Abstract
The Raf/MEK/ERK cascade is a therapeutic target in human cancers with deregulated Ras signaling, which includes tumours that have inactivated the Nf1 tumour suppressor1. Nf1 encodes neurofibromin, a GTPase activating protein that terminates Ras signalling by stimulating hydrolysis of Ras•GTP. We compared the effects of inhibitors of MEK in a myeloproliferative disorder (MPD) initiated by inactivating Nf1 in mouse bone marrow and in acute myeloid leukaemias (AMLs) in which cooperating mutations were induced by retroviral insertional mutagenesis. Here we show that MEK inhibitors are ineffective in MPD, but induce objective regression of many Nf1-deficient AMLs. Drug resistance developed due to outgrowth of AML clones that were present before treatment. We cloned clone-specific retroviral integrations to identify candidate resistance genes including Rasgrp1, Rasgrp4, and Mapk14, which encodes p38α. Functional analysis implicated increased RasGRP1 levels and reduced p38 kinase activity in resistance to MEK inhibitors. This approach represents a robust strategy for identifying genes and pathways that modulate how primary cancer cells respond to targeted therapeutics and for probing mechanisms of de novo and acquired resistance.
Aberrant Ras signalling contributes to the pathogenesis of myeloid malignancies and can result from acquired RAS mutations or from alternative genetic mechanisms that include FLT3 internal tandem duplications, the BCR-ABL fusion, PTPN11 mutations, and NF1 inactivation (reviewed in2). Children with neurofibromatosis type 1 (NF1) have a 200–500 fold excess incidence of juvenile myelomonocytic leukaemia (JMML), an aggressive MPD characterized by leukocytosis, splenomegaly, and tissue infiltration (reviewed in3). The bone marrows of affected patients frequently show loss of the normal parental NF1 allele and elevated extracellular signal-related kinase (ERK) activity4. Biallelic inactivation of murine Nf1 causes a MPD in Mx1-Cre, Nf1flox/flox mice that closely models JMML5.
We injected the MOL4070LTR retrovirus6 into Mx1-Cre, Nf1flox/flox pups to identify genes and pathways that might cooperate with Nf1 inactivation to induce progression of MPD to AML2. These mice developed acute leukaemia sooner and at a higher rate than control Mx1-Cre-negative littermates that retain normal Nf1 function, (Fig. 1a). Nf1-deficient AMLs are highly aggressive (Figs. 1b, 1c); show variable expression of myeloid surface markers; are transplantable into sublethally irradiated recipients; and form cytokine-dependent blast colonies in methylcellulose cultures.
Figure 1. Retroviral mutagenesis induces AML in Mx1-Cre, Nf1flox/flox mice and alters response to MEK inhibition.
a, Mx1-Cre, Nf1flox/flox mice that were infected with MOL4070LTR (Nf1 + MOL; n=47) had markedly reduced survival compared to control littermates that received this virus (WT + MOL; n=49; p < 0.0001). Survival curves for Mx1-Cre, Nf1flox/flox (Nf1) and WT mice that were not injected with MOL4070LTR are also shown. b and c, Myeloblasts in the peripheral blood (b) and infiltrating into lung tissues (c) of Mx1-Cre, Nf1flox/flox mice with AML. d, Myeloid colony growth from the bone marrows of WT mice (closed circles, n=12) Mx1-Cre, Nf1flox/flox mice with MPD (closed triangles, n=6), and Mx1-Cre, Nf1flox/flox mice with AML (open squares, n=8) over a range of CI-1040 concentrations (log scale). Colony growth was assayed in the presence of a saturating concentration of GM-CSF. Error bars represent s.e.m.
The MEK inhibitor CI-10407 reduced the growth of myeloid progenitor colonies from the bone marrows of Mx1-Cre, Nf1flox/flox mice with MPD and WT controls to a similar extent (Fig. 1d). By contrast, blast colony growth from many Nf1-deficient AMLs was exquisitely sensitive with abrogation of colony growth at 10–100 fold lower drug concentrations (Fig. 1d). These in vitro data suggested that cooperating mutations render Nf1 mutant AMLs more dependent on Raf/ERK/MEK signalling. To pursue this question, we first determined the maximally tolerated dose (MTD) of CI-1040 to be 100 mg/kg twice daily in WT mice, collected bone marrow at defined time points after a single drug dose, and showed that CI-1040 treatment transiently reduced the ability of graunlocyte-macrophage colony stimulating factor (GM-CSF) to increase phosphorylated ERK (pERK) levels (Supplementary Fig. 1a). We then treated control or Mx1-Cre, Nf1flox/flox mice with MPD for 28 days (n=5 per group). Consistent with the in vitro data, CI-1040 had no beneficial therapeutic index in mice with MPD (Supplementary Figs. 1b, 1c, and data not shown). Biochemical analysis of bone marrow obtained 2–8 hours after the 56th and final dose of CI-1040 revealed reduced ERK phosphorylation that was similar to the responses of WT mice that received a single drug dose (Supplementary Figs. 1a, 1d).
To investigate the unexpected in vitro sensitivity of Nf1 mutant AMLs to CI-1040, we transplanted 4 independent leukaemias into 23 recipients. Mice with AML blasts in the peripheral blood were assigned to treatment with either vehicle (n = 11) or CI-1040 (n = 12) at the same dose and schedule that was ineffective in the MPD. CI-1040 treatment induced rapid and extensive reductions in blood leukocyte counts (Fig. 2a) with clearance of blasts and reappearance of normal neutrophils (data not shown). Survival was increased greater than three-fold (Fig. 2b). However, recipients of Mx1-Cre, Nf1flox/flox AMLs invariably died with recurrent leukaemia despite ongoing treatment (Fig. 2b). These relapsed AMLs had similar morphologic and immunophenotypic features as the parental leukaemias, but demonstrated in vitro resistance to MEK inhibitors and were refractory to treatment in secondary recipients (Fig. 2c and data not shown). Sensitive AMLs showed a greater reduction in 5’-bromodeoxyuridine incorporation following CI-1040 exposure than either WT or resistant leukaemia cells (Supplementary Fig. 2). We also exposed pairs of sensitive and resistant AMLs to CI-1040 in vitro to ask if resistance is associated with reactivation of MEK. Importantly, ERK phosphorylation in response to GM-CSF was inhibited at the same concentration of CI-1040 (Figs. 2d, 2e).
Figure 2. Response and resistance to CI-1040 in Mx1-Cre, Nf1flox/flox mice with AML.
a, Leukocyte counts were markedly decreased in mice treated with CI-1040 (n=13) compared to the vehicle (n=12). Error bars represent s.e.m. b, Survival was prolonged by approximately three-fold in mice that received CI-1040 (OR 3.1; CI 2.7–3.6; p < 0.0001). c, Myeloid colony growth from the bone marrows of mice with recurrent leukaemia are less sensitive to CI-1040 inhibition than the parental AMLs. Colony growth in methylcellulose is compared for WT bone marrow (closed squares, n=2), for primary AML cells harvested from vehicle-treated mice (open squares, n=4), and for AML cells obtained after relapse in recipients that were treated with CI-1040 (closed triangles, n=3). Error bars represent s.e.m. d and e, CI-1040 abrogates the ability of GM-CSF to stimulate ERK phosphorylation in both sensitive and resistant AMLs as assessed by Western blotting (d) or phospho-flow cytometry (e).
PD0325901 is a MEK inhibitor with more favourable pharmacologic characteristics than CI-10408. We defined a MTD of 12.5 mg/kg/day for PD0325901, and found that doses > 5 mg/kg/day reduced ERK activation in response to GM-CSF for 24 hours (Supplementary Fig. 3a). To ask if prolonging the duration of MEK inhibition affects drug response and resistance in vivo, we administered PD0325901 to mice that were transplanted with two AMLs from the CI-1040 trial (AMLs #6554 and #6537). Recipients that received PD0325901 demonstrated the same pattern of response, relapse, and overall survival (Supplementary Fig. 3b). We also tested two other Nf1-deficient AMLs for response to PD0325901. AML #7723 was less sensitive to MEK inhibition in methylcellulose cultures and administering PD0325901 to recipient mice neither prolonged survival nor changed the IC50 for blast colony formation (Supplementary Fig. 4). AML #7710 showed an in vitro response to PD0325901 that was similar to sensitive leukaemias; however, in vivo treatment did not prolong survival or select for clones with altered drug responsiveness (Supplementary Fig. 4). Together, these studies define a heterogeneous response of Nf1-deficient AMLs to MEK inhibition.
Digesting DNA from AML #6554 with restriction enzymes and Southern blot analysis with a MOL4070LTR-specific probe revealed clonal evolution of the resistant leukemia (Figs. 3a). To identify candidate genes that might influence response to CI-1040, we exploited a shotgun cloning strategy9 to characterize MOL4070LTR integrations. Sensitive parental AML #6554 and resistant leukaemias from CI-1040 and PD0325901-treated recipient mice shared some common integrations as well as unique insertions (Fig. 3a and Supplementary Table 1). Importantly, resistant leukaemias from independent recipients that were treated with CI-1040 or PD0325901 invariably showed the same integration patterns (Fig. 3a and Supplementary Table 1). These data and the rapid emergence of resistance infer that resistant subclones that are present within the initial AML are selected in vivo during MEK inhibitor treatment.
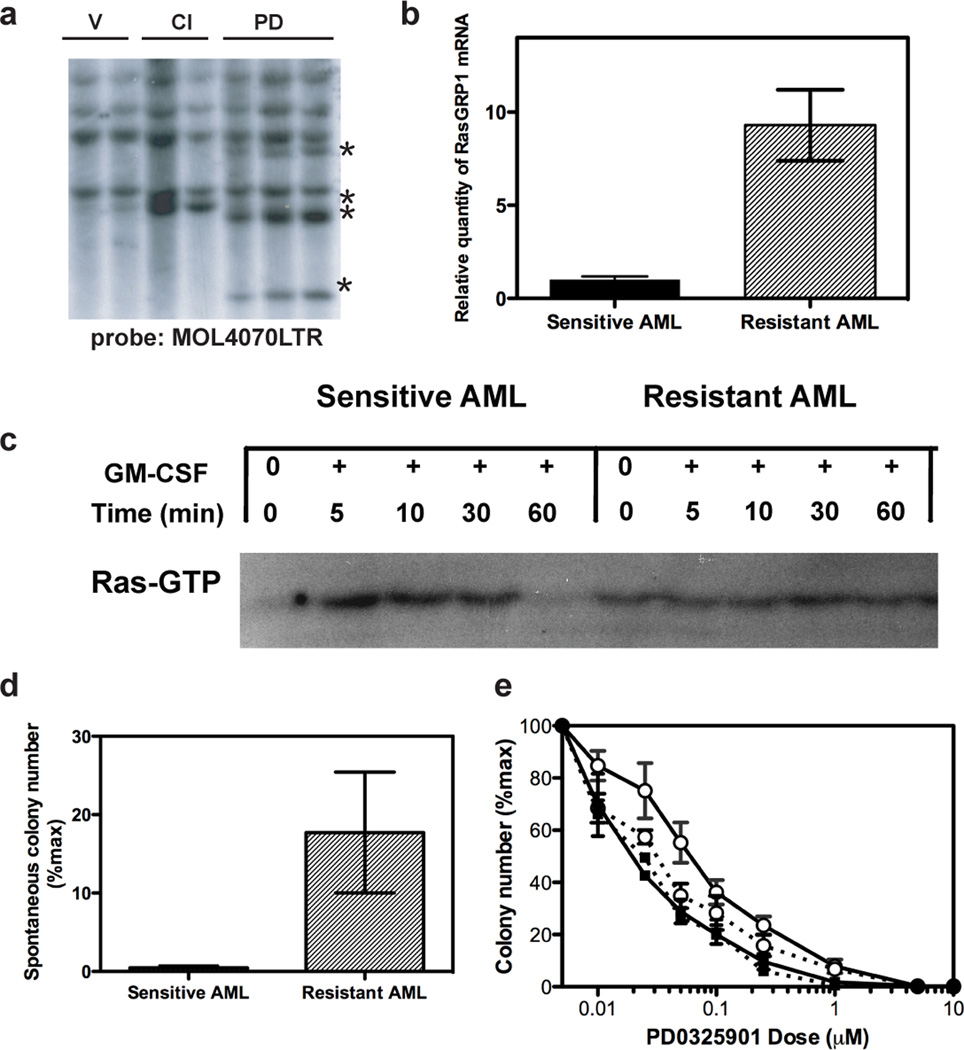
Figure 3. Genetic and functional analysis implicate Rasgrp1 overexpression as a resistance mechanism in AML #6554.
a, Retroviral integrations in DNA samples from AML #6554 in independent mice following treatment with vehicle (V), CI-1040 (CI) or PD0325901 (PD). Asterisks denote restriction fragments that are present in resistant AMLs, but are not seen in the sensitive parental leukaemia. b, Quantitative PCR analysis of Rasgrp1 expression in sensitive and resistant AML #6554 (n=3 in each group, mean with SD, p < 0.002 student’s t-test). c, Ras-GTP levels in AML #6554 show constitutive activation in the resistant leukemia. d, Resistant AML #6554 demonstrates substantial cytokine-independent blast colony growth (n=5), which is not seen in the sensitive leukaemia (n=6) (p=0.0350, t-test). e, Resistant AML #6554 that is infected with a RasGRP1 shRNA (open circles dotted line, n=7) demonstrates an IC50=0.03 µM (95% CI 0.02–0.03 µM), which is significantly lower than the IC50 of resistant cells infected with the control vector (IC50=0.06 µM, 95% CI 0.05–0.07 µM; open circles, solid, n=7). The IC50 of parental sensitive AML infected with shRNA vector to RasGRP1 (IC50=0.02 µM, 95% CI 0.017–0.024 µM; closed squares, dotted line, n=3) or control vector (IC50=0.02 µM, 95% CI 0.017–0.028 µM; closed squares, solid line n=3) are not statistically significantly different than Resistant AML #6554 infected with RasGRP1 shRNA. Error bars represent s.e.m. in d and e.
The patterns of MOL4070LTR integrations in sensitive and resistant AML #6554 suggested that Rasgrp genes might contribute to drug resistance (Supplementary Table 1). We developed quantitative real time PCR (RT-PCR) assays to correlate Rasgrp1 insertion copy number and RNA expression in AML #6554 with the integration data shown in Supplementary Table 1. Whereas analysis of sensitive AML #6554 from 8 independent recipients revealed only low levels of a unique integration junction fragment, four independent PD0325901 –resistant leukaemias demonstrated 1,000 fold (range 500–1,500 fold) increases in DNA copy number. Similarly, Rasgrp1 messenger RNA levels were increased an average of 10 fold (range 6.5–19 fold) in resistant versus sensitive leukaemias (Fig. 3b). RasGRP proteins stimulate guanine nucleotide exchange on Ras, which increases Ras•GTP levels (reviewed in10). We reasoned that increased Rasgrp1 expression might result in elevated Ras•GTP levels in resistant AML #6554. Indeed, whereas basal Ras•GTP levels were low in the parental leukaemia and returned to baseline 60 minutes after exposure to GM-CSF, we observed constitutive Ras activation in the resistant AML (Fig. 3c). Resistant AML #6554 also formed cytokine-independent blast colonies in methylcellulose (Fig 3d). This observation is consistent with biochemical and computational analyses in lymphocytes, which predict that Ras signalling becomes independent of receptor input when RasGRP levels are elevated11. To further assess the functional importance of Rasgrp1 over-expression in resistant AML #6554, we infected the sensitive and resistant leukaemias with a lentiviral vector encoding both a short hairpin RNA that efficiently reduces RasGRP1 protein (Supplementary Fig. 5) and a green fluorescent protein (GFP) marker. GFP-positive cells were isolated by sorting and plated in methylcellulose medium containing GM-CSF and a range of PD0325901 concentrations. Neither the Rasgrp1 virus nor a control virus altered the sensitivity of parental AML blast colony forming cells to the MEK inhibitor (Fig. 3e). By contrast, infecting resistant AML #6554 with the Rasgrp1 shRNA lentivirus restored the sensitivity of these cells to PD0325901, with a shift of the IC50 into the same range as in the sensitive parental AML (Fig. 3e).
Multiple recipients that were transplanted with AML #6537 relapsed with the same resistant clone after treatment with either CI-1040 or PD0325901 (Fig. 4a). This resistant leukaemia showed a distinct retroviral integration pattern, including one within Mapk14, which encodes p38α (Supplementary Table 2). RT-PCR of proviral/host DNA junctions confirmed that the inserted allele copy number is increased 1,000 fold (range 1,000–10,000 fold) in the resistant AML. The proviral insertion is in the anti-sense orientation and Southern blot analysis supports inactivation of one Mapk14 allele (Fig. 4a, 4b). Consistent with this prediction, basal p38 kinase activity is reduced in resistant AML #6537 (Fig. 4c). To further investigate if decreased p38α activity modulates resistance to MEK inhibitors in primary leukaemia cells, we enumerated blast colonies from AML #6537 in the presence of either CI-1040, SB202190 (a specific inhibitor of p38α)12 or both drugs. SB202190 strongly antagonized the inhibitory effects of CI-1040 on the sensitive leukaemia, but had minimal effects on the resistant AML (Fig. 4d).
Figure 4. Genetic and functional analysis of AML #6537 associate reduced p38α kinase activity with resistance to MEK inhibition.
a, Southern blot analysis of MOL4070LTR integrations of AML #6537 following treatment with vehicle (V), CI-1040 (CI) or PD0325901 (PD). Asterisks denote restriction fragments that are present in all the resistant AMLs, but are not seen in the sensitive leukaemia. b, Southern blot analysis of paired sensitive and resistant AML #6537 with a Mapk14 probe. The resistant leukaemias shown in lanes CI and PD show a Mapk14 hybridization fragment, which overlaps with one of the MOL4070LTR bands in the resistant leukaemias shown in panel a. c, Basal p38 kinase activity of resistant AML #6537 cells (solid line) is reduced in comparison to the parental leukaemia (heavy dashed line), but remains elevated above WT bone marrow cells (dotted line). This is a representative example of 3 independent experiments. d, SB202190 (SB), a p38α inhibitor, antagonizes the ability of CI-1040 (CI) to reduce blast colony inhibition in parental AML #6537 (solid white bars, n=7) in the presence of GM-CSF (GM). Blast colony growth of sensitive AML #6537 is significantly increased by the addition of SB to CI-1040 (*p= 0.0043; unpaired t-test). By contrast, CI-1040 (2.5 µM) has a inhibitory effect on WT CFU-GM colony (solid black bars, n=8) and resistant AML #6537 (chequered bars, n=5) blast colony growth, which is not affected by 2.5 µM SB202190. Error bars represent the s.e.m.
Cooperating mutations that induce progression of MPD to AML render these more aggressive leukaemias highly dependent upon MEK for proliferation and survival. The transient remisisons induced by imatinib in advanced (“blast crisis”) CML13 are remarkably similar to the responses of Mx1-Cre, Nf1flox/flox AMLs to MEK inhibition. Relapse of blast crisis CML and of Nf1 mutant murine leukaemias is due to outgrowth of a minor population of drug-resistant cells that is present before treatment with targeted agents14. Similarly, “backtracking” experiments in acute lymphoblastic leukaemias (ALL) showed that the dominant clone at relapse is frequently detectable before treatment15. Relapsed ALL clones differ from the predominant clone at diagnosis by a few genetic changes16. Diverse mutations of genes involved in lymphoid developmental programs, cell cycle control, and DNA damage responses are enriched in drug resistant clones, while alterations in drug import, export, or metabolism are relatively uncommon16. Understanding how preexisting drug-resistant clones underlie cancer relapse has fundamental implications for developing better therapeutic strategies.
As MEK is one component of a complex network of Ras effectors, it is possible to envision multiple mechanisms of acquired resistance. Our data support this idea. A provocative implication of our studies of AML #6554 showing that Rasgrp1 over-expression modulates the response to MEK inhibitors is that globally increasing Ras•GTP levels can overcome the effects of inhibiting a major Ras effector in some cancers. We implicated reduced p38 kinase activity as an alternative mechanism of MEK resistance in AML #6537. Interestingly, cancer cell lines that undergo apoptosis in response to oncoprotein inhibition show a rapid decrease in pERK followed by an increase in phosphorylated p38 levels17. As Nf1-deficient AMLs have elevated basal p38 kinase activity, our studies suggest that inhibiting oncogenic Raf/MEK/ERK signalling results in unopposed p38 kinase activity, which contributes to cell death. This general idea is consistent with the observation that p38 inhibitors induce imatinib resistance in cultured CML cells17,18. The AMLs that we have characterized to date showed diverse retroviral integrations (Supplementary Tables 1–3), and comprehensive genetic and preclinical analyses are required to uncover the full spectrum of mutations associated with drug resistance. Despite this limitation, a broad implication of our data is that cell lineage, the nature of cooperating mutations, and the order in which they are acquired in specific cancers will modulate the efficacy of targeted inhibitors of Ras effector pathways. Insertional mutagenesis in genetically accurate mouse cancer models is a powerful tool for dissecting the molecular basis of cellular responses to oncogenic stress and for uncovering genes that contribute to drug sensitivity and resistance.
Methods Summary
Inhibitors
CI-1040 and PD0325901 (Pfizer, Ann Arbor, MI) were administered as described in Supplementary Methods. SB202190 (Calbiochem) was diluted in 100% DMSO.
Mouse Strains and RIM
Mice All procedures involving mice were approved by the UCSF Committee on Animal Research. MOL4070LTR stocks were prepared as described6. Mice received a single intraperitoneal injection containing 105 viral particles in 100 µL admixed with 500 µg of pI-pC (to activate Mx1-Cre expression) between days 3 and 5 of life. Mice with leukemia were killed and bone marrow was cryopreserved. Leukaemias were classified based on established morphologic and flow cytometric criteria19.
Adoptive Transfer and Treatment
Cryopreserved AMLs were injected intravenously (106 cells/mouse) into 6–8 week old recipient mice that received a single radiation dose of 450 cGy. Recipients with leukaemic cells in the peripheral blood were randomly assigned to receive either CI-1040/PD0325901 or control vehicle. Mice were weighed weekly to adjust the drug dose, observed daily, and killed when they became moribund or on the 28th day of treatment.
Myeloid Progenitor Growth and Pharmacodynamic Studies
CFU-GM and blast colonies were grown in methylcellulose medium M3231 (Stem Cell Technologies) and scored by indirect microscopy. MEK inhibition was assessed by stimulating bone marrow cells with GM-CSF and then measuring pERK levels by Western blotting ( also see Supplementary Methods).
Retroviral Integrations and Quantitative RT-PCR
Junction fragments corresponding to retroviral integrations were identified as described20. PCR primers were designed to detect integration junctions21 (also see Supplementary Methods).
p38 Kinase Assay
Kinase activity of mice was quantified in 100 µg lysates in with the Omnia Plate IP kit (BioSource) in which activation of a MAPKAPK2 evokes a change in fluorescent properties due to chelation-enhanced flurophore. A fluorescent plate reader in real time kinetic mode quantified p38 kinase activities.
Supplementary Material
Acknowledgements
We are grateful to B. Braun, J. Downing, S. Lowe, and C. Sawyers for discussion and advice throughout the project. We are thankful for Catherine Hartzell studies on RasGRP protein expression. This work was supported by NIH grants U01 CA84221, R37 CA72614, T32 CA09043, T32 HD044331 and K08 CA119105, by a Specialized Center of Research award from the Leukemia and Lymphoma Society (LLS 7019-04), by the U.S. Army Neurofibromatosis Research Program (Project DAMD 17-02-1-0638), by the Ronald McDonald House Charities of Southern California/Couples Against Leukemia, by the Jeffrey and Karen Peterson Family Foundation, and by the Frank A. Campini Foundation. S.C.K. is a Scholar of the Leukemia and Lymphoma Society of America. J.P.R. is a Kimmel Foundation Scholar. The Intramural Research Program of the National Cancer Institute’s Center for Cancer Research supports research in the laboratory of L.W. at the National Institutes of Health.
Footnotes
Author Contributions J.O.L and K.M.S. designed and performed experiments, analyzed data and wrote the paper. D.K, D.T.L., M.C., K.K., K.W., and J.M.B designed experiments, conducted studies, analyzed data and provided input to manuscript. K.A. performed bioinformatics analysis of retroviral insertion sequences. Q.L, K.M.C., E. D-F. M.G., M.T. designed and conducted experiments. J.P.R. provided critical reagents, assisted in experimental design and edited the manuscript. N.C., N.J., and L.W. provided retroviral reagents and training in experimental protocols. L.P. provided the mouse strain and input into the manuscript. J. S-L. and S.P. developed protocols for suspending and administering the MEK inhibitor and provided the drug used in all studies.
References
- 1.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]; Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer. 2007;7(4):295. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- 2.Van Etten RA, Shannon KM. Focus on myeloproliferative diseases and myelodysplastic syndromes. Cancer Cell. 2004;6(6):547. doi: 10.1016/j.ccr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Lauchle JO, Braun BS, Loh ML, Shannon K. Inherited predispositions and hyperactive Ras in myeloid leukemogenesis. Pediatr Blood Cancer. 2006;46(5):579. doi: 10.1002/pbc.20644. [DOI] [PubMed] [Google Scholar]
- 4.Shannon KM, et al. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N Engl J Med. 1994;330:597. doi: 10.1056/NEJM199403033300903. [DOI] [PubMed] [Google Scholar]; Bollag G, et al. Loss of NF1 results in activation of the Ras signaling pathway and leads to aberrant growth in murine and human hematopoietic cells. Nat Genet. 1996;12:144. doi: 10.1038/ng0296-144. [DOI] [PubMed] [Google Scholar]; Side L, et al. Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N Engl J Med. 1997;336(24):1713. doi: 10.1056/NEJM199706123362404. [DOI] [PubMed] [Google Scholar]
- 5.Le DT, et al. Somatic inactivation of Nf1 in hematopoietic cells results in a progressive myeloproliferative disorder. Blood. 2004;103(11):4243. doi: 10.1182/blood-2003-08-2650. [DOI] [PubMed] [Google Scholar]
- 6.Wolff L, Koller R, Hu X, Anver MR. A Moloney murine leukemia virus-based retrovirus with 4070A long terminal repeat sequences induces a high incidence of myeloid as well as lymphoid neoplasms. Journal of virology. 2003;77(8):4965. doi: 10.1128/JVI.77.8.4965-4971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sebolt-Leopold JS, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo [see comments] Nat Med. 1999;5(7):810. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 8.Brown AP, Carlson TC, Loi CM, Graziano MJ. Pharmacodynamic and toxicokinetic evaluation of the novel MEK inhibitor, PD0325901, in the rat following oral and intravenous administration. Cancer Chemother Pharmacol. 2007;59(5):671. doi: 10.1007/s00280-006-0323-5. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy AJ, et al. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436(7048):221. doi: 10.1038/nature03691. [DOI] [PubMed] [Google Scholar]
- 10.Stone JC. Regulation of Ras in lymphocytes: get a GRP. Biochem Soc Trans. 2006;34(Pt 5):858. doi: 10.1042/BST0340858. [DOI] [PubMed] [Google Scholar]
- 11.Das J, et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136(2):337. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain J, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408(3):297. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fabian MA, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23(3):329. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 13.Druker BJ, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 15.Choi S, et al. Relapse in children with acute lymphoblastic leukemia involving selection of a preexisting drug-resistant subclone. Blood. 2007;110(2):632. doi: 10.1182/blood-2007-01-067785. [DOI] [PubMed] [Google Scholar]
- 16.Mullighan CG, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma SV, et al. A common signaling cascade may underlie "addiction" to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10(5):425. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar S, et al. Role of the p38 mitogen-activated protein kinase pathway in the generation of the effects of imatinib mesylate (STI571) in BCR-ABL-expressing cells. J Biol Chem. 2004;279(24):25345. doi: 10.1074/jbc.M400590200. [DOI] [PubMed] [Google Scholar]
- 19.Kogan SC, et al. Bethesda proposals for classification of nonlymphoid hematopoietic neoplasms in mice. Blood. 2002;100(1):238. doi: 10.1182/blood.v100.1.238. [DOI] [PubMed] [Google Scholar]
- 20.Akagi K, et al. RTCGD: retroviral tagged cancer gene database. Nucleic acids research. 2004;32(Database issue):D523. doi: 10.1093/nar/gkh013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtiss NP, et al. Isolation and analysis of candidate myeloid tumor suppressor genes from a commonly deleted segment of 7q22. Genomics. 2005;85(5):600. doi: 10.1016/j.ygeno.2005.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.