Abstract
Fasting suppresses functioning of the hypothalamic-pituitary-gonadal (HPG) axis by mechanisms that are incompletely understood. In 2003, hypothalamic kisspeptin-Kiss1r signaling was discovered to play a significant role in regulating the HPG axis. We have recently shown that in adult male macaques, short-term fasting attenuates the response of the HPG axis to an exogenous kisspeptin challenge. In the present study, we explored the mechanism underlying this attenuated response by examining the modulation of the hypothalamic expression of Kiss1 and Kiss1r under short-term fasting and normal feeding conditions in the adult male macaques. Hypothalamic mRNA was extracted from normal fed (n=3) and 48-h fasted (n=3) monkeys. Kiss1, Kiss1r, and GnRH1 mRNA was quantified by reverse transcription followed by real-time polymerase chain reaction. In addition, blood samples were collected for measurement of plasma concentrations of glucose, cortisol, leptin, and testosterone. In contrast to fed animals, plasma glucose, leptin, and testosterone levels decreased and cortisol levels increased in fasted animals. The hypothalamic expression of Kiss1 and Kiss1r mRNA was significantly lower (P<0.05) in fasted monkeys compared to fed monkeys while hypothalamic GnRH1 mRNA expression was comparable between the two groups. Thus, our results demonstrate that expression of hypothalamic Kiss1 and Kiss1r decrease after a short-term fasting in monkeys. This decrease may contribute to the suppression of the HPG axis during fasting conditions in primates. In addition, our finding of lower expression of Kiss1r in fasted monkeys provides an explanation for the attenuation in the HPG axis response to peripheral kisspeptin challenge during short-term fasting.
Keywords: Fasting, HPG axis, GnRH1, Kiss1, Kiss1r, Testosterone, Monkey
Introduction
Kisspeptin is a family of peptide hormones which belongs to RFamide superfamily of peptides [1,2]. All members of kisspeptin family are encoded by the Kiss1 (non-human)/ KISS1 (human) gene [1]. All kisspeptins act through a G protein couple receptor, GPR54 (also named as AXOR12 or hOT7T175), which is now consensually familiarized as KISS1R/ Kiss1r [1-4]. Kisspeptin is involved in many important physiological processes including a prominent role in the regulation of reproduction [5-10].
Kisspeptin is a powerful stimulator of the hypothalamic-pituitary-gonadal (HPG) axis in several vertebrate species including mammals [11-16]. Kisspeptin exerts its stimulatory effect by increasing signaling through GnRH receptor because pre-treatment with acyline, a GnRH receptor antagonist, blocks kisspeptin induced stimulation of the HPG axis [13,15,17]. Kiss1r is expressed in several regions of hypothalamus including GnRH neurons in several mammalian species [18-20]. Similarly, many studies have demonstrated Kiss1 expression in the hypothalamus of non-primate [14,21], non-human primate [15], and human [3] species.
The hypothalamic expression of Kiss1 and Kiss1r mRNA responds to both external and internal cues including steroid milieu, seasonal variation and metabolic status [22-25]. In the rat, short-term fasting decreases hypothalamic Kiss1 and increases Kiss1r mRNA expression [26]. In contrast, in the mouse, short-term fasting decreases expression of both Kiss1 and Kiss1r genes [27]. Like wise, diabetic rats (by STZ injection) display decreased expression of Kiss1 mRNA in the hypothalamus [28]. Lactation is also known to significantly reduce expression of hypothalamic Kiss1 in rats [29].
Quantification of hypothalamic Kiss1 and Kiss1r expression in states of energy imbalance in primates has not yet been examined. Recently, we demonstrated that short-term fasting cause attenuation in the response of the HPG axis to exogenous kisspeptin administration in the adult male rhesus monkey [30] but the mechanism underlying this attenuated response is unclear. Therefore, the purpose of this study was to examine hypothalamic Kiss1r mRNA expression under feeding ad libitum and short-term fasting conditions in the adult male rhesus monkey. We hypothesized that Kiss1 and Kiss1r mRNA contents will be lower in fasted monkeys as compared to fed ad libitum monkeys.
MATERIAL AND METHODS
Animals
Six adult intact male rhesus monkeys (Macaca mulatta), 6-9 years old, weighing 8.3 −14.7 kg were used in this study. The animals were housed in individual cages, in the same room, under temperature (25±3°C)- and light (06:00-18:00)- controlled conditions in the Department of Animal Sciences Primate Facility. The animals were fed daily with fresh fruits (0900-0930 h), hard boiled eggs (1100 h) and bread (1300-1330 h). Water was available ad libitum. All animal experiments were approved by the Departmental Committee for Care and Use of Animals.
Hypothalamic tissues and blood samples collection
Hypothalamic tissues were obtained from six monkeys. Three of the animals were 48 hours fasted and three were normal fed at the time of euthanasia. The animals were sacrificed after intramuscular injection of ketamine hydrochloride (Ketler, Astarapin, Germany 10 mg/kg BW i.m.) and followed by a 3-5 ml intravenous injection. The brain was removed from the cranium immediately. The hypothalami (MBH and POA) were then extracted out from the brain using dissection scheme described for the rhesus monkey before [31]. Each hemi-hypothalamus was transferred to TRIzol (1ml; Invitrogen) filled tubes and placed on −180°C until RNA extraction. In addition, blood samples were taken from these animals for hormones analysis.
Real-Time PCR
Kiss1, Kiss1r, and GnRH1 mRNA expression in hypothalamic tissues from male rhesus monkeys were quantified by real-time PCR. After reverse transcription of total RNA, 1 μl of 10 ng/μl cDNA was used for real-time PCR. Quantity of each gene mRNA expression was determined from a relative standard curve. Relative standard curves were generated by diluting one hypothalamic sample, having highest mRNA expression, 1:0, 1:1, 1:2, 1:4, 1:8 times. Each sample was run in triplicate along with a positive control (rhesus monkey normal tissue placental cDNA; BioChain Institute, Hayward, CA 94545, USA) and a negative control. The threshold cycle number (CT) from each sample was referred to standard curve to estimate the corresponding RNA content, and each RNA value was then normalized for procedural losses by using the ß-actin RNA values estimated from the relative standard curve.
The ACTB, GnRH1, Kiss1r, and Kiss1 primers and fluorescent probes used were designed to target a segment comprised within the cloned cDNA. All primers and fluorescent probes for real-time PCR were selected by use of the program Primer3 (Primer3 Web site; [32]). The accession number of genes used for primers and probes selection are; Kiss1 (AY823262), Kiss1r (AY823261), GnRH1 (S75918), and ACTB (AY369786). The primer sequences (Integrated DNA Technologies) for Kiss1r were 5′ forward (5′-CTCGCTGGTCATCTACGTCA-3′) and 3′ reverse (5′-CGAACTTGCACATGAAATCG-3′). The internal fluorescent oligodeoxynucleotide probe sequence was 5′-ACAAGCCGATGCGGACCGTGA-3′ (Integrated DNA Technologies) and was covalently linked to the fluorescent dye HEX at the 5′ end and to the quencher dye Black Hole Quencher-1 at the 3′ end. The Kiss1 real-time PCR primers used were 5′ forward (5′-CTGGAATCCCTGGACCTCTC-3′) and 3′ reverse (5′-TTGTAGTTCGGCAGGTC CTT-3′). The internal fluorescent oligodeoxynucleotide probe was 5′-GAGGAAGCCGTCTGCTACTG-3′. The GnRH1 real-time PCR primers used were 5′ forward (5′-AGATGCCGAAAATTTGATGG-3′) and 3′ reverse (5′-TTTCCAGAGCTCCTTTCAGG-3′). The internal fluorescent oligodeoxynucleotide probe was 5′-AACTGGCAGAAACCCAACAC-3′. The ACTB real-time PCR primers used were 5′ forward (5′-GCTTCTAGGCGGACTGTGAC-3′) and 3′ reverse (5′-CACCTTCACCGTTCCAGTTT-3′). The internal fluorescent oligodeoxynucleotide probe was (5′-GATGAGATTGGCATGGCTTT-3′).
Real-time PCRs were performed in a total volume of 20 μl, each reaction containing 1 μl of the reverse transcribed sample or 1, 0.5, 0.25, 0.125, 0 dilution of one of hypothalamic sample, 10 μl of AmpliTaq Gold PRC Master Mix (Applied Biosystems/Roche Molecular System), 0.8 μl of each primers and probe, 0.3 μl of reference dye, and 5.3 μl DEPC water. The real-time PCR program consisted of an initial annealing period of 2 min at 50°C, followed by 10 min of denaturing at 95°C and 40 cycles of 15 sec at 95°C and 1 min at 60°C.
Hormone and blood glucose measurements
Plasma T and cortisol concentrations were determined by specific RIAs. The T and cortisol RIA kits were purchased from Immunotech Marselle Cedex 9, France and Immunotech Prague 10, Czech Republic, respectively. The RIAs were done as per manufacturer instructions. The bound radioactivity was determined by using Beckman Gamma counter (Gamma 5500). Sensitivity of the T assay was 0.025 ng/ml and intra- and inter-assay coefficients of variation were both below 15%. Sensitivity of the cortisol assay was 0.82 μg/dl and the intra-assay coefficient of variation was below 6%. Leptin was measured using ELISA kits (AssayMax Human ELISA; Assaypro 41 Triad south drive St. Charles, MO). The sensitivity of the leptin assay was < 150 pg/ml; the intra- and inter-assay coefficients of variation were below 8%. Glucose concentrations were measured in drops of whole blood by using Oncall-plus blood glucometer (ACON laboratories, San Diego, CA, USA).
Statistical analysis
Statistical comparisons for the mean glucose, cortisol, leptin, and T levels under fasting and fed conditions were made by paired student’s t tests. Kiss1, Kiss1r, and GnRH1 mRNA contents were also compared by paired student’s t tests. All data are presented as mean (±SEM). Results were considered statistically significant at P<0.05.
Results
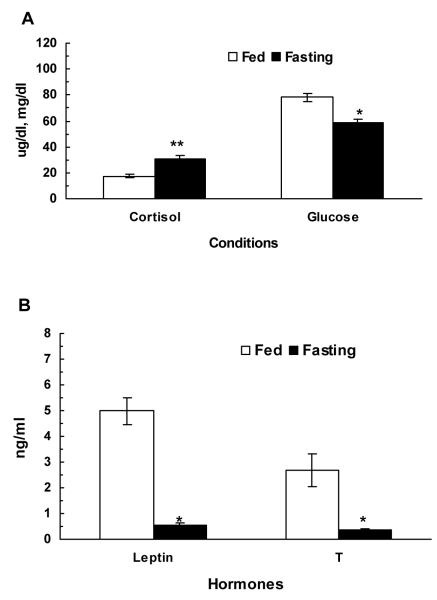
Mean basal plasma glucose, cortisol, leptin, and T are shown in figure 1. As compared to fed monkeys, mean plasma glucose and testosterone levels decreased and cortisol levels increased in fasted animals.
Figure 1.
Comparison of overall mean (±SEM) basal plasma cortisol (μg/dl) and glucose (A), leptin (ng/ml) and T (ng/ml) (B) concentrations in normal fed and 48-h fasted adult male rhesus monkeys (n=3). Fasting resulted in a significant reduction in mean plasma leptin and T levels (*P<0.01) while it significantly (**P<0.01) increased plasma cortisol levels (n=3). Blood glucose levels also significantly decreased in fasted animals.
The comparison of hypothalamic Kiss1, Kiss1r, and GnRH1 mRNA contents under fed and fasting conditions are illustrated in figures 2-4. The hypothalamic expression of Kiss1 mRNA was significantly lower (P<0.01) in fasted monkeys compared to fed monkeys (fig.2). Similarly, the hypothalamic expression of Kiss1r mRNA was also significantly (P<0.03) lower in fasted monkey compared to fed monkeys (fig.3). While no difference was observed between hypothalamic GnRH1 mRNA contents of fed and fasted animals fig.4.
Figure 2.
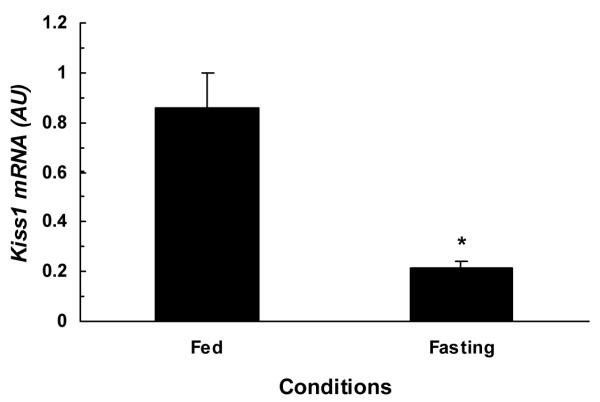
Comparison of mean (±SEM) hypothalamic Kiss1 mRNA contents in fed (1.27, 2.23, 1.64 AU) and 48-h fasted (0.47, 0.50, 0.08 AU) adult male monkeys (n=3). Hypothalamic Kiss1 mRNA contents significantly (*P<0.01) reduced in fasted monkeys as compared to fed ad libitum monkeys.
Figure 4.
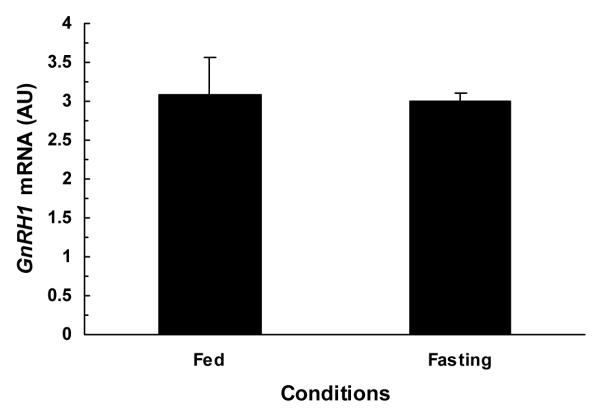
Comparison of mean (±SEM) hypothalamic GnRH1 mRNA contents in fed (2.34, 4.00, 2.88 AU) and 48-h fasted (2.97, 3.20, 2.82 AU) adult male monkeys (n=3). Hypothalamic GnRH1 mRNA contents were comparable between fasted and fed ad libitum monkeys.
Figure 3.
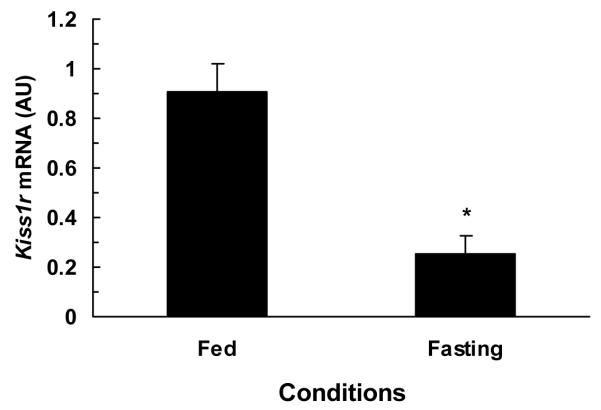
Comparison of mean (±SEM) hypothalamic Kiss1r mRNA contents in fed (0.68, 0.99, 1.05 AU) and 48-h fasted (0.39, 0.22, 0.15 AU) adult male rhesus monkeys (n=3). Hypothalamic Kiss1r mRNA contents were significantly (*P<0.03) reduced in fasted monkeys as compared to fed ad libitum monkeys.
Discussion
Kisspeptin has been hypothesized to serve as a major conduit for relaying information regarding metabolic status to centres of the brain that control the HPG axis [25]. Conditions of energy imbalance suppress the HPG axis, in part, through a decrease in the hypothalamic expression of Kiss1 and or Kiss1r mRNA [25,27,33]. To the best of our knowledge, there is no study available on the quantification of hypothalamic Kiss1 and Kiss1r mRNA in metabolically deficient conditions like fasting, in primates. In the present study, we quantified hypothalamic Kiss1 and Kiss1r mRNA under normal feeding and short-term fasting conditions in the adult male rhesus monkey. Our results demonstrated that the expression of hypothalamic Kiss1 and Kiss1r mRNA was significantly lower in the fasted animals as compared to fed animals. In contrast, no significant difference was observed between the hypothalamic GnRH1 mRNA content of fed and fasted animals.
This decrease in hypothalamic Kiss1 expression may contribute to the suppression of the HPG axis during fasting conditions in primates. Recently, a large number of studies in rodents demonstrated that lower expression of hypothalamic Kiss1 mRNA was associated with reproductive dysfunctions in metabolic conditions of energy imbalance [reviewed in 25]. While exogenous administration of kisspeptin reversed the effect of energy imbalance on the HPG axis by significantly increasing the reproductive hormones secretion [25]. In the adult male rhesus monkey, recently, we have shown that exogenous kisspeptin averted the effect of short-term fasting on the HPG axis [30]. Kisspeptin administration significantly increased plasma T secretion in fasted monkeys in the latter study. Taken together, these findings suggest that low expression of Kiss1 in fasting conditions can be a factor that may lead to decrease in the endogenous drive of kisspeptin to the HPG axis while exogenous kisspeptin may compensate for this decrease in the endogenous drive. However in our earlier study [30], several aspects of T response to KP were significantly reduced in fasted monkeys. Mean plasma T concentrations as well as T AUC for the 3 h after KP administration period was significantly reduced in fasted animals indicating lesser T response to KP stimulation. Similarly, the time taken to elicit first significant rise in T secretion following administration of KP was significantly greater in fasted animals, indicating that T response to KP administration was significantly delayed by fasting [30]. The mechanism for this attenuation in the HPG axis response to peripheral kisspeptin challenge was not clear. However, the causation of the attenuation was of hypothalamic origin as pituitary response to GnRH and testicular response to hCG were comparable in fed and fasted monkeys [30,34-36]. Our finding in the present study that the hypothalamic Kiss1r mRNA expression is also significantly decreased, therefore, provides an explanation for the attenuation in the HPG axis response to peripheral kisspeptin challenge during short term fasting condition in the adult male rhesus monkey.
Available evidences suggest that the HPG axis remains intact during fasting conditions. Testicular response to hCG and pituitary response to GnRH remained normal during short-term fasting conditions [30,34-36]. Similarly, hypothalamic GnRH1 mRNA and contents are not affected by fasting-related metabolic deficiency [37-40]. Our present results also showed that GnRH1 expression was comparable between fed and fasted monkeys, suggesting that suppression of the HPG axis during fasting conditions did not involve modulation of GnRH1 mRNA expression.
The metabolic signal generated by fasting condition that may cause decreased expression of hypothalamic Kiss1 and Kiss1r mRNA is not known. The negative energy balance and the stress can be important potential factors in this regard. The design of the present study precluded distinction between the two. Literature indicates role of hormones of both negative energy balance and stress on hypothalamic Kiss1 expression [27,33,41-43]. Recently, leptin has been shown as a major factor responsible for the regulation of Kiss1 expression [33]. Similarly, cortisol has also been shown to modulate the expression of Kiss1and Kiss1r [41,42], while ghrelin has been implicated more recently to decrease expression of hypothalamic Kiss1 [43]. Preliminary findings showed that insulin did not affect Kiss1 expression [27]. Fasting leads to hypoleptinema but increased levels of cortisol and ghrelin. Therefore, it is possible that hormonal signals of negative energy balance and stress or both entrain reduction in expression of hypothalamic Kiss1 and Kiss1r in monkeys.
In summary, our results show that the hypothalamic expression of Kiss1 and Kiss1r mRNA is decreased during short-term fasting conditions in the adult male macaque. In contrast, no change in the hypothalamic expression of GnRH1 is observed. Our findings provide an explanation for the earlier observed attenuation in the monkey HPG axis response to peripheral kisspeptin challenge after short-term fasting. The decrease in hypothalamic Kiss1 and Kiss1r expression may be one of the major contributing factors in causing suppression of the HPG axis during fasting conditions in primates.
Reference
- 1.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 2.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 3.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. Journal of Biological Chemistry. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 4.Gottsch ML, Clifton DK, Steiner RA. From KISS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30:4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. PNAS. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno J, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 7.Wahab F, Tanzeela R, Shahab M. Study of the effect of peripheral kisspeptin administration on basal and glucose-induced insulin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca mulatta) Horm Metab Res. 2010 doi: 10.1055/s-0030-1268458. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Makri A, Pissimissis N, Lembessis P, Polychronakos C, Koutsilieris M. The kisspeptin (KiSS-1)/GPR54 system in cancer biology. Cancer Treat Rev. 2008;34:682–692. doi: 10.1016/j.ctrv.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Hiden U, Bilban M, Knöfler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord. 2007;8:31–39. doi: 10.1007/s11154-007-9030-8. [DOI] [PubMed] [Google Scholar]
- 10.Wahab F, Bano R, Jabeen S, Irfan S, Shahab M. Effect of peripheral kisspeptin administration on adiponectin, leptin, and resistin secretion under fed and fasting condition in the adult male rhesus monkey (Macaca mulatta) Horm Metab Res. 2010;42:570–574. doi: 10.1055/s-0030-1252016. [DOI] [PubMed] [Google Scholar]
- 11.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 12.Thompson EL, Patterson M, Murphy KG, Smith KL, Dhillo WS, Todd JF, Ghatei MA, Bloom SR. Central and peripheral administration of kisspeptin-10 stimulates the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol. 2004;16:850–858. doi: 10.1111/j.1365-2826.2004.01240.x. [DOI] [PubMed] [Google Scholar]
- 13.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 14.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 15.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. PNAS. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary-gonadal axis in human males. The Journal of Clinical Endocrinology and Metabolism. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 17.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Nogueiras R, Vazquez MJ, Barreiro ML, Magni P, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146:156–163. doi: 10.1210/en.2004-0836. [DOI] [PubMed] [Google Scholar]
- 18.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. PNAS. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 20.Shibata M, Gibbs RB, Shahab M, Plant TM. GnRH neurons in the peripubertal male rhesus monkey (Macaca mulatta) express GPR54: Implication for the control of primate puberty. Program of the 87th Annual Meeting of the Endocrine Society; San Diego, CA, USA. 2005. pp. 1–98. (abstract) [Google Scholar]
- 21.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 22.Simonneaux V, Ansel L, Revel FG, Klosen P, Pévet P, Mikkelsen JD. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides. 2009;30:146–153. doi: 10.1016/j.peptides.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30:94–102. doi: 10.1016/j.peptides.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellano JM, Roa J, Luque RM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. KiSS-1/kisspeptins and the metabolic control of reproduction: physiologic roles and putative physiopathological implications. Peptides. 2009;30:139–145. doi: 10.1016/j.peptides.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in Hypothalamic KiSS-1 System and Restoration of Pubertal Activation of the Reproductive Axis by Kisspeptin in Undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 27.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: Analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- 28.Castellano JM, Navarro VM, Fernández-Fernández R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 29.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda KI, Tsukamura H. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148:2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- 30.Wahab F, Aziz F, Irfan S, Waheed-uz Zaman, Shahab M. Short-term fasting attenuates the response of the HPG axis to kisspeptin challenge in the adult male rhesus monkey (Macaca mulatta) Life Sci. 2008;83:633–637. doi: 10.1016/j.lfs.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19:432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 32.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa, NJ: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 34.Bergendahl M, Perheentupa A, Huhtaniemi I. Starvation-induced suppression of pituitary-testicular function in rats is reversed by pulsatile gonadotropin-releasing hormone substitution. Biol Reprod. 1991;44:413–419. doi: 10.1095/biolreprod44.3.413. [DOI] [PubMed] [Google Scholar]
- 35.Cameron JL, Nosbisch C. Suppression of pulsatile luteinizing hormone and testosterone secretion during short term food restriction in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 1991;128:1532–1540. doi: 10.1210/endo-128-3-1532. [DOI] [PubMed] [Google Scholar]
- 36.Aloi JA, Bergendahl M, Iranmanesh A, Veldhuis JD. Pulsatile intravenous gonadotropin-releasing hormone administration averts fasting-induced hypogonadotropism and hypoandrogenemia in healthy, normal weight men. Journal of Clinical Endocrinology and Metabolism. 1997;82:1543–1548. doi: 10.1210/jcem.82.5.3947. [DOI] [PubMed] [Google Scholar]
- 37.Ebling FJ, Wood RI, Karsch FJ, Vannerson LA, Suttie JM, Bucholtz DC, Schall RE, Foster DL. Metabolic interfaces between growth and reproduction: III. Central mechanisms controlling pulsatile luteinizing hormone secretion in the nutritionally growth-limited female lamb. Endocrinology. 1990;126:2719–2727. doi: 10.1210/endo-126-5-2719. [DOI] [PubMed] [Google Scholar]
- 38.I’Anson H, Terry SK, Lehman MN, Foster DL. Regional differences in the distribution of gonadotropin-releasing hormone cells between rapidly growing and growth-restricted prepubertal female sheep. Endocrinology. 1997;138:230–236. doi: 10.1210/endo.138.1.4882. [DOI] [PubMed] [Google Scholar]
- 39.McShane TM, Petersen SL, McCrone S, Keisler DH. Influence of food restriction on neuropeptide-Y, proopiomelanocortin, and luteinizing hormone-releasing hormone gene expression in sheep hypothalami. Biol Reprod. 1993;49:831–839. doi: 10.1095/biolreprod49.4.831. [DOI] [PubMed] [Google Scholar]
- 40.Cameron JL. Regulation of reproductive hormone secretion in primates by short-term changes in nutrition. Reviews of Reproduction. 1996;1:117–126. doi: 10.1530/ror.0.0010117. [DOI] [PubMed] [Google Scholar]
- 41.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, Milligan SR, Lightman SL, O’Byrne KT. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21:20–29. doi: 10.1111/j.1365-2826.2008.01807.x. [DOI] [PubMed] [Google Scholar]
- 42.Iwasa T, Matsuzaki T, Murakami M, Shimizu F, Kuwahara A, Yasui T, Irahara M. Decreased expression of kisspeptin mediates acute immune/inflammatory stress-induced suppression of gonadotropin secretion in female rat. J Endocrinol Invest. 2008;31:656–659. doi: 10.1007/BF03345620. [DOI] [PubMed] [Google Scholar]
- 43.Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–147. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]