Abstract
Although the contribution of Hedgehog (Hh) signalling to stem cell development and oncogenesis is well-recognised, its importance for spermatogonial stem cells (SSCs) has not been established. Here we interrogate adult rat SSCs using an established model in which only undifferentiated spermatogonial cells remain in the testis at 15 weeks following irradiation, and spermatogonial differentiation is induced within 4 weeks by gonadotrophin releasing hormone antagonist (GnRH-ant) administration. Synthesis of Hh pathway components in untreated adult rat testes was compared with that in irradiated testes prior to and after GnRH-ant exposure using in situ hybridization. In adult testes with complete spermatogenesis, the Desert hedgehog ligand transcript, Dhh, was detected in Sertoli cells, some spermatogonia and in spermatocytes by in situ hybridization. Spermatogenic cells were identified as sites of Hh signalling through detection of transcripts encoding the Hh receptor, Ptc2, transcripts and proteins for the key downstream target of Hh signalling, Gli1, and the Hh transcriptional activator, Gli2. Remarkably, the undifferentiated spermatogonia present in irradiated adult rat testes contained Dhh in addition to Ptc2, Gli1 and Gli2, revealing the potential for an autocrine Hh signalling loop to sustain undifferentiated spermatogonial cells. These transcripts became undetectable by in situ hybridization following GnRH-ant induction of spermatogonial differentiation, however detection of Gli1 protein in spermatogonia in all groups indicates that Hh signalling is sustained. This is the first evidence of active Hh signalling in mammalian male germline stem cells, as has been documented for some cancer stem cells.
Keywords: spermatogonial stem cells, testicular cancer, adult stem cells
Introduction
The Hedgehog (Hh) protein was originally discovered in Drosophila due to its role in segment polarity (Nusslein-Volhard and Wieschaus 1980). Genes encoding three vertebrate Hh ligands were first described in the early 1990s and named Sonic Hh (encoded by Shh), Indian Hh (Ihh), and Desert Hh (Dhh). Hh proteins are now recognized to be key regulators of cell proliferation, differentiation and cell-cell communication, and as morphogens they have important roles in embryogenesis, stem cell maintenance and oncogenesis. Hh signalling in mammals has been most widely studied in the context of specific cancers (Boonen et al 2005, Couve-Privat et al 2004, Dahmane et al 1997, Grachtchouk et al 2000, Kinzler et al 1987, Nilsson et al 2000, Raffel et al 1997, Taylor et al 2002), and its critical role in the maintenance of cancer stem cells has emerged from analysis of small cell lung cancer (Watkins et al 2003), gastric and upper gastrointestinal tract cancer (Berman et al 2003), and cancers of the pancreas (Thayer et al 2003), prostate (Karhadkar et al 2004, Sanchez et al 2004), skin (Adolphe et al 2004) and ovary (Chen et al 2007). The capacity of Hh ligands to affect particular aspects of testis growth and spermatogenesis has been amply demonstrated (Bitgood et al 1996, Szczepny et al 2006, Szczepny et al 2009), and the present study investigates for the first time the potential for Hh signalling to influence spermatogonial stem cells (SSCs) in the mammalian testis.
The transcript encoding the sole murine testicular ligand, Dhh, was shown previously to be produced only in Sertoli cells from embryonic day (E) 11.5 (Bitgood and McMahon 1995) through to adulthood (Szczepny et al 2006). Dhh appears to be essential for normal differentiation of Leydig, myoid and endothelial cells in the fetal testis (Barsoum and Yao 2011, Buehr et al 1993, Martineau et al 1997), governing basal lamina deposition and cord formation (Franco and Yao 2012). Spermatogenesis is severely reduced in Dhh−/− testes after birth, with spermatogonia and spermatocytes being rare. This may be a consequence of early functions of Dhh in germ cell proliferation or instead arise from Leydig cell deficiency which indirectly impairs spermatogenesis (Kawai et al 2011).
Several lines of evidence indicate that the Hh pathway functions within germ cells in the postnatal testis. Localization of glioma-associated oncogene homologue (Gli) transcription factor-1 (Gli1) in spermatogonia, spermatocytes and spermatids indicates these cells have active Hh signalling (Kroft et al 2001, Makela et al 2011), due to the fact that Gli1 is a direct target of pathway activity (Scales and de Sauvage 2009). Transcripts and/or proteins encoding the transmembrane receptors Patched (Ptc)-1, Ptc2 and Smoothened (Smo), and the positive pathway regulator, Fused (Fu), are present in both mitotic and meiotic murine germ cells (Morales et al 2009, Szczepny et al 2006), demonstrating these cells have the machinery required for Hh ligand responsiveness. It appears that Hh signalling is regulated during the latter stages of spermatogenesis, as the transcript encoding Suppressor of Fused (SuFu), a repressor of pathway activity, is abundant in round spermatids and the protein readily detected only in elongating spermatids. The observation that ectopic Gli1 synthesis in spermatocytes caused meiotic arrest (Kroft et al 2001) provides additional evidence that Hh pathway activity must be regulated for normal fertility. More recently, short term culture experiments with Hh signalling inhibitors have demonstrated that Hh pathway activity affects mitotic and meiotic germ cells as well as Sertoli cells (Makela et al 2011, Szczepny et al 2009).
Spermatogonial stem cells and their undifferentiated progeny are functionally unique cells which sustain fertility throughout adulthood because they retain the potential for self-renewal along with the capacity to differentiate into spermatozoa (Nakagawa et al 2010). While existing evidence implicates Hh signalling in several stages of germ cell maturation, its potential to affect adult SSCs has not been addressed. An important model for examining SSCs is the adult irradiated rat testis, in which all differentiating germ cells are lost except for spermatogonia which have the capacity to repopulate the testis. In this model, spermatogenic recovery can be reliably stimulated within 4 weeks after irradiation treatment by suppression of testosterone using GnRH antagonists (GnRH-ant) (Shuttlesworth et al 2000). We provide here new information that compares the cellular sites of Hh signalling activity in the adult rat testis with what is observed during the maintenance of adult SSCs following testicular damage and in the initial stages of spermatogenic recovery. These findings reveal a previously cryptic role for Hh signalling activity that may function during normal spermatogenesis as well as during conditions of damage in which maintenance of SSCs is essential to an individual’s future fertility.
Materials and Methods
Ethics Statement
Use of Sprague Dawley adult male rats was approved by the Monash University Standing Committee on Ethics in Animal Experimentation. All procedures for adult LBNF1 male rat experiments were approved by the M.D. Anderson Cancer Institutional Animal Care and Use Committee. All rats were humanely killed at a designated establishment by inhalation of CO2 followed by cervical dislocation.
Animals
Sprague Dawley adult male rats were obtained from Monash University Central Animal Service and killed by cervical dislocation before collection of testes for routine histological processing and RNA analyses (as in (Szczepny et al 2006)). For studies on irradiated testes, adult LBNF1 male rats (hybrids between Lewis and Brown–Norway) were purchased from Harlan Sprague–Dawley, Inc. (Indianapolis, IN, USA). Animals were allowed to acclimatize for 1 week before initiation of the experiment. Rat testes were collected for routine histological approaches. All histological specimens were preserved in Bouin’s fixative as described in previous studies (Shuttlesworth et al 2000). These samples have been previously studied by us to describe the expression profile of c-kit in the rat testis (Prabhu et al, 2006).
Irradiation and hormone treatment
Animals were anaesthetized with 0.72 mg ketamine/kg and 0.022 mg acepromazine/kg (i.m.), and then a single 6-Gy dose was delivered to the lower part of the abdomen by a Co γ-ray unit (Eldorado 8; Atomic Energy of Canada, Ltd, Ottawa, Canada) (Shuttlesworth et al 2000). GnRH-ant treatment was performed as previously (Shuttlesworth et al 2000) in which 15 weeks after irradiation, animals were given simultaneous injections of 1.5 mg Cetrorelix pamoate and 1.5 mg Cetrorelix acetate (ASTA Medica, AG Frankfurt, Germany), each at a different site in the upper portion of the dorsal region. A second Cetrorelix pamoate injection was given 3.3 weeks after the first. Hormone doses were based on results in Sprague Dawley rats, in which Cetrorelix pamoate at 1.5 mg/animal produced uniform suppression of testosterone levels for 27 days (Shuttlesworth et al 2000). Animals were killed and testes collected at specific times between 3 days and 4 weeks following the start of GnRH-ant treatment. At least three animals were used for each time point.
In situ Hybridization
The cDNAs corresponding to target genes were previously generated, validated by sequencing and Northern blot analysis, and used as described (Szczepny et al 2006) to examine the Hh pathway in mouse samples. The high sequence similarity between rat and mouse sequences corresponding to the probe (98%) enabled us to use these new constructs with confidence that they would work on rat samples. Briefly, sense and antisense DIG-labelled cRNA probes were synthesized by in vitro transcription from plasmid cDNA with digoxigenin-UTP (Roche Diagnostics) using T7 and SP6 RNA polymerases (Promega) and quantified by dot blot according to the DIG-UTP manufacturer’s instructions. Sections were treated with Proteinase K (0–5 ng/ml) for 30 min at 37°C. Hybridization was performed with 100–300 ng/μl of each DIG-cRNA probe at 50°C overnight. Both antisense and sense (control) probes were used in each experiment on duplicate samples under identical conditions. Sections were developed with NBT-BCIP (Pierce, Rockford, IL), counterstained with Harris Hematoxylin and mounted with GVA mounting solution (Zymed, San Francisco, CA) under glass coverslips. Each experiment was repeated at least three times and performed on at least three independent samples, with normal adult testis specimens used as positive controls.
Immunohistochemistry
Immunohistochemistry with the anti-SuFu antibody (goat polyclonal, sc-10933, Santa Cruz Biotechnology, Santa Cruz, CA), anti-DDX4/MVH antibody (rabbit polyclonal, ab13840 Abcam, UK), anti-Gli1 antibody (rabbit polyclonal sc20687, Santa Cruz Biotechnology), anti-Gli2 antibody (rabbit polyclonal, ab7195, Abcam) and anti-PLZF antibody (rabbit polyclonal, sc-22839, Santa Cruz) was performed as previously (Szczepny et al 2006). Briefly, antigen retrieval was performed in 50 mM glycine (pH 3.5; ≥90°C maintained for 8 min) and the primary antibodies were applied at 1.0–2.0 μg/ml for overnight incubation in 0.1% bovine serum albumin (BSA)/TBS. Negative control sections were incubated with 0.1% BSA/TBS lacking primary antibodies. Subsequent steps were performed at room temperature, with TBS washes (3 × 5 min) between incubations. Primary antibody binding was detected using a biotinylated rabbit anti-goat antibody for anti-SuFu (DAKO, 1:500 dilution, 1 hr) and a biotinylated goat anti-rabbit antibody for anti-DDX4/MVH, anti-Gli1, anti-Gli2 and anti-PLZF antibodies (Vector Laboratories, Burlingame, CA, 1:500, 1 hr) then with the Vectastain Elite ABC kit according to the manufacturer’s instructions (Vector Laboratories). Antibody binding was detected as a brown precipitate following development with 3,3′-diaminobenzidine tetrahydrochloride, with Harris Hematoxylin used as counterstain. Sections were mounted under glass coverslips in Depex (BDH Laboratories, Poole, UK). At least three independent samples were examined.
Results
Cellular expression of Hedgehog components in adult rat testes
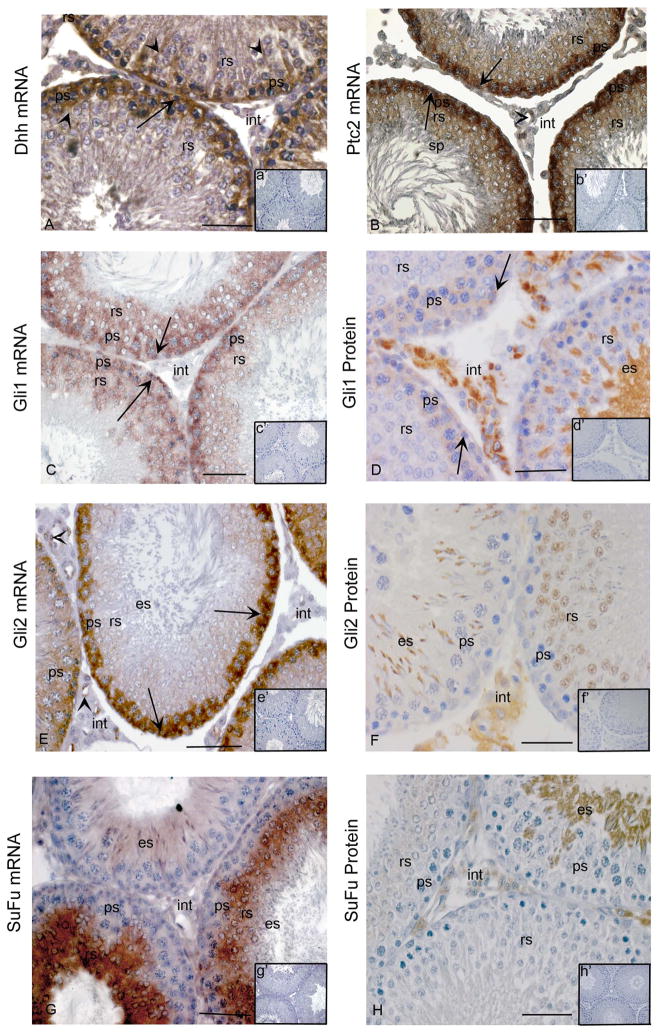
Previous microarray data have indicated that, amongst the 3 mammalian Hedgehog ligands, Dhh is the only one produced in the postnatal testis (Small et al 2005, Szczepny et al 2006, Zhou et al 2010). In situ hybridization analysis of adult rat testis showed the mRNA encoding Dhh is detectable in the Sertoli cell cytoplasm and in some spermatogonia and spermatocytes, while transcripts encoding signalling machinery were detected in multiple germ cell types, in agreement with previous studies (Fig. 1A). Ptc2, Gli1 and Gli2 transcripts were readily detected in spermatogonia and spermatocytes. Much less intense signals for each of these were evident in round spermatids, particularly for Gli2 (Fig. 1B, C, E). Reagents suitable for localization of Gli proteins have recently become available, and we applied these for the first time to delineate the cellular expression profile of Gli1 and Gli2 proteins in the adult rat testis (Fig. 1D, F). The detection of the Gli1 protein, in spermatogonia, spermatocytes, elongating spermatids and many interstitial cells provides an indication that these cells have Hh signalling activity, as Gli1 is a direct target that is strongly upregulated by Hh signalling. Localization of Gli2 protein to the nucleus of round spermatids and in the cytoplasm of elongating spermatids and some interstitial cells identifies these as cells with active Hh signalling; this transcription factor is typically active as a transcriptional activator when present in its full length isoform. The strong SuFu signal observed in round spermatids and detection of SuFu protein in round and elongating spermatids (Fig. 1G, H) matches what has been reported in adult mouse testis (Szczepny et al 2006) and proposed to indicate that this is upregulated to suppress Hh activity in the latest stages of spermatogenic differentiation. Detection of the Ptc2, Gli1, Gli2 mRNAs in endothelial cells of the adult rat testis reveals these as an additional site of Hh function.
Figure 1. Cellular localization of Hedgehog signalling components in adult rat testes.
In situ hybridization with anti-sense cRNAs (A–C, E, G) and immunohistochemistry (D, F, H) were used to detect mRNAs and proteins within cells of the adult testis. Insets in each panel indicate sense controls (a′-c′,e′,g′) and controls lacking primary antibody (D, F, H). (A) Dhh mRNA in Sertoli cells (arrow heads), spermatogonia (arrow) and spermatocytes (ps) (A, anti-sense; a′ [inset], sense control). (B) Ptc2 mRNA in spermatogonia (arrows), pachytene spermatocytes (ps) and round spermatids (rs) (B, anti-sense; b′, sense). (C) Gli1 mRNA in spermatogonia (arrows), pachytene spermatocytes (ps) and round spermatids (rs) (C, anti-sense; c′ [inset], sense control). (D) Gli1 protein in spermatogonia (arrows), spermatocytes (ps), elongating spermatids (es) and some interstitial cells (int) (D, positive staining; d′ [inset], negative control). (E) Gli2 mRNA in spermatogonia (arrows) and pachytene spermatocytes (ps), endothelial cells (arrow heads) (E, anti-sense; e′ [inset], sense control). (F), Gli2 protein in round spermatids (rs), elongating spermatids (es) and interstitial cells (int) (F, positive staining; f′ [inset], negative control) (G) SuFu mRNA in round spermatids (rs) and elongating spermatids (es) (G, anti-sense; g′ [inset], sense control). (H) SuFu protein in round spermatids (rs), elongating spermatids (es) and interstitial cells (int) (H, positive staining; h′ [inset], negative control). Bars indicate 50 μm. All analyses were conducted on at least three independent specimens and yielded consistent results, with representative images shown. Positive signals are brown; all sections have blue counterstain from Hemotoxylin.
Cellular expression of Hedgehog pathway components in irradiated rat testes
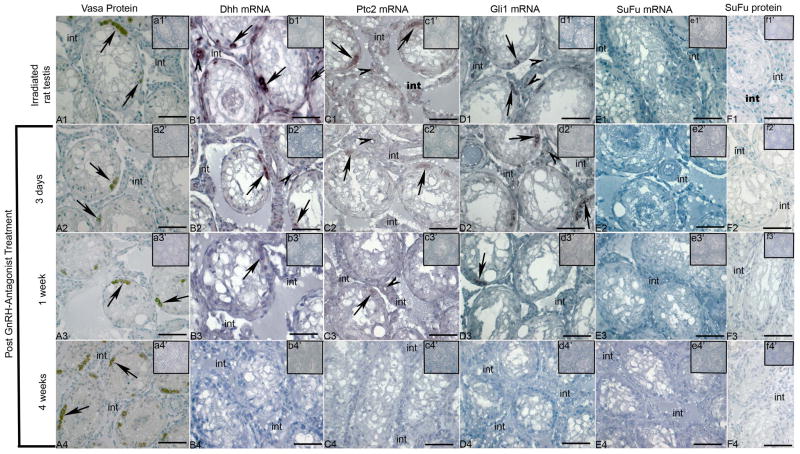
An antibody to DDX4/MVH was used to mark the germ cells in all groups of irradiated rat testis samples (Fig. 2A1–A4; Vasa Protein). Although the mRNA encoding Dhh is present in Sertoli cells of normal adult rat testis, it was detected in undifferentiated spermatogonia, in peritubular myoid cells, in endothelial cells and in some interstitial cells of irradiated rat testis samples by in situ hybridization. This Dhh signal was observed in samples from rats prior to GnRH-ant treatment (Fig. 2B1) and at 3 days after GnRH-ant treatment (Fig. 2B2). A faint signal was also detected in samples collected 1 week after treatment (Fig. 2B3), but this was absent at 4 weeks after treatment, when differentiation of some spermatogonia is apparent (Fig. 2B4).
Figure 2. Cellular expression of Hedgehog signalling components in irradiated adult rat testes.
Immunohistochemistry (A1-A4, F1-F4) and in situ hybridization with anti-sense cRNAs (B1-B4, C1-C4, D1-D4, E1-E4) were used to detect proteins and mRNAs, respectively, within cells of the irradiated adult rat testis. Insets in each panel illustrate controls lacking primary antibody (a1′-a4′, f1′-f4′) and sense probe controls for in situ hybridization (b1′-b4′, c1′ ′-c4′, d1′-d4′, e1′-e4′). (A1-A4) Vasa protein in spermatogonia (arrows) prior to antagonist treatment (A1), 3 days after treatment (A2), 1 week after treatment (A3) and 4 weeks after treatment (A4)), (B1-B4) Dhh mRNA expression in spermatogonia (arrows) and endothelial cells (arrow head) prior to antagonist treatment (B1), 3 days after treatment (B2), 1 week after treatment (B3), no positive signal at 4 weeks after treatment (B4) (B1-B4, anti-sense; b1′-b4′ [inset], sense control) (C1-C4) Ptc2 mRNA expression in spermatogonia (arrows) and endothelial cells (arrow head) prior to antagonist treatment (C1), 3 days after treatment (C2), 1 week after treatment (C3), no positive signal at 4 weeks after treatment (C4) (C1-C4, anti-sense; c1′-c4′ [inset], sense control) (D1-D4) Gli1 mRNA expression in spermatogonia (arrows) and endothelial cells (arrow head) prior to antagonist treatment (D1), 3 days after treatment (D2), 1 week after treatment (D3), no positive signal at 4 weeks after treatment (D4) (D1-D4, anti-sense; d1′-d4′ [inset], sense control) (E1-E4) SuFu mRNA expression was not observed in all studied groups (E1-E4, anti-sense; e1′-e4′ [inset], sense control). (F1-F4), SuFu protein was not observed in all studied groups (F1-F4, positive staining; f1′-f4′ [inset], negative controls). Bars indicate 50μm. All analyses were conducted on three independent specimens and yielded consistent results, with representative images shown. Positive signals are brown; all sections have blue counterstain from Hemotoxylin.
Similar to Dhh, Ptc2 and Gli1 mRNA signals were apparent in undifferentiated spermatogonia, endothelial cells and in some interstitial cells prior to antagonist treatment (Fig. 2C1 and 2D1, respectively) and at 3 days after treatment (Fig. 2C2 and 2D2, respectively). These signals were less intense in the 1 week post-treatment group (Fig. 2C3 and 2D3, respectively) and were not detectable by in situ hybridization from samples collected 4 weeks after treatment (Fig. 2C4 and 2D4, respectively). The same tissue blocks were previously used to study expression of the KIT mRNA in adult spermatogonial cell differentiation (Prabhu et al 2006), and these experiments were repeated in parallel in the present study to ensure the integrity of mRNAs in the 4 week post-treatment group samples; KIT mRNA was detected in spermatogonia in all sample groups (data not shown). In contrast to all other Hh signalling pathway components examined in this study, the SuFu mRNA and protein were undetectable across all groups in the irradiated adult rat testis (Fig. 2E1–E4 and 2F1–F4, respectively).
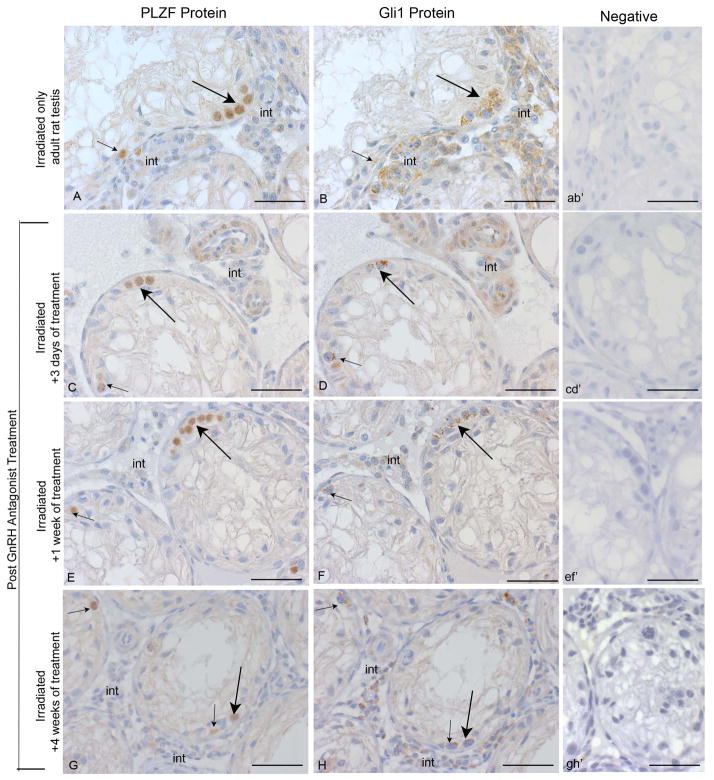
PLZF is a marker of undifferentiated spermatogonia and spermatogonial stem cells (Costoya et al 2004). To unequivocally determine whether these cells contain Gli1, immunohistochemistry was performed with serial sections from all treatment groups. In every sample, many cells with the rounded shape and chromatin features characteristic of spermatogonia were observed to contain both PLZF and Gli1 proteins (Fig. 3A–H).
Figure 3. Gli1-PLZF double staining in serial sections of irradiated adult rat testes.
Immunohistochemistry with PLZF (A, C, E, G) and Gli1 (B, D, F, H) antibodies was used to examine the irradiated adult rat testis. Right hand panels illustrate controls lacking primary antibody (ab′, cd′, ef′, gh′). PLZF protein detected in spermatogonia (arrows) and some interstitial cells (int) (A) prior to antagonist treatment, (C) 3 days after treatment, (E) 1 week after treatment, and (G) 4 weeks after treatment. Gli1 protein detected in spermatogonia (arrows) and some interstitial cells (int) (B) prior to antagonist treatment, (D) 3 days after treatment, (F) 1 week after treatment, and (H) 4 weeks after treatment. Bars indicate 50 μm. All analyses were conducted on three independent specimens and yielded consistent results, with representative images shown.
Discussion
The key outcome from this study was the discovery that mRNAs encoding essential Hh signalling components are produced by cells with spermatogonial stem cell capacity in the adult testis. Detection of the Gli1 mRNA and protein in these cells indicates they are potential sites of active Hh signalling. By identifying spermatogonia containing both Dhh and Gli1 in the damaged testis, we provide new evidence suggesting that an autocrine Hh signalling loop may sustain these cells prior to their differentiation, but this does not appear to be sustained during their recovery. It will be informative to learn what controls this switch.
In the irradiated adult rat testis model with atrophic tubules, undifferentiated spermatogonia are the only germ cell type present. These cells can proliferate, but they fail to differentiate so that further transit amplification steps in spermatogenesis are absent. However, if GnRH-ant treatment is applied that causes a substantial reduction in intratesticular testosterone, then spermatogenic recovery begins as early as the fourth week after treatment (Shuttlesworth et al 2000). This model enables identification of the undifferentiated spermatogonia, a population not readily visible in the normal adult testis due to its scarcity, as the site of expression of Hh signalling machinery. The latter persists for between 3 days and 1 week post-treatment, but disappears by the fourth week with the onset of differentiation by some spermatogonia. The presence of the Gli1 transcript demonstrates for the first time that Hh signalling may be active in undifferentiated spermatogonia and is shut down when they begin to differentiate in this model. Although there are differentiated spermatogonia present at 4 weeks, there are also still undifferentiated spermatogonia at that time. We hypothesize that this reflects a role for Hh signalling in maintaining SSCs in a state in which they are incapable of differentiation, similar to what we observe in gonocytes prior to their transformation into spermatogonia (data not shown).
In the context of cancer stem cells, it has been suggested that the Hh ligand promotes the expansion of multiple myeloma cells without differentiation, whereas Hh pathway blockade is accompanied by terminal differentiation of multiple myeloma cells (Peacock et al 2007). During spermatogenic recovery in the irradiated testis, production of Hh pathway transcripts appears to cease when spermatogonia differentiate in response to an altered hormonal environment, and it will be interesting to explore whether transcription of one or several of these transcripts are directly affected by specific hormones.
In normal tissues, Hh-induced progenitor cell proliferation is transient and tightly regulated, preventing continuous regeneration. However, constitutive Hh signalling activation results in unregulated self-renewal of progenitor cells in association with several human cancers. For example, aberrant activation of Hh signalling mainly due to a Ptc mutation is responsible for Gorlin’s syndrome, which is predominantly characterized by basal cell carcinoma, medulloblastoma and rhabdomyosarcoma (Varjosalo and Taipale 2008). The initiation and progression of a range of cancers has also been related to other components of the Hh signalling pathway, with Gli1 most notably found to be amplified in human glioma (Kinzler et al 1987). Abnormal activation of Hh signalling through mutations in Smo and Gli2 is also found in basal cell carcinoma (Regl et al 2004, Reifenberger et al 1998, Xie et al 1998). In addition, loss-of-function mutations in negative regulators of the Hh signalling pathway, including Ptc and SuFu, are linked with tumorigenesis (Chi et al 2006, Sheng et al 2004, Taylor et al 2002, Tostar et al 2006). Thus, the loss in capacity to regulate cellular differentiation and proliferation explains the involvement of aberrant Hh signalling in distinct subsets of tumours that feature mutations in individual pathway components.
Hh signalling pathway serves homeostatic roles in tissues through stem cell maintenance (Michel et al 2012). The present study reinforces previous evidence that Hh signalling pathway is modulated by transcriptional regulation of the mRNAs encoding pathway receptors, ligands and intracellular signalling modulators (Farzan et al 2009), including during progressive differentiation of male germ cells (Makela et al 2011, Morales et al 2009, Szczepny et al 2006). Both spermatogonia and spermatocytes in the adult rat testis contain Ptc2, Gli1 and Gli2 mRNAs, supporting other data which identify mitotic and meiotic male germ cells as active in Hh signalling (Kroft et al 2001, Szczepny et al 2009). The restricted expression of SuFu to the more mature post-meiotic haploid germ cells is consistent with the proposed requirement for down-regulation of Hh signalling during progression of male germ cells through spermiogenesis (Szczepny et al 2006). Thus controlled regulation of Hh signalling pathway appears to be an inherent property of normal spermatogenesis, and understanding the mechanisms by which this occurs will shed light on the processes that control normal and perturbed spermatogenesis, as well as illuminating mechanisms by which Hh activity is controlled. Furthermore, it will be interesting to consider the potential for Gli transcription factors to mediate or be influenced by crosstalk between Hedgehog and other signalling pathways, as transforming growth factor-β (via Smad3), RAS (via KRAS mutations) and PI3 Kinase (via AKT) (Dennler et al 2007, Lauth and Toftgard 2007, Riobo et al 2006).
The new evidence provided here demonstrates the potential for Hh signalling pathway to influence stem cells in the adult rat testis and establishment of normal spermatogenesis. These findings indicate that tightly controlled down-regulation of other Hh pathway components and up-regulation of SuFu regulates Hh pathway activity and signalling to enable full spermatogenesis. Given the well-documented contribution of aberrant Hh signalling pathway to several kinds of cancers, knowledge of how this pathway is normally modulated, as demonstrated here for the testis, will help to identify new treatments for patients in which stem cell behaviour is disturbed.
Acknowledgments
Support from the NHMRC of Australia, ID545916 to KL; 3236566 to KL, EMcL), the Australian Research Council (#348239 to KL, EMcL) and the NIH (#R01 ES08075 from the NIH to MM) is hereby acknowledged
Footnotes
Authors’ Contribution
- Zeliha Sahin – conception, design and implementation of the work, analysis and interpretation of data, preparation of the manuscript, approval of the final version of the manuscript.
- Anette Szczepny - design of the work, interpretation of data, critical revision and approval of the final version of the manuscript.
- Eileen A. McLaughlin – conception of the work, interpretation of data, critical revision and approval of the final version of the manuscript.
- Marvin Meistrich – conception and design of the work, analysis and interpretation of data, critical revision and approval of the final version of the manuscript.
- Wei Zhou – implementation of the work, analysis and interpretation of data, critical revision and approval of the final version of the manuscript.
- Ismail Ustunel - critical revision and approval of the final version of the manuscript.
- Kate L. Loveland – conception and design of the work, analysis and interpretation of data, drafting, critical revision and final approval of the manuscript.
References
- Adolphe C, Narang M, Ellis T, Wicking C, Kaur P, Wainwright B. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development. 2004;131:5009–5019. doi: 10.1242/dev.01367. [DOI] [PubMed] [Google Scholar]
- Barsoum I, Yao HH. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal Leydig cells. Biology of Reproduction. 2011;84:894–899. doi: 10.1095/biolreprod.110.088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Boonen SE, Stahl D, Kreiborg S, Rosenberg T, Kalscheuer V, Larsen LA, et al. Delineation of an interstitial 9q22 deletion in basal cell nevus syndrome. American Journal of Medical Genetics. 2005;132A:324–328. doi: 10.1002/ajmg.a.30422. [DOI] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Chen X, Horiuchi A, Kikuchi N, Osada R, Yoshida J, Shiozawa T, et al. Hedgehog signal pathway is activated in ovarian carcinomas, correlating with cell proliferation: it’s inhibition leads to growth suppression and apoptosis. Cancer Sci. 2007;98:68–76. doi: 10.1111/j.1349-7006.2006.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S, Huang S, Li C, Zhang X, He N, Bhutani MS, et al. Activation of the hedgehog pathway in a subset of lung cancers. Cancer Lett. 2006;244:53–60. doi: 10.1016/j.canlet.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Couve-Privat S, Le Bret M, Traiffort E, Queille S, Coulombe J, Bouadjar B, et al. Functional analysis of novel sonic hedgehog gene mutations identified in basal cell carcinomas from xeroderma pigmentosum patients. Cancer Research. 2004;64:3559–3565. doi: 10.1158/0008-5472.CAN-03-4040. [DOI] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, et al. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Research. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Stegman MA, Ogden SK, Ascano M, Jr, Black KE, Tacchelly O, et al. A quantification of pathway components supports a novel model of Hedgehog signal transduction. The Journal of Biological Chemistry. 2009;284:28874–28884. doi: 10.1074/jbc.M109.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Yao HH. Sex and hedgehog: roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Research. 2012;20:247–258. doi: 10.1007/s10577-011-9254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Noguchi J, Akiyama K, Takeno Y, Fujiwara Y, Kajita S, et al. A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction. 2011;141:217–225. doi: 10.1530/REP-10-0006. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O’Brien SJ, et al. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- Kroft TL, Patterson J, Won Yoon J, Doglio L, Walterhouse DO, Iannaccone PM, et al. GLI1 localization in the germinal epithelial cells alternates between cytoplasm and nucleus: upregulation in transgenic mice blocks spermatogenesis in pachytene. Biology of Reproduction. 2001;65:1663–1671. doi: 10.1095/biolreprod65.6.1663. [DOI] [PubMed] [Google Scholar]
- Lauth M, Toftgard R. Non-canonical activation of GLI transcription factors: implications for targeted anti-cancer therapy. Cell Cycle. 2007;6:2458–2463. doi: 10.4161/cc.6.20.4808. [DOI] [PubMed] [Google Scholar]
- Makela JA, Saario V, Bourguiba-Hachemi S, Nurmio M, Jahnukainen K, Parvinen M, et al. Hedgehog signalling promotes germ cell survival in the rat testis. Reproduction. 2011;142:711–721. doi: 10.1530/REP-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Michel M, Kupinski AP, Raabe I, Bokel C. Hh signalling is essential for somatic stem cell maintenance in the Drosophila testis niche. Development. 2012;139:2663–2669. doi: 10.1242/dev.075242. [DOI] [PubMed] [Google Scholar]
- Morales CR, Fox A, El-Alfy M, Ni X, Argraves WS. Expression of Patched-1 and Smoothened in testicular meiotic and post-meiotic cells. Microscopy Research and Technique. 2009;72:809–815. doi: 10.1002/jemt.20733. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu SM, Meistrich ML, McLaughlin EA, Roman SD, Warne S, Mendis S, et al. Expression of c-Kit receptor mRNA and protein in the developing, adult and irradiated rodent testis. Reproduction. 2006;131:489–499. doi: 10.1530/rep.1.00968. [DOI] [PubMed] [Google Scholar]
- Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Research. 1997;57:842–845. [PubMed] [Google Scholar]
- Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, et al. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Research. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Research. 1998;58:1798–1803. [PubMed] [Google Scholar]
- Riobo NA, Lu K, Emerson CP., Jr Hedgehog signal transduction: signal integration and cross talk in development and cancer. Cell Cycle. 2006;5:1612–1615. doi: 10.4161/cc.5.15.3130. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Sheng T, Li C, Zhang X, Chi S, He N, Chen K, et al. Activation of the hedgehog pathway in advanced prostate cancer. Mol Cancer. 2004;3:29. doi: 10.1186/1476-4598-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttlesworth GA, de Rooij DG, Huhtaniemi I, Reissmann T, Russell LD, Shetty G, et al. Enhancement of A spermatogonial proliferation and differentiation in irradiated rats by gonadotropin-releasing hormone antagonist administration. Endocrinology. 2000;141:37–49. doi: 10.1210/endo.141.1.7272. [DOI] [PubMed] [Google Scholar]
- Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene xpression during the differentiation and development of the murine embryonic gonad. Biology of Rreproduction. 2005;72:492–501. doi: 10.1095/biolreprod.104.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepny A, Hime GR, Loveland KL. Expression of hedgehog signalling components in adult mouse testis. Dev Dyn. 2006;235:3063–3070. doi: 10.1002/dvdy.20931. [DOI] [PubMed] [Google Scholar]
- Szczepny A, Hogarth CA, Young J, Loveland KL. Identification of Hedgehog signaling outcomes in mouse testis development using a hanging drop-culture system. Biology of Reproduction. 2009;80:258–263. doi: 10.1095/biolreprod.108.067926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tostar U, Malm CJ, Meis-Kindblom JM, Kindblom LG, Toftgard R, Unden AB. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- Varjosalo M, Taipale J. Hedgehog: functions and mechanisms. Genes & Development. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- Xie J, Murone M, Luoh SM, Ryan A, Gu Q, Zhang C, et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- Zhou W, Bolden-Tiller OU, Shetty G, Shao SH, Weng CC, Pakarinen P, et al. Changes in gene expression in somatic cells of rat testes resulting from hormonal modulation and radiation-induced germ cell depletion. Biology of Reproduction. 2010;82:54–65. doi: 10.1095/biolreprod.109.078048. [DOI] [PMC free article] [PubMed] [Google Scholar]