]. Interestingly, in return, NK cells themselves also may attract Tregs through the CCR4/CCL22 interaction. In a Lewis lung cancer (LLC) implantation model, mouse lungs bearing LLC secrete CCL22 to recruit Tregs to suppress the proliferation of endogenous CD4(+)CD25(−) cells and the only cell type in the lung to produce CCL22 is NK cells [140]. CCR4/CCL22 even induces Tregs to selectively infiltrate into a particular site in the tumor, such as the area of lymphoid aggregates where Tregs are activated and proliferate in response to tumor-associated antigens presented by DCs. However, this process does not occur in the tumor bed [98, 141]. In addition, there are other GPCRs and ligands that may recruit Tregs, such as CCR5/CCL5 in colorectal cancer (CRC) and pancreatic cancer [104, 106], CCR6/CCL20 in HCC and breast cancer [108, 110], and CCR10/CCL28 in ovarian cancer [35], while CXCR3 and CXCR6 are expressed by Tregs infiltrating renal cell carcinoma [107]. Since Tregs are believed to be one of the major suppressive host cells that interfere with antitumor immune response, targeting GPCRs should be one of the effective measures to diminish Treg infiltration of the tumor environment thereby restoring tumor immunity.
2.2.2. TAMs
In addition to the complicated interaction between Tregs and other tumor suppressing immune cells in the microenvironment, there are also other tumor supporting immune cells as important constituents. In a mouse CRC model, CCR6(+) Tregs are recruited into the tumor by responding to CCL20 secreted not only by tumor cells but also by TAMs. After targeted deletion of TAMs, Treg recruitment was abrogated with reduced tumor growth [109].
Macrophages are a major tumor infiltrating immune cell type that may affect tumor growth by either anti- or protumor effects [142]. Blood-derived monocytes infiltrate tumor tissues and differentiate into macrophages followed by further polarization into M1 or M2 phenotype, which differs in their patterns of cytokine secretion and biological function [143]. M1 macrophages mediate tumor suppression through type I cytokine production and tumor antigen acquisition and presentation [142, 144], whereas M2 macrophages promote tumor progression by producing type II cytokines [145]. Unfortunately, TAMs largely are of the M2 phenotype and promote the progression of almost all known solid tumors. Tumors produce many cytokines and other mediators that propel TAMs into the M2 phenotype [146]. Chemoattractant GPCRs are critical for TAM infiltration in the tumor, including chemokine GPCRs and the classical GPCR PAFR [84]. In certain tumor models, phagocytosis of apoptotic tumor cells by macrophages may induce M2 polarization, with the production of anti-inflammatory mediators [84, 147]. The main GPCR and ligand favoring TAM accumulation are CCR2/CCL2, which occurs in numerous tumors, such as pancreatic cancer, cervical cancer, papillary thyroid cancer, and prostate cancer [86, 91–93]. Some tumors also secrete other CCR2 ligands to recruit TAMs, such as HBD-3 in oral cancer [90]. In breast cancer, CCR2/CCL2 interaction recruits macrophages into the lung, where the cells “create” an appropriate microenvironment to facilitate tumor cell lodging and the development of metastatic foci [148]. FPR2 is also a GPCR expressed mainly on macrophages and neutrophils with the capacity to respond to bacterial chemotactic peptides [12]. In the mouse LLC model, tumors implanted subcutaneously grow more rapidly in mice deficient in Fpr2, the orthologue of human FPR2, and show significantly increased infiltration of TAMs with M2 polarization. Macrophages derived from Fpr2 deficient mice express higher levels of the chemokine GPCR, CCR4, which in cooperation with CCR2 mediate a marked increase in macrophage chemotaxis in response to CCL2. In addition, macrophages from Fpr2 deficient mice are more prone to M2 polarization after stimulation with LLC-derived supernatant. In contrast, in the presence of Fpr2, some macrophages develop an M1 phenotype after conditioning with LLC supernatant. Therefore, Fpr2 appears to sustain M1 differentiation of macrophages which participate in anti-LLC host responses [94]. Similarly, mice deficient in the chemokine GPCR CXCR3 exhibit polarization of TAMs into M2 phenotype in breast cancer [42]. Another chemokine GPCR, CX3CR1, and its ligand, CX3CL1, recruit TAMs and sustain the survival of TAMs to promote tumor metastasis [96, 149]. Therefore, chemoattractant GPCRs, in addition to mediating TAM recruitment, also favor TAM polarization to the M2 phenotype in response to tumor microenvironmental factors that promote tumor growth.
2.2.3. MDSCs
Another type of immunosuppressive cells that shape the protumor microenvironment is MDSCs, which consist of subsets of immature myeloid cells with either monocytic or granulocytic morphology [150]. MDSCs are recruited into tumors via the chemokine GPCRs CCR2, CXCR2, or CXCR4 and are believed to promote tumor progression, such as facilitating metastasis in CRC [151, 152]. MDSCs exert their protumor activity by suppressing antitumor effectors, as by inhibiting T cell function via iNOS and arginase [80, 153, 154]. Deletion of CCR2(+) MDSCs using a toxin-mediated ablation strategy increased recruitment of activated CD8(+) T cells into the tumor and thus restored antitumor defense [150]. MDSCs are also capable of sustaining a protumor microenvironment by recruiting Tregs via chemoattractant GPCRs and ligands. For instance, MDSCs release CCL3, CCL4, and CCL5, which activate CCR5 expressed by Tregs and result in their recruitment in both in vitro and in vivo experimental models [105]. In addition to recruiting Tregs, a group of CD11b(+)CCR8(+) myeloid cells similar to MDSCs recruited by CCR8/CCL1 interaction in urothelial and renal carcinomas also “educate” tumor infiltrating T cells to express FoxP3, a marker for Tregs [31]. Thus, MDSCs have been recognized as an important component in the tumor microenvironment that are regulated by chemoattractant GPCRs and ligands. MDSCs also utilize the GPCR/ligand interactions to amplify protumor host response.
2.2.4. Other Tumor Infiltrating Cells
In addition to immune cells, stromal cells in the tumor microenvironment also take part in the regulation of tumor growth. Mesenchymal stem cells (MSCs) are one of the major components in the tumor stroma and are believed to be the precursors of CAFs [155, 156]. MSCs may be recruited into the tumor through FPR2, CCR2, CXCR1, CXCR2, CXCR4, CXCR6, and CX3CR1 depending on the types and locations [125, 126, 128, 133]. Tumor-resident MSCs are often constantly exposed to immune cells and inflammatory cytokines in the microenvironment. They may have acquired functions distinct from normal tissue MSCs that alter the balance of host tumor interaction [88]. For example, compared with bone marrow MSCs, MSCs isolated from spontaneous mouse lymphomas (L-MSCs) promote tumor growth in association with recruitment of large numbers of CD11b(+) Ly6C(+) monocytes, F4/80(+) macrophages, and CD11b(+) Ly6G(+) neutrophils into the tumor. Depletion of monocytes/macrophages, but not neutrophils, completely abolishes the tumor promoting activity of L-MSCs. Such tumor infiltrating monocytes/macrophages are recruited by CCL2 produced by L-MSCs and CCR2 expressed on TAMs [88]. Similarly, CAFs are associated with immune suppressive microenvironment. In Hodgkin lymphoma and cutaneous T cell lymphoma, CAFs secrete the chemokines CCL11 and CCL26 that recruit CCR3(+) T lymphocytes into the tumor and produce high levels of IL-4, a signature of a Th2-dominant microenvironment [157].
In conclusion, GPCRs and ligands are critical for the recruitment of a variety of immune and nonimmune cells into the tumor microenvironment where these cells interact to establish host responses, which, unfortunately, mostly tip the balance to protumor elements.
3. The Role of Chemoattractant GPCRs Expressed by Tumor Cells
While chemoattractant GPCRs contribute to tumor growth by promoting the recruitment of protumor stromal cells and angiogenesis, many tumor cells also express a variety of GPCRs, which, by responding to autocrine and/or paracrine agonists produced in the microenvironment, directly stimulate tumor cell proliferation and tumor spread and expansion (Table 3).
Table 3.
The functions of chemoattractant GPCRs expressed by tumor cells.
| GPCRs | Tumor | Function | References |
|---|---|---|---|
| FPR | |||
| FPR1 | Colorectal cancer | Invasion | [158] |
| Gastric cancer | Invasion | [159] | |
| Glioblastoma | Growth, invasion, vasculogenesis, angiogenesis | [160–163] | |
| FPR2 | Gastric cancer | Invasion | [159] |
| Ovarian cancer | Invasion | [164] | |
| FPR3 | Gastric cancer | Invasion | [159] |
| PAFR | Breast cancer | Migration, proliferation, angiogenesis | [165] |
| Melanoma | Metastasis | [166] | |
| Ovarian cancer | Proliferation, invasion | [167] | |
| C5aR | Bile duct cancer, colorectal cancer | Invasion | [168] |
| Non-small-cell lung cancer | Metastasis | [169] | |
| LTB4R | |||
| BLT1 | Colorectal cancer | Proliferation | [170] |
| BLT2 | Bladder cancer | Metastasis, antiapoptosis | [171, 172] |
| Breast cancer | Metastasis | [173] | |
| Pancreatic cancer | Growth, migration | [174, 175] | |
| Prostate cancer | Antianoikis, antiapoptosis | [176, 177] | |
| Ovarian cancer | Metastasis | [178] | |
| CCR | |||
| CCR1 | Breast cancer | Invasion | [179] |
| Glioma | Proliferation, tumorigenesis | [180] | |
| Hepatocellular carcinoma | Migration, invasion | [181, 182] | |
| Oral squamous cell carcinoma | Migration | [183] | |
| Ovarian cancer | Invasion | [184] | |
| CCR2 | Bladder cancer | Migration, invasion | [185] |
| Breast cancer | Migration, proliferation, antiapoptosis | [186] | |
| Hepatocellular carcinoma | Migration, invasion | [181] | |
| Multiple myeloma | Growth | [187] | |
| Ovarian cancer | Invasion, adhesion, proliferation | [188, 189] | |
| Prostate cancer | Proliferation, migration, invasion | [190, 191] | |
| CCR3 | Lymphoma | Growth | [192] |
| Glioma | Proliferation, tumorigenesis | [180] | |
| Oral squamous cell carcinoma | Migration, invasion | [183] | |
| Ovarian cancer | Invasion, proliferation | [184, 189] | |
| Renal cell carcinoma | Growth, dissemination | [193] | |
| CCR4 | Breast cancer | Growth, metastasis, angiogenesis | [139, 194] |
| Colorectal cancer | Migration | [195] | |
| Gastric cancer | Migration | [196] | |
| Melanoma | Metastasis | [197] | |
| Squamous cell carcinoma of the head and neck | Metastasis | [198] | |
| CCR5 | Breast cancer | Proliferation, metastasis | [25, 199–202] |
| Colorectal cancer | Growth | [203] | |
| Gastric cancer | Metastasis | [204] | |
| Glioma | Proliferation, tumorigenesis | [180] | |
| Hodgkin lymphoma | Growth, metastasis | [205] | |
| Oral cancer | Migration | [206] | |
| Ovarian cancer | Invasion, proliferation | [189] | |
| CCR6 | Colorectal cancer | Proliferation, metastasis | [207, 208] |
| Endometrial adenocarcinoma | Proliferation | [27] | |
| Hepatocellular carcinoma | Metastasis | [209, 210] | |
| Non-small-cell lung cancer | Metastasis | [211] | |
| Pancreatic cancer | Invasion | [212–214] | |
| Squamous cell carcinoma of the head and neck | Metastasis | [215, 216] | |
| CCR7 | Breast cancer | Metastasis, antianoikis | [217, 218] |
| Colorectal cancer | Metastasis | [219, 220] | |
| Melanoma | Growth, metastasis, tumorigenesis | [221, 222] | |
| Non-small-cell lung cancer | Proliferation, antiapoptosis, metastasis | [29, 223–226] | |
| Oral squamous cell carcinoma | Metastasis | [227] | |
| Pancreatic ductal adenocarcinoma | Metastasis | [228] | |
| Prostate cancer | Metastasis | [229] | |
| Squamous cell carcinoma of the head and neck | Proliferation, antiapoptosis, metastasis, adhesion | [230–236] | |
| T cell lymphoma | Dissemination | [237] | |
| CCR8 | Melanoma, breast cancer, leukemia | Metastasis | [32] |
| CCR9 | Breast cancer | Migration, invasion | [238] |
| Colorectal cancer | Inhibiting metastasis | [239] | |
| Ovarian cancer | Migration, invasion | [240] | |
| Pancreatic cancer | Proliferation, invasion | [34, 241] | |
| Prostate cancer | Antiapoptosis | [242] | |
| CCR10 | Melanoma | Growth, metastasis | [243, 244] |
| CXCR | |||
| CXCR1 | Breast cancer | Stem cell self-renewal | [245] |
| Cervical carcinoma | Proliferation | [246] | |
| Colorectal cancer | Metastasis, antiapoptosis, angiogenesis | [247] | |
| Gastric cancer | Invasion | [248] | |
| Glioblastoma | Growth, migration, invasion | [249, 250] | |
| Melanoma | Growth, migration, invasion, angiogenesis, tumorigenesis | [251–253] | |
| Prostate cancer | Growth, angiogenesis | [254] | |
| Renal cell carcinoma | Growth, angiogenesis | [37] | |
| Thyroid carcinoma | Metastasis | [255] | |
| CXCR2 | Breast cancer | Migration, invasion, stem cell self-renewal | [245, 256, 257] |
| Cervical carcinoma | Proliferation | [246] | |
| Colorectal cancer | Proliferation, migration, invasion, angiogenesis | [258–261] | |
| Gastric cancer | Metastasis | [262, 263] | |
| Glioblastoma | Growth, migration | [249, 264] | |
| Melanoma | Growth, migration, invasion, angiogenesis, tumorigenesis | [251–253] | |
| Nasopharyngeal carcinoma | Growth | [265] | |
| Non-small-cell lung cancer | Growth, metastasis, angiogenesis | [266, 267] | |
| Ovarian cancer | Growth, angiogenesis | [268] | |
| Pancreatic cancer | Invasion, angiogenesis | [269] | |
| Prostate cancer | Growth, angiogenesis | [254] | |
| Renal cell carcinoma | Growth, angiogenesis | [37] | |
| Thyroid carcinoma | Metastasis | [255] | |
| CXCR3 | Breast cancer | Metastasis; inhibiting growth | [270–273] |
| Colorectal cancer | Metastasis | [274] | |
| Glioma | Growth | [275, 276] | |
| Lung adenocarcinoma | Metastasis | [226] | |
| Melanoma | Migration | [277] | |
| Myeloma | Inhibiting/promoting proliferation and apoptosis | [43] | |
| Ovarian cancer | Growth, metastasis | [278] | |
| Prostate cancer | Metastasis | [279] | |
| Renal cell carcinoma | Growth, metastasis | [280, 281] | |
| CXCR4 | At least 23 haematopoietic and solid cancers | Growth, metastasis, angiogenesis | [1, 44] |
| CXCR5 | Breast cancer | Metastasis | [282] |
| Colorectal cancer | Growth, migration | [283] | |
| Neuroblastoma | Inhibiting/promoting metastasis | [45, 284] | |
| Prostate cancer | Proliferation, invasion, migration, adhesion | [285–288] | |
| CXCR6 | Colorectal cancer | Growth, migration, invasion | [289] |
| Hepatocellular carcinoma | Growth, metastases, angiogenesis | [3] | |
| Melanoma | Stem cell self-renewal | [290] | |
| Nasopharyngeal carcinoma | Metastasis | [291] | |
| Pancreatic ductal adenocarcinoma | Invasion | [292] | |
| Prostate cancer | Proliferation, metastasis | [293–295] | |
| Renal cell carcinoma | Inhibiting migration | [296] | |
| CXCR7 | Breast cancer | Inhibiting invasion; growth, angiogenesis | [297] |
| Cervical carcinoma | Growth, adhesion | [298] | |
| Glioma | Growth, migration, sphere and tube formation | [49, 299] | |
| Hepatocellular carcinoma | Growth, metastasis, angiogenesis | [300, 301] | |
| Lymphoma | Growth, adhesion | [298] | |
| Nasopharyngeal carcinoma | Metastasis | [291] | |
| Neuroblastoma | Inhibiting growth; metastasis | [50, 302] | |
| CX3CR | |||
| CX3CR1 | Epithelial ovarian carcinoma | Proliferation, migration, adhesion | [303] |
| Glioma | Inhibiting invasion | [304] | |
| Neuroblastoma | Migration | [305] | |
| Pancreatic ductal adenocarcinoma | Migration | [306, 307] | |
| Prostate cancer | Metastasis | [308–310] | |
| Renal cell carcinoma | Metastasis | [311] | |
| XCR | |||
| XCR1 | Epithelial ovarian carcinoma | Proliferation, metastasis | [312] |
| Oral squamous cell carcinoma | Proliferation, migration, invasion | [313] |
In anaplastic large cell lymphomas, the CCR3/CCL11 interaction promotes tumor cell proliferation and inhibits apoptosis through ERK1/2, Bcl-xL and the production of survivin [192]. Similarly, through an AKT signaling pathway, CCR7 and its ligands CCL19 and CCL21 induce squamous cell carcinoma of the head and neck growth in vitro and in vivo [230]. In addition, CCR6/CCL20 interaction in endometrial adenocarcinoma, CXCR1/2/CXCL7 interaction in clear cell renal cell carcinoma, CXCR2/CXCL8 interaction in nasopharyngeal carcinoma, and CXCR6/CXCR16 interaction in HCC are reported to promote tumor cell growth [3, 27, 37, 265]. Hypoxia, which occurs during tumor expansion, induces the production of GPCR ligands that promote tumor cell proliferation in an autocrine manner. In cervical carcinoma, hypoxia stimulates tumor cells to express high levels of CXCR1/2 and CXCL8 that respond to ligands in the microenvironment by proliferating [246]. Actually, numerous chemoattractant GPCRs, such as CCR1, CCR5, CXCR5, CXCR7, and PAFR, are expressed by various types of tumor cells and are implicated in tumor growth [1]. In the case of the same GPCR, CXCR3, its two variants have opposite functions. CXCR3-A promotes cells growth but CXCR3-B mediates growth-inhibitory signals and induces apoptosis in various tumors [270].
In addition to tumor cells, stromal cells in the microenvironment also secrete GPCR ligands that stimulate the receptors on tumor cells in a paracrine manner which may represent a more important yet complicated stimulating loop. This is exemplified by observations in human glioma in which CXCR4/CXCL12 interaction favors an autocrine or paracrine loop for tumor cell proliferation [314, 315]. CXCR4/CXCL12 growth stimulating effects were also detected in glioma stem cells via an AKT-mediated prosurvival and self-renewal pathway. Highly malignant human glioblastoma cells (GBM) express a classical chemoattractant GPCR, FPR1, which recognizes a ligand, Annexin A1, released by necrotic GBM cells that mediates the proliferation of live GBM cells to increase their invasiveness and the production of angiogenic factors vascular endothelial growth factor (VEGF) and CXCL8 (IL-8), which stimulate VEGF receptor (VEGFR) and CXCR1/CXCR2 on vascular ECs to promote their migration and formation of new vasculature [316, 317]. It is interesting to note that FPR1 in GBM cells does not act alone; instead, the GPCR transactivates EGFR which accounts for part of the GBM growth stimulating activity of FPR1. GBM cells are able to maximally exploit the supportive mediators in the microenvironment to their advantage [1, 318]. By stimulating GPCR, tumor cells may even change the phenotype of neighboring stromal cells. Breast tumor cells secrete CCL20 to activate the ERK1/2/MAPK pathway in surrounding “normal” breast epithelial cells via CCR6 and promote their malignant transformation [319].
CAFs have been recognized as important regulators of tumor initiation by secreting CXCL12 to activate CXCR4 on breast cancer cells and stimulate tumor growth [320]. Studies have also shown that, after activation by CXCL12, breast cancer cells secrete another chemokine CCL20 that activates CCR6 expressed by tumor cells and facilitates their proliferation [321], while, in Hodgkin lymphoma, CAFs from tumor-involved lymph nodes cocultured with Reed-Sternberg cells produce CCL5, which activates CCR5 on tumor cells to stimulate tumor growth [205]. Multiple myeloma (MM) cells and osteoclasts (OCs) form yet another example of tumor promoting activity of GPCR/ligand interactions. MM growth in the bone marrow niche depends on bone resorption and interaction with active OCs [322, 323]. MM cells secrete CCL3 to activate OCs through its receptor CCR1 [324]. CCR1/CCL3 interaction inhibits the function of osteoblasts (OBs), resulting in the loss of OB/OC balance, which could facilitate MM growth [325]. Also, OCs in the tumor microenvironment sustain MM cell proliferation through production of chemokine that activate CCR2 on tumor cells [187]. These pathways culminate in MM outgrowth.
Based on these observations, it is now clear that chemokine GPCRs expressed by tumor cells and autocrine or paracrine ligands form a formidable interaction in the microenvironment that orchestrates the crisscross interaction between tumor cells and stromal cells stimulating further growth of the tumors.
4. The Role of Chemoattractant GPCRs in Tumor Metastasis
Metastasis is the major cause of cancer death. In order for cancer cells to metastasize, the cells should acquire a motile phenotype and be able to detach from the primary tumor mass to degrade basement membrane and intravasate into the blood or lymph vessels. After trafficking in the blood or lymphatic vessels, tumor cells tend to form emboli extravasating into distant organs or lymph nodes [1, 326]. Nearly each step of metastasis is heavily dependent on the tumor microenvironment and chemoattractant GPCRs are active participants in the processes.
A historical discovery of the role of chemoattractant GPCR/ligand interactions in promoting cancer metastasis was reported in 1998, in which the chemokine CCL2 (MCP-1) was shown to mediate kidney specific metastasis of a subpopulation of a murine experimental lymphoma [327]. This was followed by a more detailed study of several human cancer cell lines including breast and lung cancer cells which metastasized into distant organs in nude mice by using several chemokine GPCRs. These findings enriched the “seed” and “soil” paradigm of cancer metastasis by including chemoattractant GPCRs as the requisite for tumor cells as qualified “seeds” and a ligand producing distant organ or draining lymph nodes as suitable “soil” [328]. Since then, studies of the role of chemoattractant GPCRs and ligands in cancer metastasis have become a burgeoning research field and many malignant tumors have been shown to utilize a variety of GPCR/ligand interactions for metastasis. For example, in lung cancer, hypoxia induces the expression of CCR7 by tumor cells that increases cell invasiveness and eventual lymph node metastasis [29]. Hypoxia also promotes lymph node metastasis of breast cancer by increasing the expression of CCR5 on tumor cells and the ligand CCL5 in lymph nodes via the transcription factor hypoxia-inducible factor- (HIF-) 1α [25]. In prostate cancer and pancreatic ductal adenocarcinoma, cancer metastasis is associated with CX3CR1 on tumor cells and the ligand CX3CL1 at metastasis site [306, 308]. The sources of chemoattractants in tumor microenvironment are from both tumor and stromal cells. In prostate cancer, hypoxia-preconditioned MSCs produce CCL21 to attract tumor cells expressing CCR7 which is associated with enhanced lymph node metastasis of the tumor [229]. Similarly, under hypoxia, MSCs promote breast cancer metastasis through CXCR3/CXCL10 interaction [271].
Chemoattractant GPCRs and their ligands reportedly involved in enhanced tumor metastasis are listed in Table 4. Recently, cancer stem cells (CSCs) have been shown to account for most of the cancer metastasis. Interestingly, chemoattractant GPCRs participate in the maintenance of the metastatic property of CSCs by forming an autocrine loop. In ovarian cancer, the invasiveness of CD133(+) CSCs is enhanced by the chemokine CCL5, which activates CCR3 and CCR5 expressed by the cells to increase matrix metalloproteinase (MMP) 9 secretion [184]. A number of studies that use exogenous chemokines to induce cell invasion are in the literature. However, there are also a small number of chemokine and GPCR interactions that may inhibit tumor cells invasion, such as CX3CR1/CX3CL1 interaction in glioma [304].
Table 4.
Chemoattractant GPCRs associated with tumor metastasis.
| Tumor type | GPCRs | Ligands | Metastatic sites |
|---|---|---|---|
| Bladder cancer | BLT2 | LTB4 | Lung [171] |
| CCR2 | CCL2 | Lung [329] | |
| CXCR6 | CXCL16 | Perineural and lymphovascular invasion [330] | |
|
| |||
| Breast cancer | BLT2 | LTB4 | Lung [173] |
| CCR2 | CCL2 | Lung [85, 148], bone [148] | |
| CCR4 | CCL17/22 | Lung [139, 194, 331] | |
| CCR5 | CCL5 | Lung [200, 201], lymph node [25, 332] | |
| CCR6 | Pleura [333] | ||
| CCR7 | CCL19/21 | Lymph node [218, 334–337] | |
| Skin [333] | |||
| CCR8 | CCL1 | Lymph node [32] | |
| CCR9 | CCL25 | Lymph nodes and gastrointestinal tract [238] | |
| CXCR1 | CXCL8 | Bone [338, 339] | |
| CXCR2 | Lung [340], bone [341] | ||
| CXCR3 | CXCL9 | Lung [342] | |
| CXCL10 | Bone [343], lung [344] | ||
| CXCR4 | CXCL12 | Lymph node [328, 336, 337, 345], bone [346–348], lung [328, 346, 349], liver [333] | |
| CXCR5 | CXCL13 | Lymph node [282] | |
| CXCR6 | CXCL16 | Lymph node [350] | |
| CXCR7 | CXCL12 | Lung, greater omentum, and lymph nodes [351] | |
| CX3CR1 | Brain [333] | ||
|
| |||
| Cervical cancer | CXCR4 | CXCL12 | Lymph node [352] |
| CXCR4/7 | CXCL12 | Lymph node [353, 354] | |
|
| |||
| Colorectal cancer | CCR1 | CCL7/9/15 | Liver [355–357] |
| CCR2 | CCL2 | Liver [151, 358], lung [359] | |
| CCL7 | Liver [356] | ||
| CCR3 | CCL7 | Liver [356] | |
| CCR5 | CCL5 | Liver and lung [203] | |
| CCR6 | CCL20 | Liver [207, 360] | |
| CCR7 | CCL21 | Lymph node [219, 220, 361] | |
| CXCR1/2 | Liver [247] | ||
| CXCR2 | CXCL1 | Lymph node [261], liver [362] | |
| CXCL8 | Skin [363] | ||
| CXCR3 | CXCL9 | Lymph node [364] | |
| CXCL10 | Lymph node [364], lung [365] | ||
| CXCL11 | Lung [365] | ||
| CXCR6 | CXCL16 | Liver [289, 366] | |
| CXCR4 | CXCL12 | Liver [367–370], lymph node [371, 372], brain [373] | |
|
| |||
| Esophageal cancer | CCR7 | CCL21 | Lymph node [374–376] |
| CXCR2 | Lymph node [377] | ||
| CXCR4 | CXCL12 | Lung [378, 379], liver [378, 379], lymph node [378, 380], peritoneum [379], retroperitoneum [379] | |
|
| |||
| Gastric cancer | FPR1/2/3 | Annexin A1 | Peritoneum [159] |
| CCR2 | CCL2 | Lymph node [381] | |
| CCR4 | CCL17 | Lymph node, lung, and bone [194] | |
| CCR5 | CCL5 | Lymph node [204] | |
| CCR7 | Lymph node [30, 382, 383] | ||
| CXCR2 | CXCL1 | Lymph node [262] | |
| CXCR4 | CXCL12 | Lymph node [382, 384–387], peritoneum [388–390], liver [387] | |
|
| |||
| Glioma | CXCR4/7 | CXCL12 | Bone marrow [299] Lymph node, distant organs [391] |
|
| |||
| Head and neck squamous cell carcinoma | CCR4 | CCL22 | Lymph node [198] |
| CCR6 | CCL20 | Lymph node [216, 392] | |
| CCR7 | CCL19/21 | Lymph node [227, 230–232, 393] | |
| CXCR2 | CXCL1/8 | Lymph node [394, 395] | |
| CXCR4 | CXCL12 | Lymph node [393, 396], lung [397, 398] | |
| CXCR5 | CXCL13 | Bone [399] | |
| XCR1 | XCL1 | Lymph node [313] | |
|
| |||
| Hepatocellular carcinoma | CCR7 | Intrahepatic metastasis, lymph node [400] | |
| CXCR4 | CXCR12 | Lung [401], bone [402, 403], lymph node [404] | |
| CXCR6 | CXCL16 | Lung [3] | |
| CXCR7 | CXCL12 | Lung [300, 405] | |
|
| |||
| Lymphoma | CCR7 | CCL21 | Lymph node [237] |
|
| |||
| Leukemia | CCR8 | CCL1 | Lymph node [32] |
| CXCR4 | CXCL12 | Extramedullary sites (liver, kidney, spleens, and peripheral blood) [406] | |
|
| |||
| Melanoma | FPR1/2/3 | Annexin A1 | Lung [407] |
| PAFR | PAF | Lung [166, 408, 409] | |
| CCR2 | CCL2 | Lung [410] | |
| CCR3 | Brain [411] | ||
| CCR4 | CCL22 | Brain [197, 411] | |
| CCR5 | CCL4 | Lung [412, 413] | |
| CCR7 | CCL21 | Lymph node [221, 222, 244, 414], liver [415] | |
| CCR8 | CCL1 | Lymph node [32] | |
| CCR9 | CCL25 | Small intestinal [416, 417] | |
| CCR10 | CCL27 | Skin [243, 244] | |
| CXCR2 | CXCL8 | Lung [418] | |
| CXCR3 | CXCL10 | Lymph node [419, 420], bone [421] | |
| CXCR4 | CXCL2 | Lung [244, 422–424] | |
|
| |||
| Neuroblastoma | CXCR3 | CXCL10 | Bone marrow [425] |
| CXCR4 | CXCL12 | Bone [426–428], liver [429, 430], kidney [430], bone marrow [428, 430] | |
| CXCR5 | CXCL13 | Bone marrow [284] | |
| CXCR4/7 | CXCL12 | Bone marrow [302] | |
| CX3CR1 | CX3L1 | Bone marrow [305] | |
|
| |||
| Non-small-cell lung cancer | C5aR | Lymph node [169] | |
| CCR4 | CCL22 | Bone [431] | |
| CCR6 | CCL20 | Adrenal specific metastasis [211] | |
| CCR7 | CCL19/21 | Lymph node [29, 226, 432] | |
| CXCR2 | CXCL5 | Hilar and mediastinal lymph nodes, chest wall, and contralateral lung; extrathoracic distant metastases (para-aortic lymph nodes, liver, adrenal glands, kidneys, spleen, and diaphragm) [266] | |
| CXCR4 | CXCL12 | Lungs, liver, bone marrow, adrenal glands [433], pleural [434], brain [433, 435] | |
| CX3CR1 | Brain and liver [436] | ||
|
| |||
| Osteosarcoma | CCR7 | CCL21 | Lymph node [334] |
| CXCR3 | CCL9/10/11 | Lung [437] | |
| CXCR4 | CXCL12 | Lung [438] | |
| CXCR7 | CXCL12 | Lung [439] | |
|
| |||
| Ovarian carcinoma | BLT2 | Diaphragm, intestine, and mesentery (intraperitoneal dissemination) [178] | |
| CCR3 | CCL5 | Liver, bowel, and spleen [184] | |
| CCR9 | CCL25 | Small intestinal [440] | |
| CXCR4 | CXCL12 | Pelvic [441], lymph node [442, 443], peritoneum [444] | |
| CXCR6 | CXCL16 | Lymph node [443] | |
| XCR1 | XCL1/2 | Diaphragm, peritoneal wall, colon, spleen, and liver [312], peritoneum [312] | |
|
| |||
| Pancreatic cancer | CCR2 | CCL2 | Liver [92, 445], peritoneal [445] |
| CCR7 | CCL21 | Lymph node [228, 446] | |
| CXCR4/7 | CXCL12 | Liver [447, 448], lung [448], lymph node [449] | |
|
| |||
| Prostate cancer | CCR2 | CCL2 | Bone [450] |
| CCR7 | CCL21 | Lymph node [229] | |
| CXCR1/2 | CXCL8 | Lymph node [451] | |
| CXCR3 | CXCL4/10 | Lymph node, liver, lung, adrenal [279] | |
| CXCR4 | CXCL12 | Bone [133, 452, 453] | |
| CXCR5 | CXCL13 | Bone [288] | |
| CXCR6 | CXCL16 | Bone [133, 294, 453], liver [294] | |
| CX3CR1 | CX3CL1 | Bone [310] | |
|
| |||
| Renal cell carcinoma | CCR1/3 | CCL15 | Bone [454] |
| CCR5 | CCL3 | Lung [326] | |
|
| |||
| Thyroid papillary cancer | CCR7 | CCL21 | Lymph node [455, 456] |
| CXCR1/2 | CXCL8 | Lymph node [255] | |
| CXCR4 | Lymph node [455, 457, 458] | ||
| CXCR7 | Lymph node [459] | ||
While the aberrant expression of chemoattractant GPCRs is an important feature for a motile phenotype of tumor cells, the next step of tumor cell metastasis from the primary mass is detachment. These cells must survive the loss of interactions with extracellular matrix (ECM) that causes anoikis for further invasion of blood or lymph vessels [217]. In breast cancer, the activation of both CXCR4/CXCL12 and CCR7/CCL21 may reduce the sensitivity of metastatic cancer cells to anoikis by upregulating antiapoptotic proteins. Consequently, blocking the chemokine and GPCR interactions attenuates breast cancer metastasis in vivo [217]. Recently, another classical chemoattractant GPCR, BLT2, has also been shown to establish resistance to anoikis in prostate cancer cells through a BLT2-NOX-ROS-NF-κB cascade [176].
Thus, accumulating evidence indicates an essential role of chemoattractant GPCRs and ligands in every step of cancer metastasis, including the acquisition of increased motility, detachment from the primary tumor mass by breaking down matrix proteins, intra- and extravasation, and lodgment in distant organs and lymph nodes. In addition, chemoattractant GPCRs and ligands also orchestrate the interaction of metastatic tumor cells with stromal cells, such as TAMs, ECs, and fibroblasts, which act either as “driving forces” for tumor cell dissemination or as “conditioners” of the “soil” that facilitates the settlement of metastatic tumor cells to develop secondary foci. Therefore, chemoattractant GPCRs and ligands provide promising molecular targets for prevention of tumor metastasis.
5. The Role of Chemoattractant GPCRs in Tumor Neovascularization
Neovascularization is critical for consolidation of the tumor microenvironment for tumor progression. Chemoattractant GPCRs provide pro- and antiangiogenic factors and receptors and are able to regulate two phases of neovascularization: vasculogenesis and angiogenesis (Table 5).
Table 5.
Chemoattractant GPCRs associated with tumor neovascularization.
| Receptors | Tumors | |
|---|---|---|
| Vasculogenesis | FPR1 | Glioma [160] |
| FPR2 | Ovarian cancer [123] | |
| CCR2 | Hepatocellular carcinoma [460] | |
| CCR5 | Hepatocellular carcinoma [460] | |
| CCR6 | Hepatocellular carcinoma [461] | |
| CXCR2 | Pancreatic cancer [462] | |
| CXCR4 | Breast cancer [320], melanoma [463] | |
|
| ||
| Angiogenesis | FPR1 | Glioma [161, 162, 316] |
| C5aR | Epithelial ovarian cancer [17] | |
| CCR1 | Hepatocellular carcinoma [464], lymphoma [465], multiple myeloma [466] | |
| CCR2 | Breast cancer [22, 467, 468], esophageal cancer [469], gastric cancer [381], melanoma [470] | |
| CCR4 | Breast cancer [194] | |
| CCR5 | Multiple myeloma [466], renal cell carcinoma [326] | |
| CCR10 | Ovarian cancer [35] | |
| CXCR1 | Prostate cancer [471], renal cell carcinoma [37] | |
| CXCR2 | Cervical cancer [472], colorectal cancer [258, 259], glioblastoma [473], lung adenocarcinoma [267, 474, 475], melanoma [418, 476], ovarian cancer [268], pancreatic cancer [269, 477–479], prostate cancer [480], renal cell carcinoma [37, 481] | |
| CXCR1/2 | Glioblastoma [482], melanoma [251, 253], multiple myeloma [483], ovarian cancer [484], pancreatic cancer [485], prostate cancer [254, 451, 486], renal cell carcinoma [37] | |
| CXCR4 | Breast cancer [487], colorectal cancer [488, 489], gastric cancer [490], glioblastoma [491–493], hepatocellular carcinoma [494], ovarian cancer [495], pancreatic cancer [269, 496], prostate cancer [497], squamous cell carcinoma [398] | |
| CXCR6 | Hepatocellular carcinoma [3], prostate cancer [295] | |
| CXCR7 | Bladder cancer [498], breast cancer [297], breast and lung cancer [499], colorectal cancer [488], hepatocellular carcinoma [301], prostate cancer [500], renal cell carcinoma [501] | |
| CX3CR1 | Breast cancer [96], colorectal cancer [149], melanoma [502] | |
5.1. Vasculogenesis
Vasculogenesis is the formation of new blood vessels from circulating bone marrow-derived endothelial progenitor cells (EPCs). Coordinated events are required for the recruitment and incorporation of EPCs into the tumor tissue, including migration, invasion, differentiation, proliferation, and formation of vessels [461]. Although VEGF is a well-known angiogenic factor taking part in the vasculogenesis, other paracrine factors, such as chemoattractants produced by tumor cells, are also involved. EPCs expressing CXCR4 are mobilized by the ligand CXCL12 in an autocrine or paracrine manner [503]. Another chemokine CCL2 also mobilizes EPCs from the bone marrow [504]. These chemokines then promote EPC proliferation and guide the cells into tumor stroma to form functional neovasculature [505]. EPCs participating in neovascularization have also been reported in HCC, in which myeloid-derived EPCs (colony forming unit-endothelial cells) as early EPCs highly express CCR6 and are mobilized by the ligand CCL20 produced by HCC cells for migration and invasion of tumor stroma to form vasculature. CCR6/CCL20 in tumor microenvironments in addition plays a crucial role in driving phenotypic switch of hematopoietic cells with increased potential for angiogenic EC differentiation and attenuated proinflammatory activity [461]. A classical chemoattractant receptor, FPR1, may also participate in vasculogenesis in human GBM. This was shown in a xenograft model in which the number of EPCs incorporated into intracranial GBM lesion was significantly reduced in tumors formed by GBM cells in which FPR1 was depleted by RNA interference. The EPC chemotactic and tubule-stimulating activities were also attenuated in the supernatant of GBM cells deficient in FPR1 [160]. Another classical chemoattractant GPCR, the FPR1 variant FPR2, has also been reported to participate in recruiting MSCs into tumor tissues to promote the formation of neovasculature in response to tumor-derived ligand LL-37 [123].
5.2. Angiogenesis
Angiogenesis is a process in which new blood vessels sprout from existing vasculature. In tumor microenvironment, various cells regulate this process through GPCRs, which are expressed on vascular ECs and mediate cell recruitment and proliferation thereby extending the new vasculature in response to the ligands produced by tumor and other stromal cells. Tumor cells, tumor stem cells, and infiltrating TAMs in particular also express GPCRs capable of promoting the release of proangiogenic factors recruiting and activating vascular ECs [1].
FPR1 selectively expressed by GBM cells when activated by exogenous and tumor derived agonists promotes tumor cells to produce proangiogenic factors VEGF and the angiogenic chemokine CXCL8 [161, 316, 506]. CXCR1/2 expressed by vascular ECs and CXCL8, the ligand produced by tumor and stromal cells, are known to promote angiogenesis through inducing EC migration and formation of tubules [484, 507]. GBM stem cells may also utilize chemoattractant GPCRs FPR1 and CXCR4 to participate in angiogenesis by releasing VEGF [162, 249].
In addition to the direct interaction between chemoattractant GPCRs expressed by ECs and ligands in the tumor microenvironment, tumors take the advantage of infiltrating stromal cells, such as CAFs, TAMs, and Tregs, to benefit angiogenesis through GPCRs. In lung cancer, CAFs express CCR5 and are activated by CCL3 to secrete hepatocyte growth factor (HGF) to accelerate angiogenesis [326]. CAFs also cooperate with tumor cells to promote angiogenesis through CXCR4 expressed by both cell types. In pancreatic cancer, tumor cells secrete CXCL8 and CAFs secrete CXCL12 to enhance the recruitment and proliferation of ECs. However, CXCL12 promotes EC infiltration and CXCL8 enhances tubule formation by ECs revealing distinct functions of the CXCR2/CXCL8 and CXCR4/CXCL12 interactions in the process [269].
In addition, TAMs are an important source of angiogenic factors in tumor. For example, CCR2 and CD40 on TAMs are activated by CCL2 and CD40L produced in gastric cancer tissues and synergistically promote VEGF production to increase microvessel density [381, 508]. Moreover, Tregs expressing CCR10 are capable of accelerating angiogenesis through secreting VEGF in response to CCL28 produced by hypoxic tumor cells for EC infiltration and participation in angiogenesis [35].
It is interesting to note that alcohol consumption contributes to increased breast cancer angiogenesis, thus promoting the growth and metastasis of tumor cells in an animal model. This involves upregulated expression of CCR2 and CCL2 by tumor cells that increase the interaction between tumor and vascular ECs [467]. Another physical and chemical factor, radiation, exerts a similar effect through CXCR4/CXCL12 interaction on tumor angiogenesis [509].
Conversely, some chemoattractant GPCRs, such as CXCR3, are reported to mediate angiostatic activity through non-ELR CXC chemokines CXCR4/9/10/11 in various tumors [1]. The controversial results of angiogenesis are also found in C5aR [16, 17]. Therefore, angiogenesis may be regulated by a complex balancing process between opposing pro- and antiangiogenic GPCR and ligand interactions.
6. Perspectives
Accumulating evidence indicates crucial roles of chemoattractant GPCRs and their ligands in tumor progression by shaping tumor microenvironment. Almost all cell types including tumor cells per se are able to take the advantage of GPCRs and ligands to affect tumor progression. Chemoattractant GPCRs and ligands are involved in almost every step of tumor development and progression such as increasing tumor cell motility, invasiveness, intra- and extravasation, dissemination, leukocyte infiltration, and angiogenesis. These render the GPCRs and ligands promising drug targets for disruption of the tumor progression cascade. Recently, new agents targeting chemoattractant GPCRs have been developed and are being tested in the clinic, such as a humanized anti-CCR4 monoclonal antibody, mogamulizumab (KW-0761), aiming at curtailing cutaneous T cell lymphoma [510]. Therefore, gaining a better understanding of the GPCRs and their ligands in tumor microenvironment is vital and will provide novel therapeutic opportunities.
Acknowledgments
This project has been funded in part by Federal funds from the National Cancer Institute, National Institutes of Health, under Contract no. HHSN261200800001E. The research was also supported in part by the Intramural Research Program of the NCI, NIH. Jiamin Zhou is funded in part by China Scholarship Council, National Natural Science Foundation of China (no. 81101566), and Scientific Funds of Shanghai Government (11DZ2280400, 12QA1400600, XYQ2011017, and 11411950500). Yi Xiang is funded in part by the National Natural Science Foundation of China (no. 81101771) and Shanghai Municipal Commission “Exchange Scholars.” The authors thank Dr. Joost J Oppenheim for critical review of the paper and Ms. Cheryl Lamb and Ms. Sharon Livingstone for secretarial assistance.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Jiamin Zhou and Yi Xiang contributed equally to this paper.
References
- 1.Huang J, Chen K, Gong W, Dunlop NM, Wang JM. G-protein coupled chemoattractant receptors and cancer. Frontiers in Bioscience. 2008;13(9):3352–3363. doi: 10.2741/2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Y, Cui Y, Iribarren P, Ying G, Wang JM. Manipulating chemoattractant and receptor genes. In Vivo. 2002;16(1):1–23. [PubMed] [Google Scholar]
- 3.Gao Q, Zhao Y, Wang X, et al. CXCR6 upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Research. 2012;72(14):3546–3556. doi: 10.1158/0008-5472.CAN-11-4032. [DOI] [PubMed] [Google Scholar]
- 4.Chakravarti N, Peddareddigari VGR, Warneke CL, et al. Differential expression of the G-protein-coupled formyl peptide receptor in melanoma associates with aggressive phenotype. The American Journal of Dermatopathology. 2013;35(2):184–190. doi: 10.1097/DAD.0b013e31825b2506. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa D, Taketomi A, Kayashima H, et al. Expression of platelet-activating factor receptor: a novel prognosticator in patients with hepatocellular carcinoma following hepatectomy. Oncology. 2008;72(5-6):381–387. doi: 10.1159/000113149. [DOI] [PubMed] [Google Scholar]
- 6.Han M, Lv S, Zhang Y, et al. The prognosis and clinicopathology of CXCR4 in gastric cancer patients: a meta-analysis. Tumour Biology. 2014;35(5):4589–4597. doi: 10.1007/s13277-013-1603-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Wu X, Liang W, Chen C, Zheng L, An H. Clinicopathological and prognostic significance of chemokine receptor CXCR4 overexpression in patients with esophageal cancer: a meta-analysis. Tumor Biology. 2014;35(4):3709–3715. doi: 10.1007/s13277-013-1490-8. [DOI] [PubMed] [Google Scholar]
- 8.Byers RJ, Sakhinia E, Joseph P, et al. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood. 2008;111(9):4764–4770. doi: 10.1182/blood-2007-10-115915. [DOI] [PubMed] [Google Scholar]
- 9.Sugasawa H, Ichikura T, Tsujimoto H, et al. Prognostic significance of expression of CCL5/RANTES receptors in patients with gastric cancer. Journal of Surgical Oncology. 2008;97(5):445–450. doi: 10.1002/jso.20984. [DOI] [PubMed] [Google Scholar]
- 10.Cassier PA, Treilleux I, Bachelot T, et al. Prognostic value of the expression of C-Chemokine Receptor 6 and 7 and their ligands in non-metastatic breast cancer. BMC Cancer. 2011;11, article 213 doi: 10.1186/1471-2407-11-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JM, Deng X, Gong W, Su S. Chemokines and their role in tumor growth and metastasis. Journal of Immunological Methods. 1998;220(1-2):1–17. doi: 10.1016/s0022-1759(98)00128-8. [DOI] [PubMed] [Google Scholar]
- 12.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends in Immunology. 2002;23(11):541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 13.Huang J, Chen K, Gong W, et al. Receptor “hijacking” by malignant glioma cells: a tactic for tumor progression. Cancer Letters. 2008;267(2):254–261. doi: 10.1016/j.canlet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira MAND, Barcelos LS, Teixeira MM, Bakhle YS, Andrade SP. Tumor growth, angiogenesis and inflammation in mice lacking receptors for platelet activating factor (PAF) Life Sciences. 2007;81(3):210–217. doi: 10.1016/j.lfs.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. Journal of Biochemistry. 2002;131(6):773–779. doi: 10.1093/oxfordjournals.jbchem.a003164. [DOI] [PubMed] [Google Scholar]
- 16.Langer HF, Chung K, Orlova VV, et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood. 2010;116(22):4395–4403. doi: 10.1182/blood-2010-01-261503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunez-Cruz S, Gimotty PA, Guerra MW, et al. Genetic and pharmacologic inhibition of complement impairs endothelial cell function and ablates ovarian cancer neovascularization. Neoplasia. 2012;14(11):994–1004. doi: 10.1593/neo.121262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Whitfeld PL, Mackay CR. Receptors for complement C5a. The importance of C5aR and the enigmatic role of C5L2. Immunology and Cell Biology. 2008;86(2):153–160. doi: 10.1038/sj.icb.7100166. [DOI] [PubMed] [Google Scholar]
- 19.Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B4 receptors. Prostaglandins Leukotrienes and Essential Fatty Acids. 2003;69(2-3):123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 20.Cheng JF, Jack R. CCR1 antagonists. Molecular Diversity. 2008;12(1):17–23. doi: 10.1007/s11030-008-9076-x. [DOI] [PubMed] [Google Scholar]
- 21.Struthers M, Pasternak A. CCR2 antagonists. Current Topics in Medicinal Chemistry. 2010;10(13):1278–1298. doi: 10.2174/156802610791561255. [DOI] [PubMed] [Google Scholar]
- 22.Salcedo R, Ponce ML, Young HA, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96(1):34–40. [PubMed] [Google Scholar]
- 23.Willems LI, IJzerman AP. Small molecule antagonists for chemokine CCR3 receptors. Medicinal Research Reviews. 2010;30(5):778–817. doi: 10.1002/med.20181. [DOI] [PubMed] [Google Scholar]
- 24.Purandare AV, Somerville JE. Antagonists of CCR4 as immunomodulatory agents. Current Topics in Medicinal Chemistry. 2006;6(13):1335–1344. doi: 10.2174/15680266106061335. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Wan S, Sun L, et al. Chemokine C-C motif receptor 5 and C-C motif ligand 5 promote cancer cell migration under hypoxia. Cancer Science. 2012;103(5):904–912. doi: 10.1111/j.1349-7006.2012.02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Oliveira CE, Oda JM, Losi Guembarovski R, et al. CC chemokine receptor 5: the interface of host immunity and cancer. Disease Markers. 2014;2014:8 pages. doi: 10.1155/2014/126954.126954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace AE, Catalano RD, Anderson RA, Jabbour HN. Chemokine (C-C) motif ligand 20 is regulated by PGF2α-F-prostanoid receptor signalling in endometrial adenocarcinoma and promotes cell proliferation. Molecular and Cellular Endocrinology. 2011;331(1):129–135. doi: 10.1016/j.mce.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Wan W, Murphy PM. Regulation of atherogenesis by chemokine receptor CCR6. Trends in Cardiovascular Medicine. 2011;21(5):140–144. doi: 10.1016/j.tcm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Qiu X, Zhang S, Zhang Q, Wang E. Hypoxia induced CCR7 expression via HIF-1α and HIF-2α correlates with migration and invasion in lung cancer cells. Cancer Biology & Therapy. 2009;8(4):322–330. doi: 10.4161/cbt.8.4.7332. [DOI] [PubMed] [Google Scholar]
- 30.Zhou S, Xu S, Tao H, et al. CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074430.e74430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eruslanov E, Stoffs T, Kim W, et al. Expansion of CCR8+ inflammatory myeloid cells in cancer patients with urothelial and renal carcinomas. Clinical Cancer Research. 2013;19(7):1670–1680. doi: 10.1158/1078-0432.CCR-12-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Das S, Sarrou E, Podgrabinska S, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. The Journal of Experimental Medicine. 2013;210(8):1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson M, Agace WW. Role of CCL25/CCR9 in immune homeostasis and disease. Expert Review of Clinical Immunology. 2006;2(5):759–773. doi: 10.1586/1744666X.2.5.759. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich EL, Arrington AK, Ko ME, et al. Paracrine activation of chemokine receptor CCR9 enhances the invasiveness of pancreatic cancer cells. Cancer Microenvironment. 2013;6(3):241–245. doi: 10.1007/s12307-013-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475(7355):226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 36.Kai H, Kadono T, Kakinuma T, et al. CCR10 and CCL27 are overexpressed in cutaneous squamous cell carcinoma. Pathology Research and Practice. 2011;207(1):43–48. doi: 10.1016/j.prp.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Grepin R, Guyot M, Giuliano S, et al. The CXCL7/CXCR1/2 axis is a key driver in the growth of clear cell renal cell carcinoma. Cancer Research. 2014;74(3):873–883. doi: 10.1158/0008-5472.CAN-13-1267. [DOI] [PubMed] [Google Scholar]
- 38.Busch-Petersen J. Small molecule antagonists of the CXCR2 and CXCR1 chemokine receptors as therapeutic agents for the treatment of inflammatory diseases. Current Topics in Medicinal Chemistry. 2006;6(13):1345–1352. doi: 10.2174/15680266106061345. [DOI] [PubMed] [Google Scholar]
- 39.Segerer S, Nelson PJ. Chemokines in renal diseases. TheScientificWorldJournal. 2005;5:835–844. doi: 10.1100/tsw.2005.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stofas A, Levidou G, Piperi C, et al. The role of CXC-chemokine receptor CXCR2 and suppressor of cytokine signaling-3 (SOCS-3) in renal cell carcinoma. BMC Cancer. 2014;14, article 149 doi: 10.1186/1471-2407-14-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veenstra M, Ransohoff RM. Chemokine receptor CXCR2: physiology regulator and neuroinflammation controller? Journal of Neuroimmunology. 2012;246(1-2):1–9. doi: 10.1016/j.jneuroim.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oghumu S, Varikuti S, Terrazas C, et al. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014 doi: 10.1111/imm.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giuliani N, Bonomini S, Romagnani P, et al. CXCR3 and its binding chemokines in myeloma cells: expression of isoforms and potential relationships with myeloma cell proliferation and survival. Haematologica. 2006;91(11):1489–1497. [PubMed] [Google Scholar]
- 44.Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A. Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. OncoTargets and Therapy. 2013;6:1347–1361. doi: 10.2147/OTT.S36109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.del Grosso F, Coco S, Scaruffi P, et al. Role of CXCL13-CXCR5 crosstalk between malignant neuroblastoma cells and Schwannian stromal cells in neuroblastic tumors. Molecular Cancer Research. 2011;9(7):815–823. doi: 10.1158/1541-7786.MCR-10-0367. [DOI] [PubMed] [Google Scholar]
- 46.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(1):200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 47.La Porta CAM. CXCR6: the role of environment in tumor progression. Challenges for therapy. Stem Cell Reviews and Reports. 2012;8(4):1282–1285. doi: 10.1007/s12015-012-9383-6. [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Cheng G, Hao M, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer and Metastasis Reviews. 2010;29(4):709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esencay M, Sarfraz Y, Zagzag D. CXCR7 is induced by hypoxia and mediates glioma cell migration towards SDF-1alpha. BMC Cancer. 2013;13, article 347 doi: 10.1186/1471-2407-13-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liberman J, Sartelet H, Flahaut M, et al. Involvement of the CXCR7/CXCR4/CXCL12 axis in the malignant progression of human neuroblastoma. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0043665.e43665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferretti E, Bertolotto M, Deaglio S, et al. A novel role of the CX3CR1/CX3CL1 system in the cross-talk between chronic lymphocytic leukemia cells and tumor microenvironment. Leukemia. 2011;25(8):1268–1277. doi: 10.1038/leu.2011.88. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Patel JM. Role of the CX3CL1-CX3CR1 axis in chronic inflammatory lung diseases. International Journal of Clinical and Experimental Medicine. 2010;3(3):233–244. [PMC free article] [PubMed] [Google Scholar]
- 53.Lei Y, Takahama Y. XCL1 and XCR1 in the immune system. Microbes and Infection. 2012;14(3):262–267. doi: 10.1016/j.micinf.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franciszkiewicz K, Boissonnas A, Boutet M, Combadière C, Mami-Chouaib F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Research. 2012;72(24):6325–6332. doi: 10.1158/0008-5472.CAN-12-2027. [DOI] [PubMed] [Google Scholar]
- 57.Iida N, Nakamoto Y, Baba T, et al. Tumor cell apoptosis induces tumor-specific immunity in a CC chemokine receptor 1- and 5-dependent manner in mice. Journal of Leukocyte Biology. 2008;84(4):1001–1010. doi: 10.1189/jlb.1107791. [DOI] [PubMed] [Google Scholar]
- 58.Charles J, Di Domizio J, Salameire D, et al. Characterization of circulating dendritic cells in melanoma: Role of CCR6 in plasmacytoid dendritic cell recruitment to the tumor. Journal of Investigative Dermatology. 2010;130(6):1646–1656. doi: 10.1038/jid.2010.24. [DOI] [PubMed] [Google Scholar]
- 59.Lu Y, Hong S, Li H, et al. Th9 cells promote antitumor immune responses in vivo. The Journal of Clinical Investigation. 2012;122(11):4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pahne-Zeppenfeld J, Schroer N, Walch-Ruckheim B, et al. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9 expressing dendritic cells. International Journal of Cancer. 2014;134(9):2061–2073. doi: 10.1002/ijc.28549. [DOI] [PubMed] [Google Scholar]
- 61.Ramanathapuram LV, Hopkin D, Kurago ZB. Dendritic cells (DC) facilitate detachment of squamous carcinoma cells (SCC), while SCC promote an immature CD16+ DC phenotype and control DC migration. Cancer Microenvironment. 2013;6(1):41–55. doi: 10.1007/s12307-011-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Middel P, Brauneck S, Meyer W, Radzun H. Chemokine-mediated distribution of dendritic cell subsets in renal cell carcinoma. BMC Cancer. 2010;10, article 578 doi: 10.1186/1471-2407-10-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C, Lou Y, Lizée G, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. Journal of Clinical Investigation. 2008;118(3):1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong JL, Berk E, Edwards RP, Kalinski P. IL-18-primed helper NK cells collaborate with dendritic cells to promote recruitment of effector CD8+ T cells to the tumor microenvironment. Cancer Research. 2013;73(15):4653–4662. doi: 10.1158/0008-5472.CAN-12-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajitani K, Tanaka Y, Arihiro K, Kataoka T, Ohdan H. Mechanistic analysis of the antitumor efficacy of human natural killer cells against breast cancer cells. Breast Cancer Research and Treatment. 2012;134(1):139–155. doi: 10.1007/s10549-011-1944-x. [DOI] [PubMed] [Google Scholar]
- 66.Hosoi A, Matsushita H, Shimizu K, et al. Adoptive cytotoxic T lymphocyte therapy triggers a counter-regulatory immunosuppressive mechanism via recruitment of myeloid-derived suppressor cells. International Journal of Cancer. 2014;134(8):1810–1822. doi: 10.1002/ijc.28506. [DOI] [PubMed] [Google Scholar]
- 67.Fialová A, Partlová S, Sojka L, et al. Dynamics of T-cell infiltration during the course of ovarian cancer: the gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. International Journal of Cancer. 2013;132(5):1070–1079. doi: 10.1002/ijc.27759. [DOI] [PubMed] [Google Scholar]
- 68.Cho S, Koizumi K, Takeno N, et al. Anti-tumor effect of combining CC chemokine 22 and an anti-CD25 antibody on myeloma cells implanted subcutaneously into mice. Molecular Medicine Reports. 2009;2(5):773–777. doi: 10.3892/mmr_00000171. [DOI] [PubMed] [Google Scholar]
- 69.Nesbeth YC, Martinez DG, Toraya S, et al. CD4+ T cells elicit host immune responses to MHC class II− ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. The Journal of Immunology. 2010;184(10):5654–5662. doi: 10.4049/jimmunol.0903247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bell D, Chomarat P, Broyles D, et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. The Journal of Experimental Medicine. 1999;190(10):1417–1425. doi: 10.1084/jem.190.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomachot MC, Bendriss-Vermare N, Massacrier C, et al. Breast carcinoma cells promote the differentiation of CD34+ progenitors towards 2 different subpopulations of dendritic cells with CD1ahighCD86−Langerin- and CD1a+CD86+Langerin+ phenotypes. International Journal of Cancer. 2004;110(5):710–720. doi: 10.1002/ijc.20146. [DOI] [PubMed] [Google Scholar]
- 72.Fushimi T, Kojima A, Moore MAS, Crystal RG. Macrophage inflammatory protein 3α transgene attracts dendritic cells to established murine tumors and suppresses tumor growth. Journal of Clinical Investigation. 2000;105(10):1383–1393. doi: 10.1172/JCI7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biragyn A, Surenhu M, Yang D, et al. Mediators of innate immunity that target immature, but not mature, dendritic cells induce antitumor immunity when genetically fused with nonimmunogenic tumor antigens. Journal of Immunology. 2001;167(11):6644–6653. doi: 10.4049/jimmunol.167.11.6644. [DOI] [PubMed] [Google Scholar]
- 74.Ohtani H, Nakayama T, Yoshie O. In situ expression of the CCL20-CCR6 axis in lymphocyte-rich gastric cancer and its potential role in the formation of lymphoid stroma. Pathology International. 2011;61(11):645–651. doi: 10.1111/j.1440-1827.2011.02717.x. [DOI] [PubMed] [Google Scholar]
- 75.Tsuge K, Takeda H, Kawada S, Maeda K, Yamakawa M. Characterization of dendritic cells in differentiated thyroid cancer. Journal of Pathology. 2005;205(5):565–576. doi: 10.1002/path.1731. [DOI] [PubMed] [Google Scholar]
- 76.Wu S, Xing W, Peng J, et al. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells. Immunobiology. 2008;213(5):417–426. doi: 10.1016/j.imbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Alfaro C, Suárez N, Martínez-Forero I, et al. Carcinoma-derived Interleukin-8 disorients dendritic cell migration without impairing T-cell stimulation. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017922.e17922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feijoó E, Alfaro C, Mazzolini G, et al. Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. International Journal of Cancer. 2005;116(2):275–281. doi: 10.1002/ijc.21046. [DOI] [PubMed] [Google Scholar]
- 79.Asfaha S, Dubeykovskiy AN, Tomita H, et al. Mice that express human interleukin-8 have increased mobilization of immature myeloid cells, which exacerbates inflammation and accelerates colon carcinogenesis. Gastroenterology. 2013;144(1):155–166. doi: 10.1053/j.gastro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan Q, Gu D, Liu H, et al. Defective TGF-beta signaling in bone marrow-derived cells prevents hedgehog-induced skin tumors. Cancer Research. 2014;74(2):471–483. doi: 10.1158/0008-5472.CAN-13-2134-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24(5):631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhuang Y, Peng LS, Zhao YL, et al. CD8+ T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143(4):951.e8–962.e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 83.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE 2-induced CXCL 12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Research. 2011;71(24):7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bachi ALL, dos Santos LC, Nonogaki S, Jancar S, Jasiulionis MG. Apoptotic cells contribute to melanoma progression and this effect is partially mediated by the platelet-activating factor receptor. Mediators of Inflammation. 2012;2012:6 pages. doi: 10.1155/2012/610371.610371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian B, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pahler JC, Tazzyman S, Erez N, et al. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10(4):329–339. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Popivanova BK, Kostadinova FI, Furuichi K, et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Research. 2009;69(19):7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 88.Ren G, Zhao X, Wang Y, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα . Cell Stem Cell. 2012;11(6):812–824. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang KF, Tan SY, Chan SH, et al. A distinct expression of CC chemokines by macrophages in nasopharyngeal carcinoma: implication for the intense tumor infiltration by T lymphocytes and macrophages. Human Pathology. 2001;32(1):42–49. doi: 10.1053/hupa.2001.20886. [DOI] [PubMed] [Google Scholar]
- 90.Jin G, Kawsar HI, Hirsch SA, et al. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE. 2010;5(6) doi: 10.1371/journal.pone.0010993.e10993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Izumi K, Fang L-Y, Mizokami A, et al. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR2-induced STAT3 activation. EMBO Molecular Medicine. 2013;5(9):1383–1401. doi: 10.1002/emmm.201202367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clinical Cancer Research. 2013;19(13):3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryder M, Gild M, Hohl TM, et al. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054302.e54302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Chen K, Wang C, et al. Cell surface receptor FPR2 promotes antitumor host defense by limiting M2 polarization of macrophages. Cancer Research. 2013;73(2):550–560. doi: 10.1158/0008-5472.CAN-12-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barashi N, Weiss ID, Wald O, et al. Inflammation-induced hepatocellular carcinoma is dependent on CCR5 in mice. Hepatology. 2013;58(3):1021–1030. doi: 10.1002/hep.26403. [DOI] [PubMed] [Google Scholar]
- 96.Reed JR, Stone MD, Beadnell TC, Ryu Y, Griffin TJ, Schwertfeger KL. Fibroblast growth factor receptor 1 activation in mammary tumor cells promotes macrophage recruitment in a CX3CL1-dependent manner. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0045877.e45877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Held-Feindt J, Hattermann K, Müerköster SS, et al. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs) Experimental Cell Research. 2010;316(9):1553–1566. doi: 10.1016/j.yexcr.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 98.Gobert M, Treilleux I, Bendriss-Vermare N, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical utcome. Cancer Research. 2009;69(5):2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 99.Battaglia A, Buzzonetti A, Martinelli E, et al. Selective changes in the immune profile of tumor-draining lymph nodes after different neoadjuvant chemoradiation regimens for locally advanced cervical cancer. International Journal of Radiation Oncology Biology Physics. 2010;76(5):1546–1553. doi: 10.1016/j.ijrobp.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 100.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Research. 2006;66(11):5716–5722. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 101.Enarsson K, Lundgren A, Kindlund B, et al. Function and recruitment of mucosal regulatory T cells in human chronic Helicobacter pylori infection and gastric adenocarcinoma. Clinical Immunology. 2006;121(3):358–368. doi: 10.1016/j.clim.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunology, Immunotherapy. 2008;57(1):123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kimpfler S, Sevko A, Ring S, et al. Skin melanoma development in ret transgenic mice despite the depletion of CD25+Foxp3+ regulatory T cells in lymphoid organs. Journal of Immunology. 2009;183(10):6330–6337. doi: 10.4049/jimmunol.0900609. [DOI] [PubMed] [Google Scholar]
- 104.Chang LY, Lin YC, Mahalingam J, et al. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8+ T cells in colon cancer by T-regulatory cells. Cancer Research. 2012;72(5):1092–1102. doi: 10.1158/0008-5472.CAN-11-2493. [DOI] [PubMed] [Google Scholar]
- 105.Schlecker E, Stojanovic A, Eisen C, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. Journal of Immunology. 2012;189(12):5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 106.Tan MCB, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. Journal of Immunology. 2009;182(3):1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oldham KA, Parsonage G, Bhatt RI, et al. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. European Urology. 2012;61(2):385–394. doi: 10.1016/j.eururo.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 108.Xu L, Xu W, Qiu S, Xiong S. Enrichment of CCR6+Foxp3+ regulatory T cells in the tumor mass correlates with impaired CD8+ T cell function and poor prognosis of breast cancer. Clinical Immunology. 2010;135(3):466–475. doi: 10.1016/j.clim.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Zhang N, Li Q, et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0019495.e19495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen K, Lin S, Zhou L, et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0024671.e24671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lamprecht B, Kreher S, Anagnostopoulos I, et al. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3α . Blood. 2008;112(8):3339–3347. doi: 10.1182/blood-2008-01-134783. [DOI] [PubMed] [Google Scholar]
- 112.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21 . Science. 2010;328(5979):749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 113.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Research. 2005;65(12):5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 114.Eikawa S, Ohue Y, Kitaoka K, et al. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6-and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6. Journal of Immunology. 2010;185(11):6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- 115.Yan M, Jene N, Byrne D, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Research. 2011;13(2) article R47 doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dürr C, Pfeifer D, Claus R, et al. CXCL12 mediates immunosuppression in the lymphoma microenvironment after allogeneic transplantation of hematopoietic cells. Cancer Research. 2010;70(24):10170–10181. doi: 10.1158/0008-5472.CAN-10-1943. [DOI] [PubMed] [Google Scholar]
- 117.Li Q, Bao JM, Li XL, Zhang T, Shen XH. Inhibiting effect of astragalus polysaccharides on the functions of CD4+CD25 hightreg cells in the tumor microenvironment of human hepatocellular carcinoma. Chinese Medical Journal. 2012;125(5):786–793. [PubMed] [Google Scholar]
- 118.Wald O, Izhar U, Amir G, et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. Journal of Immunology. 2006;177(10):6983–6990. doi: 10.4049/jimmunol.177.10.6983. [DOI] [PubMed] [Google Scholar]
- 119.Grauer OM, Nierkens S, Bennink E, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglionia immune responses in vivo. International Journal of Cancer. 2007;121(1):95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 120.Wei S, Kryczek I, Edwards RP, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Research. 2007;67(15):7487–7494. doi: 10.1158/0008-5472.CAN-07-0565. [DOI] [PubMed] [Google Scholar]
- 121.Righi E, Kashiwagi S, Yuan J, et al. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Research. 2011;71(16):5522–5534. doi: 10.1158/0008-5472.CAN-10-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Parsonage G, MacHado LR, Hui JW, et al. CXCR6 and CCR5 localize T lymphocyte subsets in nasopharyngeal carcinoma. The American Journal of Pathology. 2012;180(3):1215–1222. doi: 10.1016/j.ajpath.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 123.Coffelt SB, Marini FC, Watson K, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Research. 2007;67(24):11687–11695. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xu F, Shi J, Yu B, Ni W, Wu X, Gu Z. Chemokines mediate mesenchymal stem cell migration toward gliomas in vitro. Oncology Reports. 2010;23(6):1561–1567. doi: 10.3892/or_00000796. [DOI] [PubMed] [Google Scholar]
- 126.Kim SM, Kim DS, Jeong CH, et al. CXC chemokine receptor 1 enhances the ability of human umbilical cord blood-derived mesenchymal stem cells to migrate toward gliomas. Biochemical and Biophysical Research Communications. 2011;407(4):741–746. doi: 10.1016/j.bbrc.2011.03.093. [DOI] [PubMed] [Google Scholar]
- 127.Kim D, Kim JH, Kwon Lee J, et al. Overexpression of CXC chemokine receptors is required for the superior glioma-tracking property of umbilical cord blood-derived mesenchymal stem cells. Stem Cells and Development. 2009;18(3):511–519. doi: 10.1089/scd.2008.0050. [DOI] [PubMed] [Google Scholar]
- 128.Liang-Kuan B, Nan Z, Cheng L, et al. Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. Urologic Oncology. 2014;32(5):607–612. doi: 10.1016/j.urolonc.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27(4):857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 130.Fakhari S, Kalantar E, Nikzaban M, et al. Effect of Helicobacter pylori infection on stromal-derived factor-1/CXCR4 axis in bone marrow-derived mesenchymal stem cells. Advanced Biomedical Research. 2014;3:p. 19. doi: 10.4103/2277-9175.124650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Park SA, Ryu CH, Kim SM, et al. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. International Journal of Oncology. 2011;38(1):97–103. [PubMed] [Google Scholar]
- 132.Behnan J, Isakson P, Joel M, et al. Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells. 2014;32(5):1110–1123. doi: 10.1002/stem.1614. [DOI] [PubMed] [Google Scholar]
- 133.Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nature Communications. 2013;4, article 1795 doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang YF, Chen MJ, Wu MH, Hung SC. The use of hypoxic cultured mesenchymal stem cell for oncolytic virus therapy. Cancer Gene Therapy. 2013;20(5):308–316. doi: 10.1038/cgt.2013.22. [DOI] [PubMed] [Google Scholar]
- 135.Jacobs JFM, Idema AJ, Bol KF, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. Journal of Neuroimmunology. 2010;225(1-2):195–199. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 136.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8+ T cells and CCR4+ regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2010;109(5):744–752. doi: 10.1016/j.tripleo.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 137.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proceedings of the National Academy of Sciences of the USA. 2013;110(44):17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Science Translational Medicine. 2013;5(200):p. 200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olkhanud PB, Baatar D, Bodogai M, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Research. 2009;69(14):5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mailloux AW, Young MRI. NK-dependent increases in CCL22 secretion selectively recruits regulatory T cells to the tumor microenvironment. The Journal of Immunology. 2009;182(5):2753–2765. doi: 10.4049/jimmunol.0801124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ménétrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Research. 2009;69(20):7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [DOI] [PubMed] [Google Scholar]
- 142.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature Reviews Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nature Reviews Immunology. 2004;4(8):641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 144.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer and Metastasis Reviews. 2007;26(3-4):373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 145.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature Reviews Immunology. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nature Reviews Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 147.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. Journal of Clinical Investigation. 2001;108(7):957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. The Journal of Biological Chemistry. 2009;284(42):29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng J, Yang M, Shao J, Miao Y, Han J, Du J. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Molecular Cancer. 2013;12(1, article 141) doi: 10.1186/1476-4598-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lesokhin AM, Hohl TM, Kitano S, et al. Monocytic CCR2+ myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Research. 2012;72(4):876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhao L, Lim SY, Gordon-Weeks AN, et al. Recruitment of a myeloid cell subset (CD11b/Gr1mid) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology. 2013;57(2):829–839. doi: 10.1002/hep.26094. [DOI] [PubMed] [Google Scholar]
- 152.Sawanobori Y, Ueha S, Kurachi M, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111(12):5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 153.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells. Advances in Experimental Medicine and Biology. 2007;601:213–223. doi: 10.1007/978-0-387-72005-0_22. [DOI] [PubMed] [Google Scholar]
- 154.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. The Journal of Clinical Investigation. 2007;117(5):1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS ONE. 2009;4(4) doi: 10.1371/journal.pone.0004992.e4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miyagaki T, Sugaya M, Fujita H, et al. Eotaxins and CCR3 interaction regulates the Th2 environment of cutaneous T-Cell lymphoma. Journal of Investigative Dermatology. 2010;130(9):2304–2311. doi: 10.1038/jid.2010.128. [DOI] [PubMed] [Google Scholar]
- 158.Babbin BA, Lee WY, Parkos CA, et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. Journal of Biological Chemistry. 2006;281(28):19588–19599. doi: 10.1074/jbc.M513025200. [DOI] [PubMed] [Google Scholar]
- 159.Cheng TY, Wu MS, Lin JT, et al. Annexin A1 is associated with gastric cancer survival and promotes gastric cancer cell invasiveness through the formyl peptide receptor/extracellular signal-regulated kinase/integrin beta-1-binding protein 1 pathway. Cancer. 2012;118(23):5757–5767. doi: 10.1002/cncr.27565. [DOI] [PubMed] [Google Scholar]
- 160.Xu CP, Zhang HR, Chen FL, et al. Human malignant glioma cells expressing functional formylpeptide receptor recruit endothelial progenitor cells for neovascularization. International Immunopharmacology. 2010;10(12):1602–1607. doi: 10.1016/j.intimp.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 161.Yao XH, Ping YF, Chen JH, et al. Production of angiogenic factors by human glioblastoma cells following activation of the G-protein coupled formylpeptide receptor FPR. Journal of Neuro-Oncology. 2008;86(1):47–53. doi: 10.1007/s11060-007-9443-y. [DOI] [PubMed] [Google Scholar]
- 162.Yao XH, Ping YF, Chen JH, et al. Glioblastoma stem cells produce vascular endothelial growth factor by activation of a G-protein coupled formylpeptide receptor FPR. Journal of Pathology. 2008;215(4):369–376. doi: 10.1002/path.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen D, Ping Y, Yu S, et al. Downregulating FPR restrains xenograft tumors by impairing the angiogenic potential and invasive capability of malignant glioma cells. Biochemical and Biophysical Research Communications. 2009;381(3):448–452. doi: 10.1016/j.bbrc.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 164.Coffelt SB, Tomchuck SL, Zwezdaryk KJ, Danka ES, Scandurro AB. Leucine leucine-37 uses formyl peptide receptor-like 1 to activate signal transduction pathways, stimulate oncogenic gene expression, and enhance the invasiveness of ovarian cancer cells. Molecular Cancer Research. 2009;7(6):907–915. doi: 10.1158/1541-7786.MCR-08-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bussolati B, Biancone L, Cassoni P, et al. PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. American Journal of Pathology. 2000;157(5):1713–1725. doi: 10.1016/S0002-9440(10)64808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Melnikova VO, Balasubramanian K, Villares GJ, et al. Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. The Journal of Biological Chemistry. 2009;284(42):28845–28855. doi: 10.1074/jbc.M109.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aponte M, Jiang W, Lakkis M, et al. Activation of platelet-activating factor receptor and pleiotropic effects on tyrosine phospho-EGFR/Src/FAK/paxillin in ovarian cancer. Cancer Research. 2008;68(14):5839–5848. doi: 10.1158/0008-5472.CAN-07-5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nitta H, Wada Y, Kawano Y, et al. Enhancement of human cancer cell motility and invasiveness by anaphylatoxin C5a via aberrantly expressed C5a receptor (CD88) Clinical Cancer Research. 2013;19(8):2004–2013. doi: 10.1158/1078-0432.CCR-12-1204. [DOI] [PubMed] [Google Scholar]
- 169.Gu J, Ding JY, Lu CL, et al. Overexpression of CD88 predicts poor prognosis in non-small-cell lung cancer. Lung Cancer. 2013;81(2):259–265. doi: 10.1016/j.lungcan.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 170.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. Journal of Pharmacological Sciences. 2007;103(1):24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 171.Kim E, Seo J, Kim C, Lee J, Lee K, Kim J. BLT2 promotes the invasion and metastasis of aggressive bladder cancer cells through a reactive oxygen species-linked pathway. Free Radical Biology and Medicine. 2010;49(6):1072–1081. doi: 10.1016/j.freeradbiomed.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 172.Seo JM, Cho KJ, Kim EY, Choi MH, Chung BC, Kim JH. Up-regulation of BLT2 is critical for the survival of bladder cancer cells. Experimental and Molecular Medicine. 2011;43(3):129–137. doi: 10.3858/emm.2011.43.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kim H, Choi J, Park G, Kim J. BLT2 up-regulates interleukin-8 production and promotes the invasiveness of breast cancer cells. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049186.e49186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hennig R, Osman T, Esposito I, et al. BLT2 is expressed in PanINs, IPMNs, pancreatic cancer and stimulates tumour cell proliferation. British Journal of Cancer. 2008;99(7):1064–1073. doi: 10.1038/sj.bjc.6604655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park MK, Park Y, Shim J, Lee HJ, Kim S, Lee CH. Novel involvement of leukotriene B4 receptor 2 through ERK activation by PP2A down-regulation in leukotriene B4-induced keratin phosphorylation and reorganization of pancreatic cancer cells. Biochimica et Biophysica Acta—Molecular Cell Research. 2012;1823(12):2120–2129. doi: 10.1016/j.bbamcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 176.Lee JW, Kim JH. Activation of the leukotriene B4 receptor 2-reactive oxygen species (BLT2-ROS) cascade following detachment confers anoikis resistance in prostate cancer cells. The Journal of Biological Chemistry. 2013;288(42):30054–30063. doi: 10.1074/jbc.M113.481283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee J, Kim G, Kim J. Androgen receptor is up-regulated by a BLT2-linked pathway to contribute to prostate cancer progression. Biochemical and Biophysical Research Communications. 2012;420(2):428–433. doi: 10.1016/j.bbrc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 178.Seo JM, Park S, Kim JH. Leukotriene B4 receptor-2 promotes invasiveness and metastasis of ovarian cancer cells through signal transducer and activator of transcription 3 (STAT3)-dependent up-regulation of matrix metalloproteinase 2. The Journal of Biological Chemistry. 2012;287(17):13840–13849. doi: 10.1074/jbc.M111.317131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Swamydas M, Ricci K, Rego SL, Dréau D. Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adhesion and Migration. 2013;7(3):315–324. doi: 10.4161/cam.25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kouno J, Nagai H, Nagahata T, et al. Up-regulation of CC chemokine, CCL3L1, and receptors, CCR3, CCR5 in human glioblastoma that promotes cell growth. Journal of Neuro-Oncology. 2004;70(3):301–307. doi: 10.1007/s11060-004-9165-3. [DOI] [PubMed] [Google Scholar]
- 181.Dagouassat M, Suffee N, Hlawaty H, et al. Monocyte chemoattractant protein-1 (MCP-1)/CCL2 secreted by hepatic myofibroblasts promotes migration and invasion of human hepatoma cells. International Journal of Cancer. 2010;126(5):1095–1108. doi: 10.1002/ijc.24800. [DOI] [PubMed] [Google Scholar]
- 182.Wu X, Fan J, Wang X, et al. Downregulation of CCR1 inhibits human hepatocellular carcinoma cell invasion. Biochemical and Biophysical Research Communications. 2007;355(4):866–871. doi: 10.1016/j.bbrc.2007.01.199. [DOI] [PubMed] [Google Scholar]
- 183.Jung DW, Che ZM, Kim J, Kim KY, Williams D, Kim J. Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. International Journal of Cancer. 2010;127(2):332–344. doi: 10.1002/ijc.25060. [DOI] [PubMed] [Google Scholar]
- 184.Long H, Xie R, Xiang T, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells. 2012;30(10):2309–2319. doi: 10.1002/stem.1194. [DOI] [PubMed] [Google Scholar]
- 185.Chiu HY, Sun KH, Chen SY, et al. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine. 2012;59(2):423–432. doi: 10.1016/j.cyto.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 186.Fang WB, Jokar I, Zou A, Lambert D, Dendukuri P, Cheng N. CCL2/CCR2 chemokine signaling coordinates survival and motility of breast cancer cells through Smad3 protein- and p42/44 mitogen-activated protein kinase (MAPK)-dependent mechanisms. The Journal of Biological Chemistry. 2012;287(43):36593–36608. doi: 10.1074/jbc.M112.365999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moreaux J, Hose D, Kassambara A, et al. Osteoclast-gene expression profiling reveals osteoclast-derived CCR2 chemokines promoting myeloma cell migration. Blood. 2011;117(4):1280–1290. doi: 10.1182/blood-2010-04-279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Furukawa S, Soeda S, Kiko Y, et al. MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Research. 2013;33(11):4785–4790. [PubMed] [Google Scholar]
- 189.Levina V, Nolen BM, Marrangoni AM, et al. Role of eotaxin-1 signaling in ovarian cancer. Clinical Cancer Research. 2009;15(8):2647–2656. doi: 10.1158/1078-0432.CCR-08-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gao J, Wang A, Zhang M, et al. RNAi targeting of CCR2 gene expression induces apoptosis and inhibits the proliferation, migration, and invasion of PC-3M cells. Oncology Research. 2014;21(2):73–82. doi: 10.3727/096504013X13775486749173. [DOI] [PubMed] [Google Scholar]
- 191.Lin TH, Liu HH, Tsai TH, et al. CCL2 increases αvα3 integrin expression and subsequently promotes prostate cancer migration. Biochimica et Biophysica Acta. 2013;1830(10):4917–4927. doi: 10.1016/j.bbagen.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 192.Miyagaki T, Sugaya M, Murakami T, et al. CCL11-CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Research. 2011;71(6):2056–2065. doi: 10.1158/0008-5472.CAN-10-3764. [DOI] [PubMed] [Google Scholar]
- 193.Jöhrer K, Zelle-Rieser C, Perathoner A, et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clinical Cancer Research. 2005;11(7):2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- 194.Li JY, Ou ZL, Yu SJ, et al. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Research and Treatment. 2012;131(3):837–848. doi: 10.1007/s10549-011-1502-6. [DOI] [PubMed] [Google Scholar]
- 195.Al-haidari AA, Syk I, Jirström K, Thorlacius H. CCR4 mediates CCL17 (TARC)-induced migration of human colon cancer cells via RhoA/Rho-kinase signaling. International Journal of Colorectal Disease. 2013;28(11):1479–1487. doi: 10.1007/s00384-013-1712-y. [DOI] [PubMed] [Google Scholar]
- 196.Lee JH, Cho Y, Lee JY, et al. The chemokine receptor CCR4 is expressed and associated with a poor prognosis in patients with gastric cancer. Annals of Surgery. 2009;249(6):933–941. doi: 10.1097/SLA.0b013e3181a77ccc. [DOI] [PubMed] [Google Scholar]
- 197.Izraely S, Klein A, Sagi-Assif O, et al. Chemokine-chemokine receptor axes in melanoma brain metastasis. Immunology Letters. 2010;130(1-2):107–114. doi: 10.1016/j.imlet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 198.Tsujikawa T, Yaguchi T, Ohmura G, et al. Autocrine and paracrine loops between cancer cells and macrophages promote lymph node metastasis via CCR4/CCL22 in head and neck squamous cell carcinoma. International Journal of Cancer. 2013;132(12):2755–2766. doi: 10.1002/ijc.27966. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Y, Yao F, Yao X, et al. Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24- phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncology Reports. 2009;21(4):1113–1121. [PubMed] [Google Scholar]
- 200.Velasco-Velázquez M, Jiao X, de La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Research. 2012;72(15):3839–3850. doi: 10.1158/0008-5472.CAN-11-3917. [DOI] [PubMed] [Google Scholar]
- 201.Velasco-Velazquez M, Pestell RG. The CCL5/CCR5 axis promotes metastasis in basal breast cancer. Oncoimmunology. 2013;2(4) doi: 10.4161/onci.23660.e23660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Murooka TT, Rahbar R, Fish EN. CCL5 promotes proliferation of MCF-7 cells through mTOR-dependent mRNA translation. Biochemical and Biophysical Research Communications. 2009;387(2):381–386. doi: 10.1016/j.bbrc.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 203.Cambien B, Richard-Fiardo P, Karimdjee BF, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRβ in colorectal carcinoma. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028842.e28842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cao Z, Xu X, Luo X, et al. Role of RANTES and its receptor in gastric cancer metastasis. Journal of Huazhong University of Science and Technology—Medical Science. 2011;31(3):342–347. doi: 10.1007/s11596-011-0378-3. [DOI] [PubMed] [Google Scholar]
- 205.Aldinucci D, Lorenzon D, Cattaruzza L, et al. Expression of CCR5 receptors on Reed-Sternberg cells and Hodgkin lymphoma cell lines: involvement of CCL5/Rantes in tumor cell growth and microenvironmental interactions. International Journal of Cancer. 2008;122(4):769–776. doi: 10.1002/ijc.23119. [DOI] [PubMed] [Google Scholar]
- 206.Chuang J, Yang W, Chen H, et al. CCL5/CCR5 axis promotes the motility of human oral cancer cells. Journal of Cellular Physiology. 2009;220(2):418–426. doi: 10.1002/jcp.21783. [DOI] [PubMed] [Google Scholar]
- 207.Rubie C, Oliveira V, Kempf K, et al. Involvement of chemokine receptor CCR6 in colorectal cancer metastasis. Tumor Biology. 2006;27(3):166–174. doi: 10.1159/000092777. [DOI] [PubMed] [Google Scholar]
- 208.Brand S, Olszak T, Beigel F, et al. Cell differentiation dependent expressed CCR6 mediates ERK-1/2, SAPK/JNK, and Akt signaling resulting in proliferation and migration of colorectal cancer cells. Journal of Cellular Biochemistry. 2006;97(4):709–723. doi: 10.1002/jcb.20672. [DOI] [PubMed] [Google Scholar]
- 209.Rubie C, Frick VO, Wagner M, et al. Chemokine expression in hepatocellular carcinoma versus colorectal liver metastases. World Journal of Gastroenterology. 2006;12(41):6627–6633. doi: 10.3748/wjg.v12.i41.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Uchida H, Iwashita Y, Sasaki A, et al. Chemokine receptor CCR6 as a prognostic factor after hepatic resection for hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2006;21(1):161–168. doi: 10.1111/j.1440-1746.2005.04157.x. [DOI] [PubMed] [Google Scholar]
- 211.Raynaud CM, Mercier O, Dartevelle P, et al. Expression of chemokine receptor CCR6 as a molecular determinant of adrenal metastatic relapse in patients with primary lung cancer. Clinical Lung Cancer. 2010;11(3):187–191. doi: 10.3816/CLC.2010.n.024. [DOI] [PubMed] [Google Scholar]
- 212.Kleeff J, Kusama T, Rossi DL, et al. Detection and localization of Mip-3alpha/LARC/Exodus, a macrophage proinflammatory chemokine, and its CCR6 receptor in human pancreatic cancer. International Journal of Cancer. 1999;81(4):650–657. doi: 10.1002/(sici)1097-0215(19990517)81:4<650::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 213.Kimsey TF, Campbell AS, Albo D, Wang TN. Co-localization of macrophage inflammatory protein-3α (Mip-3α) and its receptor, CCR6, promotes pancreatic cancer cell invasion. Cancer Journal. 2004;10(6):374–380. doi: 10.1097/00130404-200411000-00007. [DOI] [PubMed] [Google Scholar]
- 214.Campbell AS, Albo D, Kimsey TF, White SL, Wang TN. Macrophage inflammatory protein-3α promotes pancreatic cancer cell invasion. Journal of Surgical Research. 2005;123(1):96–101. doi: 10.1016/j.jss.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 215.Wang J, Xi L, Hunt JL, et al. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Research. 2004;64(5):1861–1866. doi: 10.1158/0008-5472.can-03-2968. [DOI] [PubMed] [Google Scholar]
- 216.Wang J, Xi L, Gooding W, Godfrey TE, Ferris RL. Chemokine receptors 6 and 7 identify a metastatic expression pattern in squamous cell carcinoma of the head and neck. Advances in Oto-Rhino-Laryngology. 2005;62:121–133. doi: 10.1159/000082501. [DOI] [PubMed] [Google Scholar]
- 217.Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death and Differentiation. 2009;16(5):664–673. doi: 10.1038/cdd.2008.190. [DOI] [PubMed] [Google Scholar]
- 218.Cunningham HD, Shannon LA, Calloway PA, et al. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the Lymph nodes in mice. Translational Oncology. 2010;3(6):354–361. doi: 10.1593/tlo.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li J, Sun R, Tao K, Wang G. The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Digestive and Liver Disease. 2011;43(1):40–47. doi: 10.1016/j.dld.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 220.Shuyi Y, Juping D, Zhiqun Z, et al. A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biology & Therapy. 2008;7(7):1037–1043. doi: 10.4161/cbt.7.7.6065. [DOI] [PubMed] [Google Scholar]
- 221.Emmett MS, Lanati S, Dunn DBA, Stone OA, Bates DO. CCR7 mediates directed growth of melanomas towards lymphatics. Microcirculation. 2011;18(3):172–182. doi: 10.1111/j.1549-8719.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fang L, Lee VC, Cha E, Zhang H, Hwang ST. CCR7 regulates B16 murine melanoma cell tumorigenesis in skin. Journal of Leukocyte Biology. 2008;84(4):965–972. doi: 10.1189/jlb.1107776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xu Y, Liu L, Qiu X, et al. CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033262.e33262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Liu J, Zhang L, Wang C. CCL21 modulates the migration of NSCL cancer by changing the concentration of intracellular Ca2+ . Oncology Reports. 2012;27(2):481–486. doi: 10.3892/or.2011.1528. [DOI] [PubMed] [Google Scholar]
- 225.Xu Y, Liu L, Qiu X, et al. CCL21/CCR7 promotes G2/M phase progression via the ERK pathway in human non-small cell lung cancer cells. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0021119.e21119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maekawa S, Iwasaki A, Shirakusa T, et al. Association between the expression of chemokine receptors CCR7 and CXCR3, and lymph node metastatic potential in lung adenocarcinoma. Oncology Reports. 2008;19(6):1461–1468. [PubMed] [Google Scholar]
- 227.Shang ZJ, Liu K, Shao Z. Expression of chemokine receptor CCR7 is associated with cervical lymph node metastasis of oral squamous cell carcinoma. Oral Oncology. 2009;45(6):480–485. doi: 10.1016/j.oraloncology.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 228.Sperveslage J, Frank S, Heneweer C, et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. International Journal of Cancer. 2012;131(4):E371–E381. doi: 10.1002/ijc.26502. [DOI] [PubMed] [Google Scholar]
- 229.Huang X, Su K, Zhou L, et al. Hypoxia preconditioning of mesenchymal stromal cells enhances PC3 cell lymphatic metastasis accompanied by VEGFR-3/CCR7 activation. Journal of Cellular Biochemistry. 2013;114(12):2834–2841. doi: 10.1002/jcb.24629. [DOI] [PubMed] [Google Scholar]
- 230.Wang J, Seethala RR, Zhang Q, et al. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. Journal of the National Cancer Institute. 2008;100(7):502–512. doi: 10.1093/jnci/djn059. [DOI] [PubMed] [Google Scholar]
- 231.Zhao Z, Peng L, Liu F, Sun L, Sun C. PKCα take part in CCR7/NF-κB autocrine signaling loop in CCR7-positive squamous cell carcinoma of head and neck. Molecular and Cellular Biochemistry. 2011;357(1-2):181–187. doi: 10.1007/s11010-011-0888-0. [DOI] [PubMed] [Google Scholar]
- 232.Liu F, Zhao Z, Li P, et al. NF-κB participates in chemokine receptor 7-mediated cell survival in metastatic squamous cell carcinoma of the head and neck. Oncology Reports. 2011;25(2):383–391. doi: 10.3892/or.2010.1090. [DOI] [PubMed] [Google Scholar]
- 233.Zhao Z, Liu F, Li P, Ding X, Zong Z, Sun C. CCL19-induced chemokine receptor 7 activates the phosphoinositide-3 kinase-mediated invasive pathway through Cdc42 in metastatic squamous cell carcinoma of the head and neck. Oncology Reports. 2011;25(3):729–737. doi: 10.3892/or.2010.1109. [DOI] [PubMed] [Google Scholar]
- 234.Li P, Liu F, Sun L, et al. Chemokine receptor 7 promotes cell migration and adhesion in metastatic squamous cell carcinoma of the head and neck by activating integrin αvβ3. International Journal of Molecular Medicine. 2011;27(5):679–687. doi: 10.3892/ijmm.2011.628. [DOI] [PubMed] [Google Scholar]
- 235.Mburu YK, Abe K, Ferris LK, Sarkar SN, Ferris RL. Human β-defensin 3 promotes NF-kB-mediated CCR7 expression and anti-apoptotic signals in squamous cell carcinoma of the head and neck. Carcinogenesis. 2011;32(2):168–174. doi: 10.1093/carcin/bgq236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li P, Zhao Z, Liu F, et al. The chemokine receptor 7 regulates cell adhesion and migration via β1 integrin in metastatic squamous cell carcinoma of the head and neck. Oncology Reports. 2010;24(4):989–995. doi: 10.3892/or.2010.989. [DOI] [PubMed] [Google Scholar]
- 237.Yang J, Wang S, Zhao G, Sun B. Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: clinical and experimental study. Journal of Experimental and Clinical Cancer Research. 2011;30(1, article 51) doi: 10.1186/1756-9966-30-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Johnson-Holiday C, Singh R, Johnson E, et al. CCL25 mediates migration, invasion and matrix metalloproteinase expression by breast cancer cells in a CCR9-dependent fashion. International Journal of Oncology. 2011;38(5):1279–1285. doi: 10.3892/ijo.2011.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen HJ, Edwards R, Tucci S, et al. Chemokine 25—induced signaling suppresses colon cancer invasion and metastasis. The Journal of Clinical Investigation. 2012;122(9):3184–3196. doi: 10.1172/JCI62110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Johnson EL, Singh R, Singh S, et al. CCL25-CCR9 interaction modulates ovarian cancer cell migration, metalloproteinase expression, and invasion. World Journal of Surgical Oncology. 2010;8, article 62 doi: 10.1186/1477-7819-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Shen X, Mailey B, Ellenhorn JDIE, Chu PG, Lowy AM, Kim J. Cc chemokine receptor 9 enhances proliferation in pancreatic intraepithelial neoplasia and pancreatic cancer cells. Journal of Gastrointestinal Surgery. 2009;13(11):1955–1962. doi: 10.1007/s11605-009-1002-8. [DOI] [PubMed] [Google Scholar]
- 242.Sharma PK, Singh R, Novakovic KR, Eaton JW, Grizzle WE, Singh S. CCR9 mediates PI3K/AKT-dependent antiapoptotic signals in prostate cancer cells and inhibition of CCR9-CCL25 interaction enhances the cytotoxic effects of etoposide. International Journal of Cancer. 2010;127(9):2020–2030. doi: 10.1002/ijc.25219. [DOI] [PubMed] [Google Scholar]
- 243.Murakami T, Cardones AR, Finkelstein SE, et al. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. Journal of Experimental Medicine. 2003;198(9):1337–1347. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Murakami T, Cardones AR, Hwang ST. Chemokine receptors and melanoma metastasis. Journal of Dermatological Science. 2004;36(2):71–78. doi: 10.1016/j.jdermsci.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 245.Singh JK, Farnie G, Bundred NJ, et al. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clinical Cancer Research. 2013;19(3):643–656. doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Liu LB, Xie F, Chang KK, et al. Hypoxia promotes the proliferation of cervical carcinoma cells through stimulating the secretion of IL-8. International Journal of Clinical and Experimental Pathology. 2014;7(2):575–583. [PMC free article] [PubMed] [Google Scholar]
- 247.Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK. Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Letters. 2011;300(2):180–188. doi: 10.1016/j.canlet.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Eck M, Schmausser B, Scheller K, Brändlein S, Müller-Hermelink HK. Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clinical & Experimental Immunology. 2003;134(3):508–515. doi: 10.1111/j.1365-2249.2003.02305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Infanger DW, Cho Y, Lopez BS, et al. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Research. 2013;73(23):7079–7089. doi: 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Raychaudhuri B, Vogelbaum MA. IL-8 is a mediator of NF-κB induced invasion by gliomas. Journal of Neuro-Oncology. 2011;101(2):227–235. doi: 10.1007/s11060-010-0261-2. [DOI] [PubMed] [Google Scholar]
- 251.Gabellini C, Trisciuoglio D, Desideri M, et al. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. European Journal of Cancer. 2009;45(14):2618–2627. doi: 10.1016/j.ejca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 252.Singh S, Nannuru KC, Sadanandam A, Varney ML, Singh RK. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. British Journal of Cancer. 2009;100(10):1638–1646. doi: 10.1038/sj.bjc.6605055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Singh S, Sadanandam A, Nannuru KC, et al. Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clinical Cancer Research. 2009;15(7):2380–2386. doi: 10.1158/1078-0432.CCR-08-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Murphy C, McGurk M, Pettigrew J, et al. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clinical Cancer Research. 2005;11(11):4117–4127. doi: 10.1158/1078-0432.CCR-04-1518. [DOI] [PubMed] [Google Scholar]
- 255.Fang W, Ye L, Shen L, et al. Tumor-associated macrophages promote the metastatic potential of thyroid papillary cancer by releasing CXCL8. Carcinogenesis. 2014 doi: 10.1093/carcin/bgu060. [DOI] [PubMed] [Google Scholar]
- 256.Bohrer LR, Schwertfeger KL. Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a Cxcr2-dependent manner. Molecular Cancer Research. 2012;10(10):1294–1305. doi: 10.1158/1541-7786.MCR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Halpern JL, Kilbarger A, Lynch CC. Mesenchymal stem cells promote mammary cancer cell migration in vitro via the CXCR2 receptor. Cancer Letters. 2011;308(1):91–99. doi: 10.1016/j.canlet.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ning Y, Labonte MJ, Zhang W, et al. The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Molecular Cancer Therapeutics. 2012;11(6):1353–1364. doi: 10.1158/1535-7163.MCT-11-0915. [DOI] [PubMed] [Google Scholar]
- 259.Lee YS, Choi I, Ning Y, et al. Interleukin-8 and its receptor CXCR2 in the tumour microenvironment promote colon cancer growth, progression and metastasis. British Journal of Cancer. 2012;106(11):1833–1841. doi: 10.1038/bjc.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kawamura M, Toiyama Y, Tanaka K, et al. CXCL5, a promoter of cell proliferation, migration and invasion, is a novel serum prognostic marker in patients with colorectal cancer. European Journal of Cancer. 2012;48(14):2244–2251. doi: 10.1016/j.ejca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 261.Ogata H, Sekikawa A, Yamagishi H, et al. GROα promotes invasion of colorectal cancer cells. Oncology Reports. 2010;24(6):1479–1486. [PubMed] [Google Scholar]
- 262.Xu J, Zhang C, He Y, et al. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. International Journal of Cancer. 2012;130(4):787–797. doi: 10.1002/ijc.26035. [DOI] [PubMed] [Google Scholar]
- 263.Cheng WL, Wang CS, Huang YH, Tsai MM, Liang Y, Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Annals of Oncology. 2011;22(10):2267–2276. doi: 10.1093/annonc/mdq739. [DOI] [PubMed] [Google Scholar]
- 264.Baron N, Deuster O, Noelker C, et al. Role of macrophage migration inhibitory factor in primary glioblastoma multiforme cells. Journal of Neuroscience Research. 2011;89(5):711–717. doi: 10.1002/jnr.22595. [DOI] [PubMed] [Google Scholar]
- 265.Lo M-C, Yip T-C, Ngan K-C, et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer Letters. 2013;335(1):81–92. doi: 10.1016/j.canlet.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 266.Saintigny P, Massarelli E, Lin S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Research. 2013;73(2):571–582. doi: 10.1158/0008-5472.CAN-12-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. Journal of Immunology. 2004;172(5):2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 268.Yang G, Rosen DG, Liu G, et al. CXCR2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clinical Cancer Research. 2010;16(15):3875–3886. doi: 10.1158/1078-0432.CCR-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Matsuo Y, Ochi N, Sawai H, et al. CXCL8/IL-8 and CXCL12/SDF-1alpha co-operatively promote invasiveness and angiogenesis in pancreatic cancer. International Journal of Cancer. 2009;124(4):853–861. doi: 10.1002/ijc.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Balan M, Pal S. A novel CXCR3-B chemokine receptor-induced growth-inhibitory signal in cancer cells is mediated through the regulation of Bach-1 protein and Nrf2 protein nuclear translocation. The Journal of Biological Chemistry. 2014;289(6):3126–3137. doi: 10.1074/jbc.M113.508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chaturvedi P, Gilkes DM, Wong CC, et al. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. The Journal of Clinical Investigation. 2013;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Mulligan AM, Raitman I, Feeley L, et al. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the ontario familial breast cancer registry. Clinical Cancer Research. 2013;19(2):336–346. doi: 10.1158/1078-0432.CCR-11-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Datta D, Flaxenburg JA, Laxmanan S, et al. Ras-induced modulation of CXCL10 and its receptor splice variant CXCR3-B in MDA-MB-435 and MCF-7 cells: relevance for the development of human breast cancer. Cancer Research. 2006;66(19):9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- 274.Murakami T, Kawada K, Iwamoto M, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. International Journal of Cancer. 2013;132(2):276–287. doi: 10.1002/ijc.27670. [DOI] [PubMed] [Google Scholar]
- 275.Liu C, Luo D, Reynolds BA, et al. Chemokine receptor CXCR3 promotes growth of glioma. Carcinogenesis. 2011;32(2):129–137. doi: 10.1093/carcin/bgq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Maru SV, Holloway KA, Flynn G, et al. Chemokine production and chemokine receptor expression by human glioma cells: Role of CXCL10 in tumour cell proliferation. Journal of Neuroimmunology. 2008;199(1-2):35–45. doi: 10.1016/j.jneuroim.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 277.Amatschek S, Lucas R, Eger A, et al. CXCL9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through CXCR3-mediated activation of melanoma cells. British Journal of Cancer. 2011;104(3):469–479. doi: 10.1038/sj.bjc.6606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lau TS, Chung TK, Cheung TH, et al. Cancer cell-derived lymphotoxin mediates reciprocal tumour-stromal interactions in human ovarian cancer by inducing CXCL11 in fibroblasts. The Journal of Pathology. 2014;232(1):43–56. doi: 10.1002/path.4258. [DOI] [PubMed] [Google Scholar]
- 279.Wu Q, Dhir R, Wells A. Altered CXCR3 isoform expression regulates prostate cancer cell migration and invasion. Molecular Cancer. 2012;11, article 3 doi: 10.1186/1476-4598-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Utsumi T, Suyama T, Imamura Y, et al. The association of CXCR3 and renal cell carcinoma metastasis. The Journal of Urology. 2014 doi: 10.1016/j.juro.2014.01.100. [DOI] [PubMed] [Google Scholar]
- 281.Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S. CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. The Journal of Biological Chemistry. 2010;285(47):36842–36848. doi: 10.1074/jbc.M110.170324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Biswas S, Sengupta S, Roy Chowdhury S, et al. CXCL13-CXCR5 co-expression regulates epithelial to mesenchymal transition of breast cancer cells during lymph node metastasis. Breast Cancer Research and Treatment. 2014;143(2):265–276. doi: 10.1007/s10549-013-2811-8. [DOI] [PubMed] [Google Scholar]
- 283.Meijer J, Zeelenberg IS, Sipos B, Roos E. The CXCR5 chemokine receptor is expressed by carcinoma cells and promotes growth of colon carcinoma in the liver. Cancer Research. 2006;66(19):9576–9582. doi: 10.1158/0008-5472.CAN-06-1507. [DOI] [PubMed] [Google Scholar]
- 284.Airoldi I, Cocco C, Morandi F, Prigione I, Pistoia V. CXCR5 may be involved in the attraction of human metastatic neuroblastoma cells to the bone marrow. Cancer Immunology, Immunotherapy. 2008;57(4):541–548. doi: 10.1007/s00262-007-0392-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.El-Haibi CP, Singh R, Gupta P, et al. Antibody microarray analysis of signaling networks regulated by Cxcl13 and Cxcr5 in prostate cancer. Journal of Proteomics and Bioinformatics. 2012;5(8):177–184. doi: 10.4172/jpb.1000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.El-Haibi CP, Sharma P, Singh R, et al. Differential G protein subunit expression by prostate cancer cells and their interaction with CXCR5. Molecular Cancer. 2013;12(1, article 64) doi: 10.1186/1476-4598-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.El-Haibi CP, Singh R, Sharma PK, Singh S, Lillard JW. CXCL13 mediates prostate cancer cell proliferation through JNK signalling and invasion through ERK activation. Cell Proliferation. 2011;44(4):311–319. doi: 10.1111/j.1365-2184.2011.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Singh S, Singh R, Sharma PK, et al. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Letters. 2009;283(1):29–35. doi: 10.1016/j.canlet.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Matsushita K, Toiyama Y, Tanaka K, et al. Soluble CXCL16 in preoperative serum is a novel prognostic marker and predicts recurrence of liver metastases in colorectal cancer patients. Annals of Surgical Oncology. 2012;19(supplement 3):S518–S527. doi: 10.1245/s10434-011-1993-8. [DOI] [PubMed] [Google Scholar]
- 290.Taghizadeh R, Noh M, Huh YH, et al. Cxcr6, a newly defined biomarker of tissue-specific stem cell asymmetric self-renewal, identifies more aggressive human melanoma cancer stem cells. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015183.e15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ou DL, Chen CL, Lin SB, Hsu CH, Lin LI. Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. Journal of Pathology. 2006;210(3):363–373. doi: 10.1002/path.2053. [DOI] [PubMed] [Google Scholar]
- 292.Wente MN, Gaida MM, Mayer C, et al. Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. International Journal of Oncology. 2008;33(2):297–308. [PubMed] [Google Scholar]
- 293.Lu Y, Wang J, Xu Y, et al. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro . Molecular Cancer Research. 2008;6(4):546–554. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- 294.Hershberger PM, Peddibhotla S, Sugarman E, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda, Md, USA: National Center for Biotechnology Information (US); 2010. Probing the CXCR6/CXCL16 axis: targeting prevention of prostate cancer metastasis. [PubMed] [Google Scholar]
- 295.Wang J, Lu Y, Wang J, Koch AE, Zhang J, Taichman RS. CXCR6 induces prostate cancer progression by the AKT/mammalian target of rapamycin signaling pathway. Cancer Research. 2008;68(24):10367–10376. doi: 10.1158/0008-5472.CAN-08-2780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 296.Gutwein P, Schramme A, Sinke N, et al. Tumoural CXCL16 expression is a novel prognostic marker of longer survival times in renal cell cancer patients. European Journal of Cancer. 2009;45(3):478–489. doi: 10.1016/j.ejca.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 297.Hernandez L, Magalhaes MAO, Coniglio SJ, Condeelis JS, Segall JE. Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Research. 2011;13(6, article R128) doi: 10.1186/bcr3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. Journal of Experimental Medicine. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu C, Pham K, Luo D, et al. Expression and functional heterogeneity of chemokine receptors CXCR4 and CXCR7 in primary patient-derived glioblastoma cells. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0059750.e59750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Xue TC, Chen RX, Han D, et al. Down-regulation of CXCR7 inhibits the growth and lung metastasis of human hepatocellular carcinoma cells with highly metastatic potential. Experimental and Therapeutic Medicine. 2012;3(1):117–123. doi: 10.3892/etm.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zheng K, Li HY, Su XL, et al. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. Journal of Experimental and Clinical Cancer Research. 2010;29(1, article 31) doi: 10.1186/1756-9966-29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ma M, Ye JY, Deng R, Dee CM, Chan GC. Mesenchymal stromal cells may enhance metastasis of neuroblastoma via SDF-1/CXCR4 and SDF-1/CXCR7 signaling. Cancer Letters. 2011;312(1):1–10. doi: 10.1016/j.canlet.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 303.Kim M, Rooper L, Xie J, Kajdacsy-Balla AA, Barbolina MV. Fractalkine receptor CX3CR1 is expressed in epithelial ovarian carcinoma cells and required for motility and adhesion to peritoneal mesothelial cells. Molecular Cancer Research. 2012;10(1):11–24. doi: 10.1158/1541-7786.MCR-11-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sciumé G, Soriani A, Piccoli M, Frati L, Santoni A, Bernardini G. CX3CR1/CX3CL1 axis negatively controls glioma cell invasion and is modulated by transforming growth factor-beta1. Neuro-Oncology. 2010;12(7):701–710. doi: 10.1093/neuonc/nop076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Nevo I, Sagi-Assif O, Meshel T, et al. The involvement of the fractalkine receptor in the transmigration of neuroblastoma cells through bone-marrow endothelial cells. Cancer Letters. 2009;273(1):127–139. doi: 10.1016/j.canlet.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 306.Zhao T, Gao S, Wang X, et al. Hypoxia-inducible factor-1α regulates chemotactic migration of pancreatic ductal adenocarcinoma cells through directly transactivating the CX3CR1 gene. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0043399.e43399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Research. 2008;68(21):9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 308.Xiao LJ, Chen YY, Lin P, et al. Hypoxia increases CX3CR1 expression via HIF-1 and NF-κB in androgen-independent prostate cancer cells. International Journal of Oncology. 2012;41(5):1827–1836. doi: 10.3892/ijo.2012.1610. [DOI] [PubMed] [Google Scholar]
- 309.Castellana D, Zobairi F, Martinez MC, et al. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: A role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Research. 2009;69(3):785–793. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 310.Jamieson WL, Shimizu S, D'Ambrosio JA, Meucci O, Fatatis A. CX3CR1 is expressed by prostate epithelial cells and androgens regulate the levels of CX3CL1/fractalkine in the bone marrow: potential role in prostate cancer bone tropism. Cancer Research. 2008;68(6):1715–1722. doi: 10.1158/0008-5472.CAN-07-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yao X, Qi L, Chen X, Du J, Zhang Z, Liu S. Expression of CX3CR1 associates with cellular migration, metastasis, and prognosis in human clear cell renal cell carcinoma. Urologic Oncology. 2014;32(2):162–170. doi: 10.1016/j.urolonc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 312.Kim M, Rooper L, Xie J, et al. The lymphotactin receptor is expressed in epithelial ovarian carcinoma and contributes to cell migration and proliferation. Molecular Cancer Research. 2012;10(11):1419–1429. doi: 10.1158/1541-7786.MCR-12-0361. [DOI] [PubMed] [Google Scholar]
- 313.Khurram SA, Whawell SA, Bingle L, Murdoch C, McCabe BM, Farthing PM. Functional expression of the chemokine receptor XCR1 on oral epithelial cells. Journal of Pathology. 2010;221(2):153–163. doi: 10.1002/path.2695. [DOI] [PubMed] [Google Scholar]
- 314.Bajetto A, Barbieri F, Dorcaratto A, et al. Expression of CXC chemokine receptors 1-5 and their ligands in human glioma tissues: Role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochemistry International. 2006;49(5):423–432. doi: 10.1016/j.neuint.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 315.Bonavia R, Bajetto A, Barbero S, Pirani P, Florio T, Schettini G. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicology Letters. 2003;139(2-3):181–189. doi: 10.1016/s0378-4274(02)00432-0. [DOI] [PubMed] [Google Scholar]
- 316.Zhou Y, Bian X, Le Y, et al. Formylpeptide receptor FPR and the rapid growth of malignant human gliomas. Journal of the National Cancer Institute. 2005;97(11):823–835. doi: 10.1093/jnci/dji142. [DOI] [PubMed] [Google Scholar]
- 317.Yang Y, Liu Y, Yao X, et al. Annexin 1 released by necrotic human glioblastoma cells stimulates tumor cell growth through the formyl peptide receptor 1. The American Journal of Pathology. 2011;179(3):1504–1512. doi: 10.1016/j.ajpath.2011.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Huang J, Hu J, Bian X, et al. Transactivation of the epidermal growth factor receptor by formylpeptide receptor exacerbates the malignant behavior of human glioblastoma cells. Cancer Research. 2007;67(12):5906–5913. doi: 10.1158/0008-5472.CAN-07-0691. [DOI] [PubMed] [Google Scholar]
- 319.Marsigliante S, Vetrugno C, Muscella A. CCL20 induces migration and proliferation on breast epithelial cells. Journal of Cellular Physiology. 2013;228(9):1873–1883. doi: 10.1002/jcp.24349. [DOI] [PubMed] [Google Scholar]
- 320.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 321.Beider K, Abraham M, Begin M, et al. Interaction between CXCR4 and CCL20 pathways regulates tumor growth. PLoS ONE. 2009;4(4) doi: 10.1371/journal.pone.0005125.e5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yaccoby S. Advances in the understanding of myeloma bone disease and tumour growth. British Journal of Haematology. 2010;149(3):311–321. doi: 10.1111/j.1365-2141.2010.08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Valentin-Opran A, Charhon SA, Meunier PJ, Edouard CM, Arlot ME. Quantitative histology of myeloma-induced bone changes. The British Journal of Haematology. 1982;52(4):601–610. doi: 10.1111/j.1365-2141.1982.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 324.Dairaghi DJ, Oyajobi BO, Gupta A, et al. CCR1 blockade reduces tumor burden and osteolysis in vivo in a mouse model of myeloma bone disease. Blood. 2012;120(7):1449–1457. doi: 10.1182/blood-2011-10-384784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Vallet S, Pozzi S, Patel K, et al. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25(7):1174–1181. doi: 10.1038/leu.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wu Y, Li Y, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. Journal of Immunology. 2008;181(9):6384–6393. doi: 10.4049/jimmunol.181.9.6384. [DOI] [PubMed] [Google Scholar]
- 327.Wang JM, Chertov O, Proost P, et al. Purification and identification of chemokines potentially involved in kidney-specific metastasis by a murine lymphoma variant: induction of migration and NFkappaB activation. International Journal of Cancer. 1998;75(6):900–907. doi: 10.1002/(sici)1097-0215(19980316)75:6<900::aid-ijc13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 328.Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 329.Said N, Theodorescu D. RhoGDI2 suppresses bladder cancer metastasis via reduction of inflammation in the tumor microenvironment. Oncoimmunology. 2012;1(7):1175–1177. doi: 10.4161/onci.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Lee JT, Lee SD, Lee JZ, Chung MK, Ha HK. Expression analysis and clinical significance of CXCL16/CXCR6 in patients with bladder cancer. Oncology Letters. 2013;5(1):229–235. doi: 10.3892/ol.2012.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Biragyn A, Bodogai M, Olkhanud PB, et al. Inhibition of lung metastasis by chemokine CCL17-mediated in vivo silencing of genes in CCR4+ Tregs. Journal of Immunotherapy. 2013;36(4):258–267. doi: 10.1097/CJI.0b013e318294357c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhang Y, Meng FY, Li WL, Zhou CX, Guan Z, Fan HY. Association of chemotactic factor receptor 5 gene with breast cancer. Genetics and Molecular Research. 2013;12(4):5289–5300. doi: 10.4238/2013.November.7.4. [DOI] [PubMed] [Google Scholar]
- 333.Andre F, Cabioglu N, Assi H, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Annals of Oncology. 2006;17(6):945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 334.Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Research. 2009;69(1):349–357. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- 335.Pan M, Hou M, Chang H, Hung W. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. Journal of Biological Chemistry. 2008;283(17):11155–11163. doi: 10.1074/jbc.M710038200. [DOI] [PubMed] [Google Scholar]
- 336.Liu Y, Ji R, Li J, et al. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. Journal of Experimental and Clinical Cancer Research. 2010;29(1, article 16) doi: 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Cabioglu N, Yazici MS, Arun B, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clinical Cancer Research. 2005;11(16):5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 338.Kamalakar A, Bendre MS, Washam CL, et al. Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans. Bone. 2014;61:176–185. doi: 10.1016/j.bone.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33(1):28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 340.Sharma B, Nawandar DM, Nannuru KC, Varney ML, Singh RK. Targeting CXCR2 enhances chemotherapeutic response, inhibits mammary tumor growth, angiogenesis, and lung metastasis. Molecular Cancer Therapeutics. 2013;12(5):799–808. doi: 10.1158/1535-7163.MCT-12-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bersini S, Jeon JS, Dubini G, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35(8):2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. Journal of Immunotherapy. 2007;30(5):490–498. doi: 10.1097/CJI.0b013e318031b551. [DOI] [PubMed] [Google Scholar]
- 343.Bu H, Shu B, Gao F, et al. Spinal IFN-γ-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Research and Treatment. 2014;143(2):255–263. doi: 10.1007/s10549-013-2807-4. [DOI] [PubMed] [Google Scholar]
- 344.Ma X, Norsworthy K, Kundu N, et al. CXCR3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Molecular Cancer Therapeutics. 2009;8(3):490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- 345.Markiewicz A, Ksiazkiewicz M, Welnicka-Jaskiewicz M, et al. Mesenchymal phenotype of CTC-enriched blood fraction and lymph node metastasis formation potential. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0093901.e93901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Roy LD, Sahraei M, Schettini JL, Gruber HE, Besmer DM, Mukherjee P. Systemic neutralization of IL-17A significantly reduces breast cancer associated metastasis in arthritic mice by reducing CXCL12/SDF-1 expression in the metastatic niches. BMC Cancer. 2014;14, article 225 doi: 10.1186/1471-2407-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Hung CS, Su HY, Liang HH, et al. High-level expression of CXCR4 in breast cancer is associated with early distant and bone metastases. Tumor Biology. 2014;35(2):1581–1588. doi: 10.1007/s13277-013-1218-9. [DOI] [PubMed] [Google Scholar]
- 348.Subik K, Shu L, Wu C, et al. The ubiquitin E3 ligase WWP1 decreases CXCL12-mediated MDA231 breast cancer cell migration and bone metastasis. Bone. 2012;50(4):813–823. doi: 10.1016/j.bone.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Shepard JB, Wilkinson RA, Starkey JR, Teintze M. Novel guanide-substituted compounds bind to CXCR4 and inhibit breast cancer metastasis. Anti-Cancer Drugs. 2014;25(1):8–16. doi: 10.1097/CAD.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 350.Lin S, Sun L, Hu J, et al. Chemokine C-X-C motif receptor 6 contributes to cell migration during hypoxia. Cancer Letters. 2009;279(1):108–117. doi: 10.1016/j.canlet.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 351.Luker KE, Lewin SA, Mihalko LA, et al. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012;31(45):4750–4758. doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Chaudary N, Mujcic H, Wouters BG, Hill RP. Hypoxia and metastasis in an orthotopic cervix cancer xenograft model. Radiotherapy and Oncology. 2013;108(3):506–510. doi: 10.1016/j.radonc.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 353.Kodama J, Kusumoto T, Seki N, et al. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Annals of Oncology. 2007;18(1):70–76. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 354.Yang YC, Lee ZY, Wu CC, Chen TC, Chang CL, Chen CP. CXCR4 expression is associated with pelvic lymph node metastasis in cervical adenocarcinoma. International Journal of Gynecological Cancer. 2007;17(3):676–686. doi: 10.1111/j.1525-1438.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 355.Itatani Y, Kawada K, Fujishita T, et al. Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology. 2013;145(5):1064–1075. doi: 10.1053/j.gastro.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 356.Cho YB, Lee WY, Choi SJ, et al. CC chemokine ligand 7 expression in liver metastasis of colorectal cancer. Oncology Reports. 2012;28(2):689–694. doi: 10.3892/or.2012.1815. [DOI] [PubMed] [Google Scholar]
- 357.Kitamura T, Fujishita T, Loetscher P, et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in amouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Lim SY, Gordon-Weeks AN, Zhao L, et al. Recruitment of myeloid cells to the tumor microenvironment supports liver metastasis. Oncoimmunology. 2013;2(3) article e23187 doi: 10.4161/onci.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wolf MJ, Hoos A, Bauer J, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. 2012;22(1):91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 360.Ghadjar P, Coupland SE, Na IK, et al. Chemokine receptor CCR6 expression level and liver metastases in colorectal cancer. Journal of Clinical Oncology. 2006;24(12):1910–1916. doi: 10.1200/JCO.2005.04.1822. [DOI] [PubMed] [Google Scholar]
- 361.Higashiguchi T, Hotta T, Takifuji K, et al. Clinical impact of matrix metalloproteinase-7 mRNA expression in the invasive front and inner surface of tumor tissues in patients with colorectal cancer. Diseases of the Colon and Rectum. 2007;50(10):1585–1593. doi: 10.1007/s10350-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 362.Yamamoto M, Kikuchi H, Ohta M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Research. 2008;68(23):9754–9762. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- 363.Kollmar O, Junker B, Rupertus K, Menger MD, Schilling MK. Studies on MIP-2 and CXCR2 expression in a mouse model of extrahepatic colorectal metastasis. European Journal of Surgical Oncology. 2007;33(6):803–811. doi: 10.1016/j.ejso.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 364.Kawada K, Hosogi H, Sonoshita M, et al. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26(32):4679–4688. doi: 10.1038/sj.onc.1210267. [DOI] [PubMed] [Google Scholar]
- 365.Cambien B, Karimdjee BF, Richard-Fiardo P, et al. Organ-specific inhibition of metastatic colon carcinoma by CXCR3 antagonism. British Journal of Cancer. 2009;100(11):1755–1764. doi: 10.1038/sj.bjc.6605078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kee J, Ito A, Hojo S, et al. Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncology Reports. 2013;29(3):975–982. doi: 10.3892/or.2012.2185. [DOI] [PubMed] [Google Scholar]
- 367.Rubie C, Frick VO, Ghadjar P, et al. CXC receptor-4 mRNA silencing abrogates CXCL12-induced migration of colorectal cancer cells. Journal of Translational Medicine. 2011;9, article 22 doi: 10.1186/1479-5876-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Drury LJ, Ziarek JJ, Gravel S, et al. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4interactions and signaling pathways. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(43):17655–17660. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Abedini F, Ismail M, Hosseinkhani H, et al. Effects of CXCR4 siRNA/dextran-spermine nanoparticles on CXCR4 expression and serum LDH levels in a mouse model of colorectal cancer metastasis to the liver. Cancer Management and Research. 2011;3(1):301–309. doi: 10.2147/CMR.S11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Ma L, Qiao H, He C, et al. Modulating the interaction of CXCR4 and CXCL12 by low-molecular-weight heparin inhibits hepatic metastasis of colon cancer. Investigational New Drugs. 2012;30(2):508–517. doi: 10.1007/s10637-010-9578-0. [DOI] [PubMed] [Google Scholar]
- 371.Silinsky J, Grimes C, Driscoll T, et al. CD133+ and CXCR4+ colon cancer cells as a marker for lymph node metastasis. Journal of Surgical Research. 2013;185(1):113–118. doi: 10.1016/j.jss.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 372.Chang SC, Lin PC, Yang SH, Wang HS, Li AF, Lin JK. SDF-1α G801A polymorphism predicts lymph node metastasis in stage T3 colorectal cancer. Annals of Surgical Oncology. 2009;16(8):2323–2330. doi: 10.1245/s10434-009-0501-x. [DOI] [PubMed] [Google Scholar]
- 373.Mongan JP, Fadul CE, Cole BF, et al. Brain metastases from colorectal cancer: risk factors, incidence, and the possible role of chemokines. Clinical Colorectal Cancer. 2009;8(2):100–105. doi: 10.3816/CCC.2009.n.016. [DOI] [PubMed] [Google Scholar]
- 374.Ding Y, Shimada Y, Maeda M, et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clinical Cancer Research. 2003;9(9):3406–3412. [PubMed] [Google Scholar]
- 375.Ishida K, Iwahashi M, Nakamori M, et al. High CCR7 mRNA expression of cancer cells is associated with lymph node involvement in patients with esophageal squamous cell carcinoma. International Journal of Oncology. 2009;34(4):915–922. doi: 10.3892/ijo_00000217. [DOI] [PubMed] [Google Scholar]
- 376.Song Y, Wang Z, Liu X, Jiang W, Shi M. CCR7 and VEGF-C: molecular indicator of lymphatic metastatic recurrence in pn0 esophageal squamous cell carcinoma after ivor-lewis esophagectomy? Annals of Surgical Oncology. 2012;19(11):3606–3612. doi: 10.1245/s10434-012-2419-y. [DOI] [PubMed] [Google Scholar]
- 377.Sui P, Hu P, Zhang T, Zhang X, Liu Q, Du J. High expression of CXCR-2 correlates with lymph node metastasis and predicts unfavorable prognosis in resected esophageal carcinoma. Medical Oncology. 2013;31, article 809 doi: 10.1007/s12032-013-0809-z. [DOI] [PubMed] [Google Scholar]
- 378.Gros SJ, Kurschat N, Drenckhan A, et al. Involvement of CXCR4 chemokine receptor in metastastic HER2-positive esophageal cancer. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047287.e47287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Gros SJ, Graeff H, Drenckhan A, et al. CXCR4/SDF-1α-mediated chemotaxis in an in vivo model of metastatic esophageal carcinoma. In Vivo. 2012;26(4):711–718. [PubMed] [Google Scholar]
- 380.Sasaki K, Natsugoe S, Ishigami S, et al. Expression of CXCL12 and its receptor CXCR4 correlates with lymph node metastasis in submucosal esophageal cancer. Journal of Surgical Oncology. 2008;97(5):433–438. doi: 10.1002/jso.20976. [DOI] [PubMed] [Google Scholar]
- 381.Futagami S, Tatsuguchi A, Hiratsuka T, et al. Monocyte chemoattractant protein 1 and CD40 ligation have a synergistic effect on vascular endothelial growth factor production through cyclooxygenase 2 upregulation in gastric cancer. Journal of Gastroenterology. 2008;43(3):216–224. doi: 10.1007/s00535-007-2151-8. [DOI] [PubMed] [Google Scholar]
- 382.Arigami T, Natsugoe S, Uenosono Y, et al. CCR7 and CXCR4 expression predicts lymph node status including micrometastasis in gastric cancer. International Journal of Oncology. 2009;35(1):19–24. doi: 10.3892/ijo_00000308. [DOI] [PubMed] [Google Scholar]
- 383.Wang W, Chen Y, Zhang Y, Hu T. The regulatory mechanism of CCR7 gene expression and its involvement in the metastasis and progression of gastric cancer. Tumor Biology. 2013;34(3):1865–1871. doi: 10.1007/s13277-013-0728-9. [DOI] [PubMed] [Google Scholar]
- 384.Feng B, Li K, Zhong H, et al. RhoE promotes metastasis in gastric cancer through a mechanism dependent on enhanced expression of CXCR4. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0081709.e81709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Xu H. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World Journal of Gastroenterology. 2011;17(19):2389–2396. doi: 10.3748/wjg.v17.i19.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Ying J, Xu Q, Zhang G, Liu B, Zhu L. The expression of CXCL12 and CXCR4 in gastric cancer and their correlation to lymph node metastasis. Medical Oncology. 2012;29(3):1716–1722. doi: 10.1007/s12032-011-9990-0. [DOI] [PubMed] [Google Scholar]
- 387.Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Research. 2009;29(11):4751–4758. [PubMed] [Google Scholar]
- 388.Koizumi K, Kato S, Sakurai H, Hashimoto I, Yasumoto K, Saiki I. Therapeutics target of CXCR4 and its downstream in peritoneal carcinomatosis of gastric cancer. Frontiers in Bioscience. 2012;4(1):269–276. doi: 10.2741/s267. [DOI] [PubMed] [Google Scholar]
- 389.Hashimoto I, Koizumi K, Tatematsu M, et al. Blocking on the CXCR4/mTOR signalling pathway induces the anti-metastatic properties and autophagic cell death in peritoneal disseminated gastric cancer cells. European Journal of Cancer. 2008;44(7):1022–1029. doi: 10.1016/j.ejca.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 390.Yasumoto K, Koizumi K, Kawashima A, et al. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Research. 2006;66(4):2181–2187. doi: 10.1158/0008-5472.CAN-05-3393. [DOI] [PubMed] [Google Scholar]
- 391.Hattermann K, Mentlein R. An Infernal Trio: The chemokine CXCL12 and its receptors CXCR4 and CXCR7 in tumor biology. Annals of Anatomy. 2013;195(2):103–110. doi: 10.1016/j.aanat.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 392.Kesselring R, Thiel A, Pries R, Trenkle T, Wollenberg B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. British Journal of Cancer. 2010;103(8):1245–1254. doi: 10.1038/sj.bjc.6605891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Ueda M, Shimada T, Goto Y, et al. Expression of CC-chemokine receptor 7 (CCR7) and CXC-chemokine receptor 4 (CXCR4) in head and neck squamous cell carcinoma. Auris Nasus Larynx. 2010;37(4):488–495. doi: 10.1016/j.anl.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 394.Warner KA, Miyazawa M, Cordeiro MMR, et al. Endothelial cells enhance tumor cell invasion through a crosstalk mediated by CXC chemokine signaling. Neoplasia. 2008;10(2):131–139. doi: 10.1593/neo.07815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Han L, Jiang B, Wu H, et al. High expression of CXCR2 is associated with tumorigenesis, progression, and prognosis of laryngeal squamous cell carcinoma. Medical Oncology. 2012;29(4):2466–2472. doi: 10.1007/s12032-011-0152-1. [DOI] [PubMed] [Google Scholar]
- 396.Albert S, Riveiro ME, Halimi C, et al. Focus on the role of the CXCL12/CXCR4 chemokine axis in head and neck squamous cell carcinoma. Head and Neck. 2013;35(12):1819–1828. doi: 10.1002/hed.23217. [DOI] [PubMed] [Google Scholar]
- 397.Uchida D, Kuribayashi N, Kinouchi M, et al. Expression and function of CXCR4 in human salivary gland cancers. Clinical and Experimental Metastasis. 2013;30(2):133–142. doi: 10.1007/s10585-012-9518-9. [DOI] [PubMed] [Google Scholar]
- 398.Yoon Y, Liang Z, Zhang X, et al. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Research. 2007;67(15):7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 399.Sambandam Y, Sundaram K, Liu A, Kirkwood KL, Ries WL, Reddy SV. CXCL13 activation of c-Myc induces RANK ligand expression in stromal/preosteoblast cells in the oral squamous cell carcinoma tumor-bone microenvironment. Oncogene. 2013;32(1):97–105. doi: 10.1038/onc.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Schimanski CC, Bahre R, Gockel I, et al. Chemokine receptor CCR7 enhances intrahepatic and lymphatic dissemination of human hepatocellular cancer. Oncology Reports. 2006;16(1):109–113. [PubMed] [Google Scholar]
- 401.Manu KA, Shanmugam MK, Ong TH, et al. Emodin suppresses migration and invasion through the modulation of CXCR4 expression in an orthotopic model of human hepatocellular carcinoma. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0057015.e57015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Xiang Z-L, Zeng Z-C, Fan J, et al. A clinicopathological model to predict bone metastasis in hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology. 2011;137(12):1791–1797. doi: 10.1007/s00432-011-1060-7. [DOI] [PubMed] [Google Scholar]
- 403.Xiang Z, Zeng Z, Tang Z, et al. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9, article 176 doi: 10.1186/1471-2407-9-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Xiang Z, Zeng Z, Tang Z, et al. Increased expression of vascular endothelial growth factor-C and nuclear CXCR4 in hepatocellular carcinoma is correlated with lymph node metastasis and poor outcome. Cancer Journal. 2009;15(6):519–525. doi: 10.1097/PPO.0b013e3181c6aa6b. [DOI] [PubMed] [Google Scholar]
- 405.Xue TC, Han D, Chen RX, et al. High expression of CXCR7 combined with alpha fetoprotein in hepatocellular carcinoma correlates with extra-hepatic metastasis to lung after hepatectomy. Asian Pacific Journal of Cancer Prevention. 2011;12(3):657–663. [PubMed] [Google Scholar]
- 406.Juarez J, Dela Pena A, Baraz R, et al. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia. 2007;21(6):1249–1257. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- 407.Rondepierre F, Bouchon B, Papon J, et al. Proteomic studies of B16 lines: involvement of annexin A1 in melanoma dissemination. Biochimica et Biophysica Acta. 2009;1794(1):61–69. doi: 10.1016/j.bbapap.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 408.Braeuer RR, Zigler M, Villares GJ, Dobroff AS, Bar-Eli M. Transcriptional control of melanoma metastasis: the importance of the tumor microenvironment. Seminars in Cancer Biology. 2011;21(2):83–88. doi: 10.1016/j.semcancer.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Melnikova VO, Villares GJ, Bar-Eli M. Emerging roles of PAR-1 and PAFR in melanoma metastasis. Cancer Microenvironment. 2008;1(1):103–111. doi: 10.1007/s12307-008-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.van Deventer HW, Palmieri DA, Wu QP, McCook EC, Serody JS. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. The Journal of Immunology. 2013;190(9):4861–4867. doi: 10.4049/jimmunol.1202857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Klein A, Sagi-Assif O, Izraely S, et al. The metastatic microenvironment: brain-derived soluble factors alter the malignant phenotype of cutaneous and brain-metastasizing melanoma cells. International Journal of Cancer. 2012;131(11):2509–2518. doi: 10.1002/ijc.27552. [DOI] [PubMed] [Google Scholar]
- 412.Van Deventer HW, Qing PW, Bergstralh DT, et al. C-C chemokine receptor 5 on pulmonary fibrocytes facilitates migration and promotes metastasis via matrix metalloproteinase 9. The American Journal of Pathology. 2008;173(1):253–264. doi: 10.2353/ajpath.2008.070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.van Deventer HW, O'Connor W, Jr., Brickey WJ, Aris RM, Ting JP, Serody JS. C–C chemokine receptor 5 on stromal cells promotes pulmonary metastasis. Cancer Research. 2005;65(8):3374–3379. doi: 10.1158/0008-5472.CAN-04-2616. [DOI] [PubMed] [Google Scholar]
- 414.Lanati S, Dunn DB, Roussigné M, et al. Chemotrap-1: an engineered soluble receptor that blocks chemokine-induced migration of metastatic cancer cells in vivo. Cancer Research. 2010;70(20):8138–8148. doi: 10.1158/0008-5472.CAN-10-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.van den T, Bosch AE, Vaarwater J, van den Berg M, de Klein A, Verdijk RM. Chemokine receptor CCR7 expression predicts poor outcome in uveal melanoma and relates to liver metastasis whereas expression of CXCR4 is not of clinical relevance. Investigative Ophthalmology & Visual Science. 2013;54(12):7354–7361. doi: 10.1167/iovs.13-12407. [DOI] [PubMed] [Google Scholar]
- 416.Letsch A, Keilholz U, Schadendorf D, et al. Functional CCR9 expression is associated with small intestinal metastasis. Journal of Investigative Dermatology. 2004;122(3):685–690. doi: 10.1111/j.0022-202X.2004.22315.x. [DOI] [PubMed] [Google Scholar]
- 417.Amersi FF, Terando AM, Goto Y, et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clinical Cancer Research. 2008;14(3):638–645. doi: 10.1158/1078-0432.CCR-07-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Singh S, Varney M, Singh RK. Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis. Cancer Research. 2009;69(2):411–415. doi: 10.1158/0008-5472.CAN-08-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Kawada K, Taketo MM. Significance and mechanism of lymph node metastasis in cancer progression. Cancer Research. 2011;71(4):1214–1218. doi: 10.1158/0008-5472.CAN-10-3277. [DOI] [PubMed] [Google Scholar]
- 420.Kawada K, Sonoshita M, Sakashita H, et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Research. 2004;64(11):4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 421.J. H. Lee. CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer Research. 2012;72(13):3175–3186. doi: 10.1158/0008-5472.CAN-12-0481. [DOI] [PubMed] [Google Scholar]
- 422.Murakami T, Maki W, Cardones AR, et al. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Research. 2002;62(24):7328–7334. [PubMed] [Google Scholar]
- 423.Takekoshi T, Ziarek JJ, Volkman BF, Hwang ST. A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4-expressing melanoma cells due to enhanced serum stability. Molecular Cancer Therapeutics. 2012;11(11):2516–2525. doi: 10.1158/1535-7163.MCT-12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Fusi A, Liu Z, Kümmerlen V, Nonnemacher A, Jeske J, Keilholz U. Expression of chemokine receptors on circulating tumor cells in patients with solid tumors. Journal of Translational Medicine. 2012;10(1, article 52) doi: 10.1186/1479-5876-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Goldberg-Bittman L, Sagi-Assif O, Meshel T, et al. Cellular characteristics of neuroblastoma cells: regulation by the ELR—CXC chemokine CXCL10 and expression of a CXCR3-like receptor. Cytokine. 2005;29(3):105–117. doi: 10.1016/j.cyto.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 426.Zhao H, Cai W, Li S, et al. Characterization of neuroblastoma bone invasion/metastasis in established bone metastatic model of SY5Y and KCNR cell lines. Child's Nervous System. 2013;29(7):1097–1105. doi: 10.1007/s00381-013-2086-8. [DOI] [PubMed] [Google Scholar]
- 427.Zhao H, Cai W, Li S, et al. Establishment and characterization of xenograft models of human neuroblastoma bone metastasis. Child's Nervous System. 2012;28(12):2047–2054. doi: 10.1007/s00381-012-1909-3. [DOI] [PubMed] [Google Scholar]
- 428.Russell HV, Hicks J, Okcu MF, Nuchtern JG. CXCR4 expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. Journal of Pediatric Surgery. 2004;39(10):1506–1511. doi: 10.1016/j.jpedsurg.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 429.Meier R, Mühlethaler-Mottet A, Flahaut M, et al. The chemokine receptor CXCR4 strongly promotes neuroblastoma primary tumour and metastatic growth, but not invasion. PLoS ONE. 2007;2(10) doi: 10.1371/journal.pone.0001016.e1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Zhang L, Yeger H, Das B, Irwinz MS, Baruchel S. Tissue microenvironment modulates CXCR4 expression and tumor metastasis in neuroblastoma. Neoplasia. 2007;9(1):36–46. doi: 10.1593/neo.06670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Nakamura ES, Koizumi K, Kobayashi M, et al. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clinical and Experimental Metastasis. 2006;23(1):9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 432.Zhang Q, Sun L, Yin L, et al. CCL19/CCR7 upregulates heparanase via specificity protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumor Biology. 2013;34(5):2703–2708. doi: 10.1007/s13277-013-0822-z. [DOI] [PubMed] [Google Scholar]
- 433.Choi YH, Burdick MD, Strieter BA, Mehrad B, Strieter RM. CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells. Molecular Cancer Research. 2014;12(1):38–47. doi: 10.1158/1541-7786.MCR-12-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Yang CL, Liu SS, Ma YG, Liu Y, Xue Y, Huang B. The influence of intraoperative pleural perfusion with matrine-cisplatin or cisplatin on stromal cell-derived factor-1 in non-small cell lung cancer patients with subclinical pleural metastasis. Medical Oncology. 2012;29(2):574–581. doi: 10.1007/s12032-011-9849-4. [DOI] [PubMed] [Google Scholar]
- 435.Chen G, Wang Z, Liu X, Liu F. High-level CXCR4 expression correlates with brain-specific metastasis of non-small cell lung cancer. World Journal of Surgery. 2011;35(1):56–61. doi: 10.1007/s00268-010-0784-x. [DOI] [PubMed] [Google Scholar]
- 436.Mauri FA, Pinato DJ, Trivedi P, Sharma R, Shiner RJ. Isogeneic comparison of primary and metastatic lung cancer identifies CX3CR1 as a molecular determinant of site-specific metastatic diffusion. Oncology Reports. 2012;28(2):647–653. doi: 10.3892/or.2012.1818. [DOI] [PubMed] [Google Scholar]
- 437.Pradelli E, Karimdjee-Soilihi B, Michiels J, et al. Antagonism of chemokine receptor CXCR3 inhibits osteosarcoma metastasis to lungs. International Journal of Cancer. 2009;125(11):2586–2594. doi: 10.1002/ijc.24665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Brennecke P, Arlt MJ, Campanile C, et al. CXCR4 antibody treatment suppresses metastatic spread to the lung of intratibial human osteosarcoma xenografts in mice. Clinical & Experimental Metastasis. 2014;31(3):339–49. doi: 10.1007/s10585-013-9632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Goguet-Surmenian E, Richard-Fiardo P, Guillemot E, et al. CXCR7-mediated progression of osteosarcoma in the lungs. British Journal of Cancer. 2013;109(6):1579–1585. doi: 10.1038/bjc.2013.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Singh R, Stockard CR, Grizzle WE, Lillard JW, Jr., Singh S. Expression and histopathological correlation of CCR9 and CCL25 in ovarian cancer. International Journal of Oncology. 2011;39(2):373–381. doi: 10.3892/ijo.2011.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Qu QX, Huang Q, Xu J, Duan LT, Zhu YB, Zhang XG. CD40 signal regulates CXCR4 mediating ovarian carcinoma cell migration: implications for extrapelvic metastastic factors. Oncology Research. 2013;20(9):383–392. doi: 10.3727/096504013X13657689382653. [DOI] [PubMed] [Google Scholar]
- 442.Wang J, Cai J, Han F, et al. Silencing of CXCR4 blocks progression of ovarian cancer and depresses canonical Wnt signaling pathway. International Journal of Gynecological Cancer. 2011;21(6):981–987. doi: 10.1097/IGC.0b013e31821d2543. [DOI] [PubMed] [Google Scholar]
- 443.Guo L, Cui Z, Zhang J, Huang Y. Chemokine axes CXCL12/CXCR4 and CXCL16/CXCR6 correlate with lymph node metastasis in epithelial ovarian carcinoma. Chinese Journal of Cancer. 2011;30(5):336–343. doi: 10.5732/cjc.010.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kajiyama H, Shibata K, Terauchi M, Ino K, Nawa A, Kikkawa F. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. International Journal of Cancer. 2008;122(1):91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 445.Mitchem JB, Brennan DJ, Knolhoff BL, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Research. 2013;73(3):1128–1141. doi: 10.1158/0008-5472.CAN-12-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Guo J, Lou W, Ji Y, Zhang S. Effect of CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human pancreatic ductal adenocarcinoma. Oncology Letters. 2013;5(5):1572–1578. doi: 10.3892/ol.2013.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Roy I, Zimmerman NP, Mackinnon AC, Tsai S, Evans DB, Dwinell MB. CXCL12 chemokine expression suppresses human pancreatic cancer growth and metastasis. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0090400.e90400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Saur D, Seidler B, Schneider G, et al. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129(4):1237–1250. doi: 10.1053/j.gastro.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 449.Zhong W, Chen W, Zhang D, et al. CXCL12/CXCR4 axis plays pivotal roles in the organ-specific metastasis of pancreatic adenocarcinoma: a clinical study. Experimental and Therapeutic Medicine. 2012;4(3):363–369. doi: 10.3892/etm.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Lu Y, Chen Q, Corey E, et al. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clinical and Experimental Metastasis. 2009;26(2):161–169. doi: 10.1007/s10585-008-9226-7. [DOI] [PubMed] [Google Scholar]
- 451.Kim SJ, Uehara H, Karashima T, Mccarty M, Shih N, Fidler IJ. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3(1):33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Yang X, Xu Z, Li D, et al. Cell surface nucleolin is crucial in the activation of the CXCL12/CXCR4 signaling pathway. Tumor Biology. 2014;35(1):333–338. doi: 10.1007/s13277-013-1044-0. [DOI] [PubMed] [Google Scholar]
- 453.Hu W, Zhen X, Xiong B, Wang B, Zhang W, Zhou W. CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Science. 2008;99(7):1362–1369. doi: 10.1111/j.1349-7006.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Kominsky SL, Abdelmagid SM, Doucet M, Brady K, Weber KL. Macrophage inflammatory protein-1δ: A novel osteoclast stimulating factor secreted by renal cell carcinoma bone metastasis. Cancer Research. 2008;68(5):1261–1266. doi: 10.1158/0008-5472.CAN-07-6122. [DOI] [PubMed] [Google Scholar]
- 455.Wagner PL, Moo TA, Arora N, et al. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid carcinoma. Annals of Surgical Oncology. 2008;15(10):2833–2841. doi: 10.1245/s10434-008-0064-2. [DOI] [PubMed] [Google Scholar]
- 456.Sancho M, Vieira JM, Casalou C, et al. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. Journal of Endocrinology. 2006;191(1):229–238. doi: 10.1677/joe.1.06688. [DOI] [PubMed] [Google Scholar]
- 457.Wang N, Luo HJ, Yin GB, et al. Overexpression of HIF-2α, TWIST, and CXCR4 is associated with lymph node metastasis in papillary thyroid carcinoma. Clinical and Developmental Immunology. 2013;2013:9 pages. doi: 10.1155/2013/589423.589423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Yasuoka H, Kodama R, Hirokawa M, et al. CXCR4 expression in papillary thyroid carcinoma: induction by nitric oxide and correlation with lymph node metastasis. BMC Cancer. 2008;8, article 274 doi: 10.1186/1471-2407-8-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Dang S, Peng Y, Ye L, et al. Stimulation of TLR4 by LMW-HA induces metastasis in human papillary thyroid carcinoma through CXCR7. Clinical and Developmental Immunology. 2013;2013:11 pages. doi: 10.1155/2013/712561.712561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Spring H, Schüler T, Arnold B, Hämmerling GJ, Ganss R. Chemokines direct endothelial progenitors into tumor neovessels. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(50):18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Shih Y, Wang MC, Peng H, et al. Modulation of chemotactic and pro-inflammatory activities of endothelial progenitor cells by hepatocellular carcinoma. Cellular Signalling. 2012;24(3):779–793. doi: 10.1016/j.cellsig.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 462.Li A, Cheng XJ, Moro A, Singh RK, Hines OJ, Eibl G. CXCR2-dependent endothelial progenitor cell mobilization in pancreatic cancer growth. Translational Oncology. 2011;4(1):20–28. doi: 10.1593/tlo.10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Liu Z, Tian R, Li Y, et al. Inhibition of tumor angiogenesis and melanoma growth by targeting vascular E-selectin. Annals of Surgery. 2011;254(3):450–457. doi: 10.1097/SLA.0b013e31822a72dc. [DOI] [PubMed] [Google Scholar]
- 464.Yang X, Lu P, Fujii C, et al. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. International Journal of Cancer. 2006;118(8):1869–1876. doi: 10.1002/ijc.21596. [DOI] [PubMed] [Google Scholar]
- 465.Rodero MP, Auvynet C, Poupel L, Combadière B, Combadière C. Control of both myeloid cell infiltration and angiogenesis by ccr1 promotes liver cancer metastasis development in mice. Neoplasia. 2013;15(6):641–648. doi: 10.1593/neo.121866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Menu E, De Leenheer E, De Raeve H, et al. Role of CCR1 and CCR5 in homing and growth of multiple myeloma and in the development of osteolytic lesions: a study in the 5TMM model. Clinical and Experimental Metastasis. 2006;23(5-6):291–300. doi: 10.1007/s10585-006-9038-6. [DOI] [PubMed] [Google Scholar]
- 467.Wang S, Xu M, Li F, et al. Ethanol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1. Breast Cancer Research and Treatment. 2012;133(3):1037–1048. doi: 10.1007/s10549-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Potter SM, Dwyer RM, Hartmann MC, et al. Influence of stromal-epithelial interactions on breast cancer in vitro and in vivo. Breast Cancer Research and Treatment. 2012;131(2):401–411. doi: 10.1007/s10549-011-1410-9. [DOI] [PubMed] [Google Scholar]
- 469.Ohta M, Kitadai Y, Tanaka S, et al. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. International Journal of Cancer. 2002;102(3):220–224. doi: 10.1002/ijc.10705. [DOI] [PubMed] [Google Scholar]
- 470.Koga M, Kai H, Egami K, et al. Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochemical and Biophysical Research Communications. 2008;365(2):279–284. doi: 10.1016/j.bbrc.2007.10.182. [DOI] [PubMed] [Google Scholar]
- 471.Araki S, Omori Y, Lyn D, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Research. 2007;67(14):6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 472.Sales KJ, Sutherland JR, Jabbour HN, Katz AA. Seminal plasma induces angiogenic chemokine expression in cervical cancer cells and regulates vascular function. Biochimica et Biophysica Acta. 2012;1823(10):1789–1795. doi: 10.1016/j.bbamcr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 473.Dwyer J, Hebda JK, Le Guelte A, et al. Glioblastoma cell-secreted interleukin-8 induces brain endothelial cell permeability via CXCR2. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0045562.e45562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Tazzyman S, Barry ST, Ashton S, et al. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. International Journal of Cancer. 2011;129(4):847–858. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]
- 475.Saijo Y, Tanaka M, Miki M, et al. Proinflammatory cytokine IL-1β promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. Journal of Immunology. 2002;169(1):469–475. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 476.Horton LW, Yu Y, Zaja-Milatovic S, Strieter RM, Richmond A. Opposing roles of murine duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Research. 2007;67(20):9791–9799. doi: 10.1158/0008-5472.CAN-07-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Wente MN, Keane MP, Burdick MD, et al. Blockade of the chemokine receptor CXCR2 inhibits pancreatic cancer cell-induced angiogenesis. Cancer Letters. 2006;241(2):221–227. doi: 10.1016/j.canlet.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 478.Matsuo Y, Raimondo M, Woodward TA, et al. CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer. International Journal of Cancer. 2009;125(5):1027–1037. doi: 10.1002/ijc.24383. [DOI] [PubMed] [Google Scholar]
- 479.Li A, King J, Moro A, et al. Overexpression of CXCL5 is associated with poor survival in patients with pancreatic cancer. The American Journal of Pathology. 2011;178(3):1340–1349. doi: 10.1016/j.ajpath.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Shen H, Schuster R, Lu B, Waltz SE, Lentsch AB. Critical and opposing roles of the chemokine receptors CXCR2 and CXCR3 in prostate tumor growth. Prostate. 2006;66(16):1721–1728. doi: 10.1002/pros.20476. [DOI] [PubMed] [Google Scholar]
- 481.Mestas J, Burdick MD, Reckamp K, Pantuck A, Figlin RA, Strieter RM. The role of CXCR2/CXCR2 ligand biological axis in renal cell carcinoma. The Journal of Immunology. 2005;175(8):5351–5357. doi: 10.4049/jimmunol.175.8.5351. [DOI] [PubMed] [Google Scholar]
- 482.Desbaillets I, Diserens A-C, de Tribolet N, Hamou M-F, van Meir EG. Upregulation of interleukin 8 by oxygen-deprived cells in glioblastoma suggests a role in leukocyte activation, chemotaxis, and angiogenesis. The Journal of Experimental Medicine. 1997;186(8):1201–1212. doi: 10.1084/jem.186.8.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Kline M, Donovan K, Wellik L, et al. Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression. Leukemia Research. 2007;31(5):591–598. doi: 10.1016/j.leukres.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 484.Agarwal A, Tressel SL, Kaimal R, et al. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Research. 2010;70(14):5880–5890. doi: 10.1158/0008-5472.CAN-09-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Hill KS, Gaziova I, Harrigal L, et al. Met receptor tyrosine kinase signaling induces secretion of the angiogenic chemokine interleukin-8/CXCL8 in pancreatic cancer. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0040420.e40420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Charalambous C, Pen LB, Su YS, Milan J, Chen TC, Hofman FM. Interleukin-8 differentially regulates migration of tumor-associated and normal human brain endothelial cells. Cancer Research. 2005;65(22):10347–10354. doi: 10.1158/0008-5472.CAN-05-0949. [DOI] [PubMed] [Google Scholar]
- 487.Liang Z, Brooks J, Willard M, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochemical and Biophysical Research Communications. 2007;359(3):716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Kollmar O, Rupertus K, Scheuer C, et al. CXCR4 and CXCR7 regulate angiogenesis and CT26.WT tumor growth independent from SDF-1. International Journal of Cancer. 2010;126(6):1302–1315. doi: 10.1002/ijc.24956. [DOI] [PubMed] [Google Scholar]
- 489.Margolin DA, Silinsky J, Grimes C, et al. Lymph node stromal cells enhance drug-resistant colon cancer cell tumor formation through SDF-1α/CXCR4 paracrine signaling. Neoplasia. 2011;13(9):874–886. doi: 10.1593/neo.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Ingold B, Simon E, Ungethüm U, et al. Vascular CXCR4 expression—a novel antiangiogenic target in gastric cancer? PLoS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010087.e10087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Salmaggi A, Gelati M, Pollo B, et al. CXCL12 in malignant glial tumors: a possible role in angiogenesis and cross-talk between endothelial and tumoral cells. Journal of Neuro-Oncology. 2004;67(3):305–317. doi: 10.1023/b:neon.0000024241.05346.24. [DOI] [PubMed] [Google Scholar]
- 492.Rempel SA, Dudas S, Ge S, Gutiérrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clinical Cancer Research. 2000;6(1):102–111. [PubMed] [Google Scholar]
- 493.Ping Y, Yao X, Jiang J, et al. The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. Journal of Pathology. 2011;224(3):344–354. doi: 10.1002/path.2908. [DOI] [PubMed] [Google Scholar]
- 494.Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. Journal of Experimental and Clinical Cancer Research. 2007;26(4):527–533. [PubMed] [Google Scholar]
- 495.Kryczek I, Lange A, Mottram P, et al. CXCL12 and vascular endothelial growth factor synergistically induce neonaniogenisis in human ovarian cancers. Cancer Research. 2005;65(2):465–472. [PubMed] [Google Scholar]
- 496.Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clinical Cancer Research. 2000;6(9):3530–3535. [PubMed] [Google Scholar]
- 497.Wang J, Wang J, Sun Y, et al. Diverse signaling pathways through the SDF-1/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cellular Signalling. 2005;17(12):1578–1592. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 498.Hao M, Zheng J, Hou K, et al. Role of chemokine receptor CXCR7 in bladder cancer progression. Biochemical Pharmacology. 2012;84(2):204–214. doi: 10.1016/j.bcp.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 499.Miao Z, Luker KE, Summers BC, et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(40):15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Wang J, Shiozawa Y, Wang Y, et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. The Journal of Biological Chemistry. 2008;283(7):4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 501.Maishi N, Ohga N, Hida Y, et al. CXCR7: a novel tumor endothelial marker in renal cell carcinoma. Pathology International. 2012;62(5):309–317. doi: 10.1111/j.1440-1827.2012.02792.x. [DOI] [PubMed] [Google Scholar]
- 502.Ren T, Chen Q, Tian Z, Wei H. Down-regulation of surface fractalkine by RNA interference in B16 melanoma reduced tumor growth in mice. Biochemical and Biophysical Research Communications. 2007;364(4):978–984. doi: 10.1016/j.bbrc.2007.10.124. [DOI] [PubMed] [Google Scholar]
- 503.Smadja DM, Bièche I, Uzan G, et al. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(11):2321–2327. doi: 10.1161/01.ATV.0000184762.63888.bd. [DOI] [PubMed] [Google Scholar]
- 504.Nolan DJ, Ciarrocchi A, Mellick AS, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes and Development. 2007;21(12):1546–1558. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Arbab AS, Pandit SD, Anderson SA, et al. Magnetic resonance imaging and confocal microscopy studies of magnetically labeled endothelial progenitor cells trafficking to sites of tumor angiogenesis. Stem Cells. 2006;24(3):671–678. doi: 10.1634/stemcells.2005-0017. [DOI] [PubMed] [Google Scholar]
- 506.Brat DJ, Mapstone TB. Malignant glioma physiology: cellular response to hypoxia and its role in tumor progression. Annals of Internal Medicine. 2003;138(8):659–668. doi: 10.7326/0003-4819-138-8-200304150-00014. [DOI] [PubMed] [Google Scholar]
- 507.Campbell LM, Maxwell PJ, Waugh DJJ. Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer. Pharmaceuticals. 2013;6(8):929–959. doi: 10.3390/ph6080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Futagami S, Hiratsuka T, Shindo T, et al. COX-2 and CCR2 induced by CD40 ligand and MCP-1 are linked to VEGF production in endothelial cells. Prostaglandins Leukotrienes and Essential Fatty Acids. 2008;78(2):137–146. doi: 10.1016/j.plefa.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 509.Brown JM. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. The British Journal of Radiology. 2014;87(1035) doi: 10.1259/bjr.20130686.20130686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Tobinai K, Takahashi T, Akinaga S. Targeting chemokine receptor CCR4 in adult T-cell leukemia-lymphoma and other T-cell lymphomas. Current Hematologic Malignancy Reports. 2012;7(3):235–240. doi: 10.1007/s11899-012-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


