Abstract
Feline immunodeficiency virus (FIV) is a naturally-occurring, large animal model of lentiviral-induced immunodeficiency syndrome, and has been used as a model of HIV pathogenesis and therapeutic interventions. HIV reservoirs in the form of latent virus remain the primary roadblock to viral eradication and cure, and FIV has been previously established an animal model of lentiviral latency. The goal of this study was to determine whether administration of the histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA) to aviremic, chronically FIV-infected cats would induce latent viral reactivation in vivo. A proof-of-concept experiment in a Transwell co-culture system demonstrated the ability of SAHA to reactivate latent virus which was replication competent and able to infect naïve cells. Oral SAHA (250 mg/m2) was administered with food to four asymptomatic, experimentally FIV-infected cats and one uninfected control cat, and a limited pharmacokinetic and pharmacodynamic analysis was performed. A statistically significant increase in cell-associated FIV RNA was detected in the cat with the greatest serum SAHA exposure, and cell-free viral RNA was detected at one time point in the three cats that achieved the highest levels of SAHA in serum. Interestingly, there was a significant decrease in viral DNA burden at 2 hours post drug administration in the same three cats. Though the sample size is small and the drug response was modest, this study provides evidence that in vivo treatment of FIV-infected cats with the HDACi SAHA can induce viral transcriptional reactivation, which may be dependent upon the concentration of SAHA achieved in blood. Importantly, alternative putative antilatency therapy drugs, and multimodal drug combinations, could be studied in this in vivo system. The FIV/cat model provides a unique opportunity to test novel therapeutic interventions aimed at eradicating latent virus in vivo.
Keywords: FIV, SAHA, antilatency therapy, HIV, animal model, HDAC inhibitor
1. Introduction
Feline immunodeficiency virus (FIV) is a lentivirus of domestic cats with a seroprevalance of approximately 1% among asymptomatic cats in the United States. Seroprevalance is much higher in symptomatic animals, and in countries such as Japan and Australia, has been as high as 30% of the total population (Courchamp and Pontier, 1994). Terminally, infected animals exhibit feline acquired immunodeficiency syndrome (FAIDS), which includes opportunistic infections, tumor formation, wasting, and death. Though some antiretroviral therapies have been shown to be effective against FIV in vitro, FIV-infected cats are typically managed clinically by isolation, symptomatic treatment, and/or euthanasia. Although multiple antiretroviral agents are known to be effective against FIV (Bisset et al., 2002; Fogle et al., 2011), lifelong antiretroviral therapy is not financially or logistically feasible for the owners of most cats.
FIV is similar to the human immunodeficiency virus (HIV) in genome structure and immunopathogenesis (Burkhard and Dean, 2003; Kanzaki and Looney, 2004), and has been utilized as the only naturally occurring animal model of immunodeficiency for HIV-infection in people (Elder et al., 2010). During highly active antiretroviral therapy (HAART), HIV persists in infected individuals as a transcriptionally inactive (latent) integrated provirus (Trono et al., 2010), which prevents viral eradication and necessitates lifelong adherence to therapy. Memory CD4+ T cells are the primary long-lived lentiviral reservoir (Blankson et al., 2002; Chomont et al., 2009). Multiple molecular mechanisms may underlie the establishment and maintenance of latently-infected cellular reservoirs (Margolis, 2010), including epigenetic modification of histone proteins in chromatin (Colin and Van Lint, 2009; Imai et al., 2010; Margolis, 2010, 2011). FIV has been established as an outbred, large animal model of lentiviral latency, reviewed in (McDonnel et al., 2013). Studies in our laboratory demonstrated that domestic cats experimentally infected with FIV exhibit a state of latency in peripheral blood CD4+ T-cells with concurrent undetectable plasma viremia approximately 8 months after inoculation (Murphy et al., 2012). We have also shown that the latent, transcriptionally inactive FIV promoter persists within in vivo-derived CD4+ T-cells in association with de-acetylated histones, consistent with a repressive chromatin structure (McDonnel et al., 2012a). FIV infection of its natural host can therefore provide critical insight into generalizable mechanisms of lentiviral latency and allow for the investigation of novel therapeutics targeting latent viral reservoirs.
Histone modifications, especially by acetylation, play an important role in the epigenetic regulation of gene expression. Augmented histone acetylation results in a “relaxed” chromatin state, allowing binding of transcription factors and polymerases, and ultimately promotes transcription of RNA. Conversion from the acetylated to the deacetylated state of chromatin is accomplished by a family of enzymes called histone deacetylases (HDAC). Suberolyanilide hydroxamic acid (SAHA) is a potent small-molecule inhibitor of HDAC classes I, II and IV (Lane and Chabner, 2009), which include all prominent active nuclear HDACs in mammalian cells. Thus, SAHA is considered a pan-HDAC inhibitor, and has been shown to cause an overall increase in global histone acetylation.
In 2006, SAHA (vorinostat, Zolinza™) became the first HDAC inhibitor (HDACi) approved by the Food and Drug Administration as a treatment for cutaneous T-cell lymphoma (CTCL) in humans. In addition to use for treatment of CTCL and other cancers, there is much interest in using HDACi such as SAHA to eradicate HIV from infected individuals. SAHA would be used as an inducer of viral promoter-associated histone acetylation, thereby reactivating viral transcription. The proposed concept is to activate transcription of latently integrated provirus in the presence of antiretroviral drugs, so as to induce death of infected cells by either direct cytopathic effect of the virus or immune surveillance, but protect uninfected cells from de novo infection (Richman et al., 2009). In theory, this strategy would purge the latent reservoir and eliminate the need for life-long HAART (Geeraert et al., 2008). We have shown in our lab that SAHA is effective in reactivating FIV transcription, protein expression, and infectious particle assembly ex vivo (McDonnel et al., 2012b). We have also examined the pharmacokinetics and pharmacodynamics of oral and intravenous-administered SAHA in cats (McDonnel et al., 2014).
Use of the FIV-infected cat model of lentiviral latency may help address questions that are either logistically or ethically not feasible in HIV-infected humans, including the investigation of histone-modifying agents and other novel therapeutic antilatency approaches (McDonnel et al., 2013). Importantly, these findings have the potential to benefit FIV-infected cats as well as HIV-infected humans. In this study, we sought to determine whether a candidate HDACi (SAHA) would be able to induce activation of viral transcription and replication in vivo. We hypothesized that treatment of aviremic, chronically FIV-infected cats with oral suberoylanilide hydroxamic acid (SAHA) would result in viral reactivation and subsequent viremia.
2. Materials and Methods
As a proof of concept experiment, freshly isolated peripheral blood mononuclear cells (PBMC) from two chronically FIV-infected cats (#165 and 187) were co-cultured ex vivo in a Transwell system (Figure 1c) with specific pathogen free (SPF) PBMC in the upper culture well separated by a 400 nM permeable polycarbonate membrane (Corning). Cells were cultured in peripheral blood leukocyte (PBL) media (Murphy et al., 2012) alone, or media containing 1 uM SAHA, SAHA and allogeneic feline SPF PBMC (i.e., the same PBMC that were used in the upper well), or SAHA, SPF PBMC, and 5 μg/mL concanavalin A. One million cells from the upper well were sampled at 7 and 14 days post ex vivo culture, and assessed for the presence of FIV gag DNA using real-time PCR as previously described (Murphy et al., 2012), indicating a productive viral infection. Cellular GAPDH DNA was used to normalize the amount of viral DNA in each sample.
Figure 1.
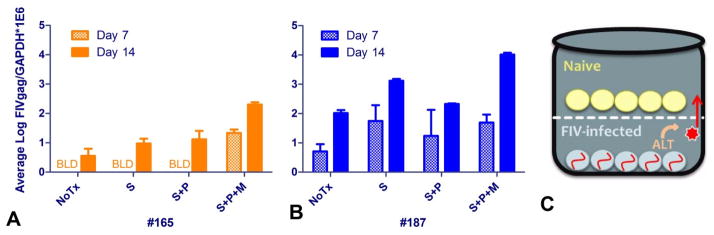
Viral DNA load from peripheral blood mononuclear cells (PBMC) in the upper culture well of the Transwell co-culture system at 7 and 14 days post ex vivo culture with PBMC from cat 165 (A) and 187 (B). Cells were cultured in media alone (NoTx), media containing 1 uM SAHA (S), SAHA and allogenic SPF PBMC (S+P), or SAHA, allogeneic PBMC, and a mitogen (5 ug/mL concanavalin A) (S+P+M). FIV gag transcripts were normalized to cellular GAPDH; the average and standard deviation of triplicate measurements are displayed (BLD – below the limit of detection). A schematic of the concept behind the Transwell co-culture system is shown in (C), where naïve SPF PBMC are co-cultured with FIV-infected PBMC separated by a 400 nm permeable membrane. Putative antilatency therapy (ALT) is used to reactivate latent virus in the lower well.
Four experimentally FIV-C infected cats (#165, 184, 186, and 187) between 4 and 4.5 years post viral inoculation and one sham-inoculated cat (#185) were administered 250 mg/m2 SAHA (>98% pure, Cayman Chemical) orally in gelatin capsules. All cats were fasted overnight and offered food (1/4 can feline Hills A/D® in addition to their normal meal) approximately 10 minutes before oral drug administration. Peripheral blood was collected by venipuncture the day before (0, pre-dose), and 1, 2, 4, 8, and 24 hours, as well as 5 days post administration into two tubes: one tube containing EDTA (for plasma collection and leukocyte isolation) and one lacking anticoagulant (for serum collection).
Serum [SAHA] was determined by liquid chromatography tandem mass spectrometry (LC-MS/MS), and histone acetylation status was quantified by Western blotting of PBMC lysates, as described previously (McDonnel et al., 2012c; McDonnel et al., 2014; Patel et al., 2008). PBMC were isolated from EDTA-anti-coagulated blood by ficoll-hypaque, and cellular DNA and RNA were extracted using a commercial kit (Qiagen AllPrep Mini kit). Viral RNA (vRNA) was isolated from double-spun (Murphy et al., 2012) plasma using another commercial kit (Qiagen viral RNA kit), and both plasma and cell-associated RNA were DNAse treated (Ambion) and reverse transcribed (Origene). Copies of FIV gag and cellular GAPDH were quantified by real-time PCR using SYBR Mastermix (5 Prime) as previously described (Murphy et al., 2012). For plasma co-culture assays, 100 μL of double-spun plasma from 0, 1, and 2 hours post SAHA administration was incubated for 7 days with 200,000 specific pathogen free feline PBMC in media containing mitogens as previously described (McDonnel et al., 2012c). DNA was then isolated as above, and viral infection was detected by the presence of FIV gag DNA using real-time PCR.
3. Results
In the ex vivo study, FIV-infected PBMC isolated from two cats were co-cultured in a Transwell system with SPF feline PBMC, using various added conditions to illicit viral reactivation. Infectious virus was released from the PBMC of both cats, and able to infect SPF PBMC through the permeable membrane (Figure 1). The addition of SAHA, allogeneic PBMC (to induce a mixed-lymphocyte reaction), and mitogen (concanavalin A) increased the viral DNA load in the naïve PBMC, indicating that these conditions augmented viral reactivation ex vivo. Because the mitogen is freely diffusible across the Transwell membrane, the possibility exists that the increased infectivity when adding this agent was due in part to its proliferative effects on recipient cells in the upper well.
Following oral SAHA administration in the in vivo study, drug concentrations in serum were evaluated by LC-MS/MS. Serum SAHA kinetics were found to be variable, though the limited sample time points precluded a complete analysis of pharmacokinetics. One cat, #165 had an observed maximum concentration, Cmax of less than 25% of the next lowest Cmax and more than an order of magnitude less than the highest Cmax (63 nM). Cat 165 was presumed to have spit out or regurgitated part or all of the capsule (though no vomitus or capsule material was found). The remaining cats’ observed Cmax ranged from 250 to 840 nM, with an average of 460 nM (Figure 2). It should be noted that the observed Cmax is not expected to closely approximate the actual Cmax in this study due to limited early sampling time points.
Figure 2.
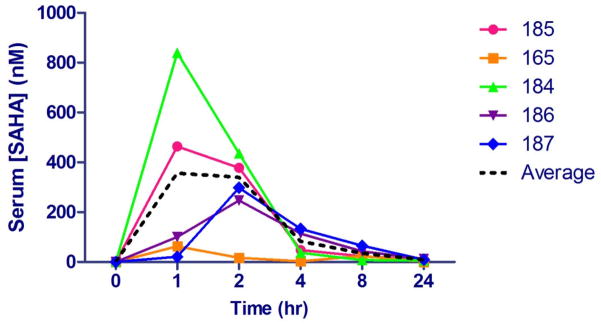
Pharmacokinetics of SAHA in 4 FIV+ cats (165, 184, 186, and 187) and 1 FIV- control cat (185) administered 250 mg/m2 oral SAHA with food. The average (dotted black line) represents data excluding cat #165 (see text). Serum samples were analyzed by UPLC coupled to tandem mass spectrometry using deuterated SAHA as an internal standard.
As a measure of the pharmacodynamic effect of SAHA, total and acetylated histone H3 were quantified at 0, 1, and 2 hours post SAHA administration using Western blotting as described previously (McDonnel et al., 2014). With the exception of cat #165, all animals demonstrated an increase in histone acetylation in PBMC following oral SAHA administration (Figure 3). The effect appeared to be somewhat dependent on the serum concentration of SAHA, with the greatest effect corresponding to the animal with the highest Cmax (cat #184).
Figure 3.
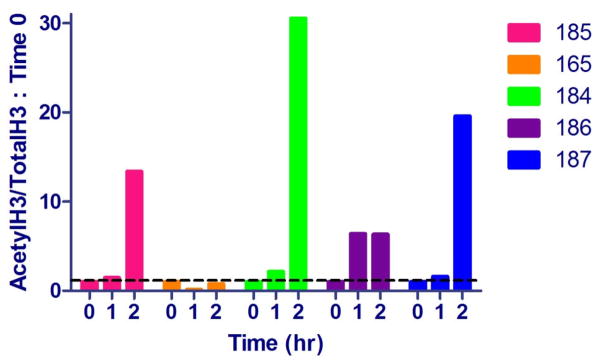
Pharmacodynamics of SAHA in 5 cats administered 250 mg/m2 oral SAHA with food. PBMC collected at 0 (pre-dose), 1, and 2 hours after SAHA administration were analyzed by Western blotting for both acetylated and total histone H3. Densitometry ratios of acetylH3/totalH3 were normalized to pre-dose controls (time 0). Values above the dashed black line (1) have a relative increase in histone H3 acetylation.
PBMC-associated viral DNA and RNA (FIV gag) were quantified by real-time PCR and normalized to cellular GAPDH. A statistically significant increase (nearly 10-fold) in cell-associated FIV RNA was detected in the cat (184) with the greatest serum drug exposure (Figure 4). Both cats 184 and 187 had detectable levels of FIV RNA in PBMC prior to drug administration. The gap in detectability of FIV RNA in Cat 187 at 1 and 2 hrs may reflect a limit of detection due to the low viral burden in these chronically-infected animals (McDonnel et al., 2012c) Alternatively, this animal could have periodically circulating FIV RNA positive cells from central lymphoid tissues. Cell-associated vRNA was below the limit of detection in cats 185 (FIV- control), 165, and 186 at all time points. Interestingly, a decrease in PBMC viral DNA load was noted at 2 hours in all cats except 165 (ie, those with Cmax ≥ 250 nM), which was statistically significant (Figure 5). Viral DNA remained relatively constant for cat 165, and was below the limit of detection in the control cat.
Figure 4.
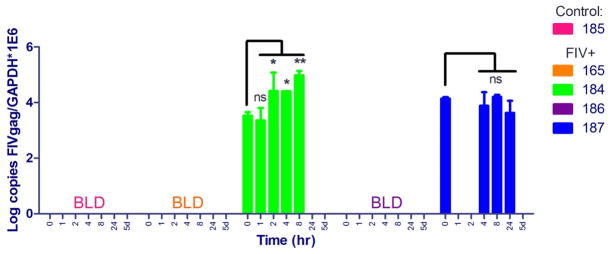
Cell (PBMC)-associated viral RNA at various time points before (0) and after oral SAHA administration. FIV gag transcripts were normalized to cellular GAPDH; the average and standard deviation of triplicate measurements are shown. A one-way ANOVA followed by Dunnett’s multiple comparison post-test using time 0 (pre-dose) as the control was performed to determine if there was a significant change in the level of vRNA after drug administration. *p < 0.05; **p < 0.01; ns = not significant; BLD = below the limit of detection, 5d = 5 days.
Figure 5.
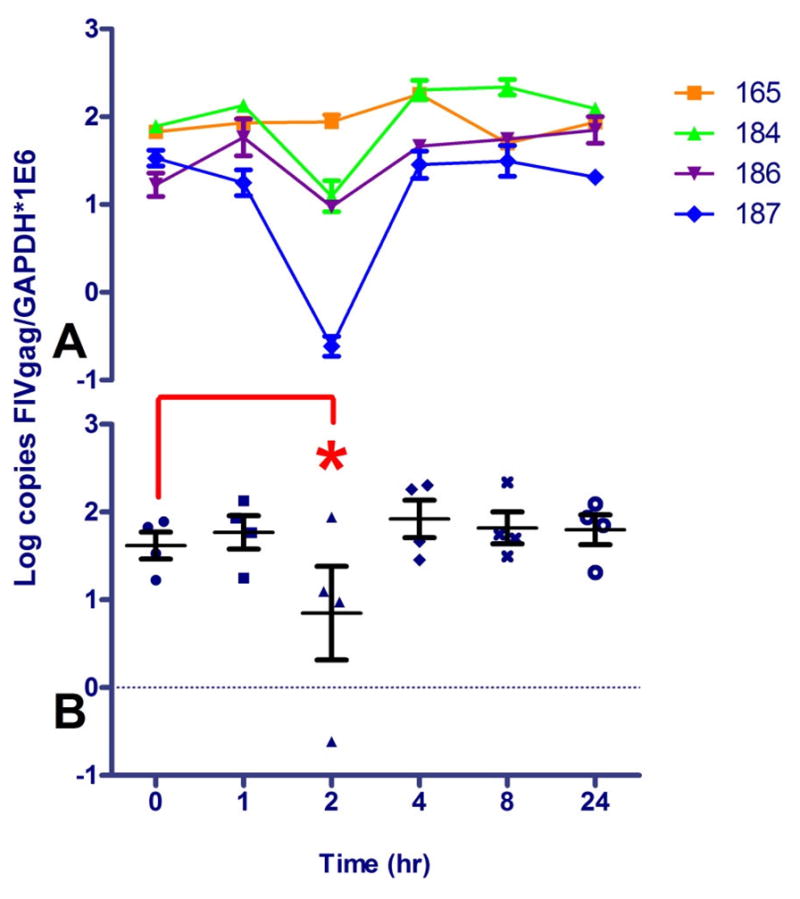
PBMC FIV DNA load at various time points before (0) and after oral SAHA administration to 4 FIV+ cats (165, 184, 186, and 187). Copies of FIV gag were normalized to cellular GAPDH. The average and standard deviation of triplicate measurements for individual animals are shown in (A), and the average and standard deviation of all 4 FIV+ animals are graphed in (B). A one-way ANOVA followed by Dunnett’s multiple comparison post-test using time 0 (pre-dose) as the control was performed to determine if there was a significant change in the level of viral DNA after drug administration; *p < 0.05. DNA from the FIV- control cat (185) was below the limit of detection of FIV gag at all time points (not shown).
Attempts were made to isolate putative viral RNA from double-spun plasma and assess FIV gag transcripts by real-time PCR. Viremia was below the limit of detection (103 copies/mL, data not shown) for all animals at all time points, except at 1 hour post treatment in cats 184 and 186, and at 2 hours in cat 187 (Table 1). All 3 of these cats had significant levels of serum SAHA exposure and demonstrated a significant decrease in viral DNA burden at 2 hours after drug administration. Double-spun plasma from 0, 1, or 2 hours post SAHA administration co-cultured for one week ex vivo with allogeneic feline PBMC did not result in detectable FIV infection at any time point for any cat (not shown).
Table 1.
Viral RNA (FIV gag copies/mL) in double-spun plasma at various time points after SAHA administration.
| Cat# | 0 | 1 hr | 2 hr | 4 hr | 8 hr | 24 hr | 5 days |
|---|---|---|---|---|---|---|---|
| 185 (−) | BLD | BLD | BLD | BLD | BLD | BLD | BLD |
| 165 | BLD | BLD | BLD | BLD | BLD | BLD | BLD |
| 184 | BLD | 3.74×104 | BLD | BLD | BLD | BLD | BLD |
| 186 | BLD | 1.86×105 | BLD | BLD | BLD | BLD | BLD |
| 187 | BLD | BLD | 5.25×104 | BLD | BLD | BLD | BLD |
Data are the average of triplicate qPCR measurements; BLD indicates samples below the limit of detection (~103 copies/mL).
4. Discussion
Here we described a novel transwell co-culture system that tests the ability of antilatency agents to reactivate latent FIV ex vivo and infect naïve cells through a permeable membrane. Conceivably, antiretroviral (ART) agents could be added to the media of this system to evaluate their ability to block infection of naïve cells after reactivation from latency, an ex vivo simulation of the proposed eradication strategy purging the latent viral reservoir in the presence of uninfected cells. This ex vivo system may be useful in screening future combinatorial ALT/ART strategies in a high-throughput platform before moving to in vivo experiments in the feline model.
In this pilot in vivo viral reactivation study, four FIV-C infected cats in the asymptomatic phase of infection and one control cat were administered the standard human dose of 250 mg/m2 oral SAHA. The degree of variability observed in the serum SAHA concentrations in these cats could be partially attributed to variability in food intake at the time of administration as animals were not force fed a specific amount. In our previous study of SAHA pharmacokinetics and pharmacodynamics in fasted cats (McDonnel et al., 2014), the maximum observed concentration in serum was not only much less (average of 160 nM) than the present study (460 nM, excluding outlier cat #165), but there was also less variability (~2-fold difference between animals) compared with animals in the fed state (~3.5 fold difference). The other obvious difference between this study and the previous one is that 4 of 5 of these cats are infected with FIV, which might conceivably affect enteric mucosal integrity and drug absorption. However, the uninfected control animal (185) had the second highest Cmax, indicating that food, rather than FIV infection status, may have played the largest role in altering pharmacokinetics. From this data, it may be inferred that the fasting vs. fed state influences the pharmacokinetics of orally administered SAHA in cats much more than in humans, and that the fed state may augment SAHA bioavailability.
Pharmacodynamics of SAHA in these cats roughly correlated with the drug kinetics in that the peak effect was noted 1–2 hours post oral administration and increased drug exposure in an individual animal corresponded to increased histone acetylation. Importantly, the pharmacodynamic effect of histone acetylation was observed in all animals with adequate serum drug exposure (i.e., all but outlier cat #165). However, neither pharmacokinetic nor pharmacodynamic profiles were thoroughly evaluated in this study due to the study design (limited number of early sampling time points). The Cmax and peak histone acetylation reported here should therefore be interpreted as a minimum rather than an approximation of their true maximum values.
A significant increase in cell-associated viral RNA was observed in the cat with the greatest observed serum SAHA concentration (#184), consistent with in vivo viral transcriptional activation in response to treatment. Moreover, a decrease in viral DNA load at 2 hours in all 3 cats with adequate serum exposure could potentially indicate death of infected cells in central lymphoid tissues. This may occur if paused RNA polymerase II is reactivated upon exposure to SAHA and results in a toxic accumulation of FIV transcripts or proteins. However, this mechanism is speculative and it is not clear why this effect would be transient, or why an increase in FIV transcripts would not be detected in peripheral leukocytes other than in one cat. One possible explanation is that the local environment of the central lymphoid tissues is different with regard to FIV infection and replication status. Another possibility is that the accumulation of toxic viral transcripts were those other than FIV gag. It should be noted that the GAPDH copy number (or Ct) in both DNA and cellular RNA was not significantly different at 1 or 2 hours than at any other time point, indicating that a similar number of cells were used and a similar amount of total nucleic acids were isolated. In addition, there was no decrease in the viral DNA load of cat #165, which may be considered a control for SAHA exposure. Because the only cat with a demonstrable increase in cell-associate FIV transcripts was also positive for FIV gag at baseline, it cannot be ruled out that SAHA may have augmented viral transcription rather than reactivated latent virus. However, this is thought to be less likely considering its mechanism of action and previous studies in vitro. It would have been optimal to isolate CD4+ T cells from the remaining PBMC as they are the primary cells of interest as latent reservoirs; however, it was not possible to collect them in sufficient numbers in this study due to limitations on the volume of blood collected at multiple time points.
Although cell-associated RNA was only significantly increased in one animal, all 3 of the cats with high serum [SAHA] demonstrated transiently detectable cell-free FIV gag transcripts in the plasma at one time point. This might suggest transcriptional reactivation in central lymphoid tissues or other sites resulting in cell lysis and release of free viral transcripts, which would corroborate the drop in viral DNA load at 2 hours after drug administration. Alternatively, the detection of this viral RNA in plasma could represent non-infectious viral exosomes (Columba Cabezas and Federico, 2013). It is possible that lower concentrations of cell-free viral transcripts were released at other time points but were undetectable by our relatively insensitive assay which has a limit of detection of approximately 103 copies/mL of plasma (sensitivity limited by the blood volume obtainable at each time point). Viral transcripts other than FIV gag were not assessed in this study. It would seem unlikely that whole, formed virions were being released as early as 2 hours after transcriptional reactivation, which would explain why plasma co-culture studies failed to demonstrate infectability and replication competence of the putative reactivated ‘virus’ in plasma at that time. Importantly, plasma viral RNA was found only in the three cats that had significant serum [SAHA], and not the uninfected cat or the cat with very low serum [SAHA].
5. Conclusion
In conclusion, this report provides evidence that in vivo treatment of FIV-infected cats with the HDAC inhibitor SAHA can result in viral transcriptional reactivation, which may be dependent upon the concentration of SAHA achieved in blood. Confirmation using a larger sample size would be necessary to confirm the findings in this pilot study. Administration of SAHA could be further optimized; for example, an alternative route of drug administration, such as subcutaneous injection, may result in greater serum drug exposure and more consistent viral reactivation. Another avenue of potential exploration would be multiple dosages of oral SAHA in these cats to determine if serial drug exposure would result in a greater or more sustained viral activity, though this pharmacologic strategy had limited success in a recent study in human patients (Archin et al., 2014). Importantly, alternative HDACi’s, other classes of putative antilatency therapy drugs, and multimodal drug combinations, could be studied in this ex vivo/in vivo system. The FIV/cat model provides a unique opportunity to test novel therapeutic interventions aimed at eradicating latent virus in vivo.
Highlights.
Treatment of FIV+ cats with SAHA can result in viral transcriptional reactivation.
Viral reactivation may depend upon the concentration of SAHA achieved in blood.
An ex vivo Transwell co-culture system to test antilatency therapy is described.
The FIV model provides a unique opportunity to test ALT/ART strategies in vivo.
Acknowledgments
The authors are grateful for the research support provided by Christina Eckstrand, DVM, DACVP, Chad Hillman, Deborah Bee, and Laurie Brignolo, DVM, DACLAM, as well as the excellent animal care provided by Monica Durden and the staff at the University of California Davis Feline Research Laboratory. Funding for this study was provided by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis, and NIH T32 training grant #5T32AI060555.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archin NM, Bateson R, Tripathy M, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, Richman DD, Robertson KR, Kashuba AD, Bosch RJ, Hazuda DJ, Kuruc JD, Eron JJ, Margolis DM. HIV-1 Expression within Resting CD4 T-Cells Following Multiple Doses of Vorinostat. J Infect Dis. 2014 doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset LR, Lutz H, Boni J, Hofmann-Lehmann R, Luthy R, Schupbach J. Combined effect of zidovudine (ZDV), lamivudine (3TC) and abacavir (ABC) antiretroviral therapy in suppressing in vitro FIV replication. Antiviral Res. 2002;53:35–45. doi: 10.1016/s0166-3542(01)00190-5. [DOI] [PubMed] [Google Scholar]
- Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1:15–29. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin L, Van Lint C. Molecular control of HIV-1 postintegration latency: implications for the development of new therapeutic strategies. Retrovirology. 2009;6:111. doi: 10.1186/1742-4690-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columba Cabezas S, Federico M. Sequences within RNA coding for HIV-1 Gag p17 are efficiently targeted to exosomes. Cell Microbiol. 2013;15:412–429. doi: 10.1111/cmi.12046. [DOI] [PubMed] [Google Scholar]
- Courchamp F, Pontier D. Feline immunodeficiency virus: an epidemiological review. C R Acad Sci III. 1994;317:1123–1134. [PubMed] [Google Scholar]
- Elder JH, Lin YC, Fink E, Grant CK. Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV. Curr HIV Res. 2010;8:73–80. doi: 10.2174/157016210790416389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogle JE, Tompkins WA, Campbell B, Sumner D, Tompkins MB. Fozivudine tidoxil as single-agent therapy decreases plasma and cell-associated viremia during acute feline immunodeficiency virus infection. J Vet Intern Med. 2011;25:413–418. doi: 10.1111/j.1939-1676.2011.0699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraert L, Kraus G, Pomerantz RJ. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu Rev Med. 2008;59:487–501. doi: 10.1146/annurev.med.59.062806.123001. [DOI] [PubMed] [Google Scholar]
- Imai K, Togami H, Okamoto T. Involvement of histone H3 lysine 9 (H3K9) methyltransferase G9a in the maintenance of HIV-1 latency and its reactivation by BIX01294. J Biol Chem. 2010;285:16538–16545. doi: 10.1074/jbc.M110.103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki LI, Looney DJ. Feline immunodeficiency virus: a concise review. Front Biosci. 2004;9:370–377. doi: 10.2741/1235. [DOI] [PubMed] [Google Scholar]
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- Margolis DM. Mechanisms of HIV latency: an emerging picture of complexity. Curr HIV/AIDS Rep. 2010;7:37–43. doi: 10.1007/s11904-009-0033-9. [DOI] [PubMed] [Google Scholar]
- Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6:25–29. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel S, Sparger EE, Luciw PA, Murphy B. Transcriptional regulation of latent feline immunodeficiency virus in peripheral CD4+ T-lymphocytes. Viruses. 2012a doi: 10.3390/v4050878. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel SJ, Sparger EE, Luciw PA, Murphy BG. Pharmacologic reactivation of latent feline immunodeficiency virus ex vivo in peripheral CD4+ T-lymphocytes. Virus Res. 2012b;170:174–179. doi: 10.1016/j.virusres.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel SJ, Sparger EE, Luciw PA, Murphy BG. Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes. Viruses. 2012c;4:878–888. doi: 10.3390/v4050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel SJ, Sparger EE, Murphy BG. Feline immunodeficiency virus latency. Retrovirology. 2013;10:69. doi: 10.1186/1742-4690-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel SJ, Tell LA, Murphy BG. Pharmacokinetics and pharmacodynamics of suberoylanilide hydroxamic acid in cats. J Vet Pharmacol Ther. 2014;37:196–200. doi: 10.1111/jvp.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B, Vapniarsky N, Hillman C, Castillo D, McDonnel S, Moore P, Luciw PA, Sparger EE. FIV establishes a latent infection in feline peripheral blood CD4+ T lymphocytes in vivo during the asymptomatic phase of infection. Retrovirology. 2012;9:12. doi: 10.1186/1742-4690-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Guichard SM, Jodrell DI. Simultaneous determination of decitabine and vorinostat (Suberoylanalide hydroxamic acid, SAHA) by liquid chromatography tandem mass spectrometry for clinical studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;863:19–25. doi: 10.1016/j.jchromb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, Chomont N. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science. 2010;329:174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]


