Abstract
The goal of this project was to evaluate the relationship between self-reported sleep habits, daytime sleepiness, and drug use variables in individuals with cocaine and methamphetamine (METH) use disorders. Participants with a cocaine or meth use disorder completed questionnaires, including the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and a Demographic/Drug use form. Participants with a cocaine (N=51) or meth use disorder (N=85) were separated into those with either high or low sleep deficits. In participants with a cocaine use disorder, ANOVA revealed significantly higher ESS scores among those defined as “poor sleepers” (with a PSQI score >5) when compared to those defined as “good sleepers” (with a PSQI score ≤5). In addition, poor sleepers reported using cocaine for more days out of the past 30 when compared to good sleepers. Interestingly, good sleepers reported using more grams of cocaine/day compared to poor sleepers. In participants with a METH use disorder, ANOVA revealed significantly higher ESS scores among poor sleepers when compared to good sleepers. Finally, individuals with a METH use disorder that endorsed elevated daytime sleepiness also had significantly higher PSQI scores when compared to those with normal daytime sleepiness. The results indicate that drug use variables, such as recent and daily use, may affect sleep quality and daytime sleepiness in individuals with stimulant use disorders; however, further investigations (i.e. in cocaine and METH users that do not meet criteria for a cocaine or METH use disorder) must be conducted in order to provide more conclusive evidence of the impact these usage variables may have on these sleep characteristics.
Keywords: cocaine, methamphetamine, sleep
1. Introduction
Both recent cocaine administration (<24h since use) and acute cocaine withdrawal (>24h since use) have been reported to adversely affect objective measures of sleep quality. For example, both recent cocaine administration and withdrawal prolong sleep onset latency (SOL), reduce total sleep time (TST) and decrease sleep efficiency (SE) (Post et al., 1974; Watson et al., 1992; Johanson et al., 1999; Schierenbeck et al., 2008). Cocaine administration decreases REM sleep (Post et al., 1974; Watson et al., 1992; Johanson et al., 1999; Schierenbeck et al., 2008), while cocaine withdrawal increases REM sleep percentage and decreases REM latency (Post et al., 1974; Kowatch et al., 1992; Watson et al., 1992; Gillin et al., 1994; Johanson et al., 1999; Schierenbeck et al., 2008); however, since sleep time in cocaine-dependent individuals is dramatically reduced when compared to healthy controls, there is not an overall increase in REM sleep.
Similarly, both recent methamphetamine (METH) administration and withdrawal have also been reported to adversely affect objective measures of sleep quality. Indeed, recent METH administration has been reported to increase SOL and markedly decrease TST (Perez et al., 2008) while acute METH withdrawal prolongs SOL, increases nighttime and daytime TST, increases awakenings during the night and reduces sleep quality (McGregor et al., 2008). Although there are few reports concerning the acute effects of either METH administration or withdrawal on REM sleep, some studies that have included both cocaine and METH users reported an increased propensity for REM sleep during acute withdrawal (Watson et al., 1972; Watson et al., 1992); however, as mentioned previously, sleep time is dramatically reduced in stimulant-dependent individuals when compared to healthy controls, so total REM sleep is not increased.
Another topic of interest is the effect of stimulant abstinence on sleep quality. In one particular study, all subjective measures of sleep improved as a function of cocaine abstinence as assessed by the sleep quality questionnaire (SQQ) (Matuskey et al., 2010). In addition, progressive weeks of cocaine abstinence are associated with decreased TST, REM sleep, and sleep efficiency and are associated with increases in sleep onset and REM latencies. Also, TST was negatively associated with years of cocaine use (Matuskey et al., 2010). Similarly, in a separate study, self-report assessments of sleep quality demonstrated robust improvement over the course of abstinence where each of the four self-reported morning sleep and alertness measures was positively correlated with number of days abstinent. However, this was incongruent with performance on objective measures (where there was the presence of decreased sleep, impaired vigilance and sleep-dependent procedural learning, and spectral activity) demonstrating that these individuals may be unaware of these sleep deficits and their subsequent impact on daily functioning (Morgan et al., 2006).
Excessive daytime sleepiness in stimulant users, which could result from poor sleep quality and/or reduced sleep time, may lead to partial sleep deprivation and deleteriously affect cognitive functioning. In addition, it has been well documented in the literature that stimulant users are more susceptible to cognitive impairment (Jovanovski et al., 2005; Scott et al., 2007; Sofuoglu et al., 2013). Thus, because partial sleep deprivation has been reported to impair cognitive function and cognitive impairments may be predictive of poor treatment retention and outcome (Aharonovich et al., 2003; Aharonovich et al., 2008), we sought to determine the effects of long-term cocaine or METH use on sleep behavior and daytime sleepiness. Specifically, we hypothesized that individuals who meet criteria for either a cocaine or METH use disorder would report poor sleep quality and increased levels of daytime sleepiness relative to literature-based controls. We also hypothesized that those individuals reporting greater or increased frequency or duration of stimulant use would report greater sleep deficits compared to those who use less frequently.
2. Methods
2.1 Participants
51 participants with a cocaine use disorder and 85 participants with a METH use disorder completed a screening packet for enrollment in ongoing inpatient studies, including self-report drug use history. All subjects were non-treatment seeking and met DSM-IV-TR criteria for a cocaine or METH use disorder (but not both), as assessed by the MINI International Neuropsychiatric Interview (MINI) (Sheehan et al., 1997). Other inclusion criteria included being between 18 and 55 years of age, at least twice-weekly use of METH or cocaine (recent use (within the past 72 hours) confirmed via urine toxicology). Exclusion criteria included diagnosis of any other Axis I psychiatric disorder, dependence on any other drugs aside from nicotine, a history of seizure disorder, head trauma, or concomitant use of any psychotropic medication. All forms were administered in private with utmost sensitivity to the volunteers, and the UCLA Institutional Review Board approved the study. Participants were compensated for their participation.
2.2 Forms
Pittsburgh Sleep Quality Index (PSQI)
The PSQI (Buysse et al., 1989) is a nineteen-item questionnaire (with a range of 0–21 where higher scores indicate decreased sleep quality) designed to gauge a patient’s sleeping habits during the past month. Individuals are asked to report their bed time, morning waking time, nighttime sleep latency, and to gauge the frequency of various impediments to healthy sleep, such as waking up in the middle of the night and not being able to fall back asleep. Each item is assigned a point value, and a PSQI score of ≤ 5 is considered normal sleep.
Epworth Sleepiness Scale (ESS)
The ESS (Johns, 1991) is an eight item questionnaire which asks individuals to gauge on a scale of 0–3 (0 being no chance, 3 being high chance) how likely they would have been in the past month to fall asleep during eight common situations such as sitting quietly after a meal or while watching TV (with a range of 0–24 where higher scores indicate increased daytime sleepiness). An ESS score of less than 10 is considered normal daytime sleepiness.
Cocaine and METH Use
Cocaine and METH use were assessed with a self-report questionnaire with frequency assessed in terms of date of last use, days used in the past 30, years of use, grams used per day, and route of administration. Recent drug use was assessed via qualitative urine toxicology (testing for cocaine, amphetamine, METH, marijuana, and opiates).
2.3 Statistical Analyses
Correlations between total PSQI, ESS and drug use variables (i.e. years, recent, daily cocaine/meth use) and demographic variables (i.e. age, education) were determined using Pearson product-moment correlations. Group comparisons between those with poor sleep quality (PSQI scores > 5) versus good sleep quality (PSQI scores ≤ 5) and demographic/drug use variables were determined using one-way ANOVA. The identical analyses were performed when comparing those with elevated daytime sleepiness (ESS > 9) and normal daytime sleepiness (ESS ≤ 9). Significance was set at p<0.05. All analyses were performed using SPSS (version 20.0).
3. Results
The primary demographic, drug use, and sleep outcome data are shown in Table 1. Participants with a cocaine use disorder were approximately 45 years of age and were predominantly male (78%). Cocaine users reported using cocaine for ~18 days out of the past 30 days, using cocaine for ~19 years and using a total of ~2 grams per day. For self-reported measures of sleep quality and daytime sleepiness, cocaine users reported a score of ~8 on the PSQI, and ~10 on the ESS. Participants with a METH use disorder were approximately 37 years of age and were predominantly male (75%). METH users reported using METH for ~18 out of the past 30 days, using METH for ~12 years and using a total of ~1 gram per day. For self-reported measures of sleep quality and daytime sleepiness, METH users reported a score of ~8 on the PSQI, and ~10 on the ESS.
Table 1.
Demographic and drug use information for individuals with cocaine and METH use disorders
| Cocaine (n=51) |
Meth (n=85) |
P | ||
|---|---|---|---|---|
| Gender (N) | ||||
| Male | 40 (78%) | 64 (75%) | ||
| Female | 11 (22%) | 21 (25%) | ||
| Age (yrs) | 45.00±0.89 | 37.29±0.96 | <0.001* | |
| Education (yrs) | 12.12±0.27 | 11.75±0.33 | 0.37 | |
| Stimulant Use | ||||
| Years of use | 18.53±1.12 | 12.04±0.79 | <0.001* | |
| Recent usea | 17.75±1.23 | 17.56±1.02 | 0.91 | |
| Grams/day | 2.05±0.21 | 1.19±0.14 | <0.001* | |
| Sleep Quality | ||||
| PSQIb | 7.77±0.61 | 8.25±0.57 | 0.58 | |
| ESS | 9.64±0.72 | 9.76±0.58 | 0.90 | |
P<0.05; Reflect Mean ± SEM
Recent use equals the number of days in which stimulant was used in the 30 days.
Number reflects global PSQI score
No significant correlations were found between demographic (i.e. age) and drug use characteristics (i.e. years of use, amount used per day, days used in the past 30) and PSQI or ESS scores in either cocaine or METH users. Age was not initially included as a covariate in the analysis; however, we did look at correlations between age and PSQI score (r scores for cocaine and methamphetamine users were 0.002 and 0.042, respectively) as well as age and ESS score (r scores for cocaine and methamphetamine users were −0.159 and 0.047, respectively). While we do agree that sleep changes as a function of age, since the correlations were weak, we did not include age as a covariate. In addition, there were no gender differences noted in PSQI or ESS scores. Using the PSQI, participants were separated into those defined as “good sleepers” (with a global PSQI score of ≤ 5) or “poor sleepers” (with a global PSQI score of > 5) as defined by Buysse et al., 1989 (Table 2). Within the individuals with a cocaine use disorder, ANOVA revealed significantly higher ESS scores among those defined as “poor sleepers” compared to those defined as “good sleepers” (F1,49=13.5, p=0.001). In addition, poor sleepers reported using cocaine for significantly more days over the past 30 when compared to good sleepers (F1,49=4.9, p=0.032), while good sleepers group reported using more grams of cocaine/day when compared to poor sleepers (F1,46=5.3, p=0.026). Within those individuals with a METH use disorder, ANOVA revealed significantly higher ESS scores among poor sleepers when compared to good sleepers (F1,83=4.2, p=0.043). Using the ESS, participants were separated into those defined as having normal levels of daytime sleepiness (with a global ESS score of ≤ 9) or elevated levels of daytime sleepiness (with a global ESS score of > 9) as defined by Ruggles and Hausman (2003) (Table 3). While there were no differences between individuals with cocaine users disorders with respect to elevated versus normal daytime sleepiness, those participants with METH use disorders who had elevated daytime sleepiness also had significantly higher PSQI scores (Table 4; F1,83=4.4, p=0.040).
Table 2.
Characteristics of Poor sleepers (as defined by PSQIa scores >5) versus Good sleepers (as defined by PSQIa scores ≤ 5) in individuals with cocaine and METH use disorders
| COCAINE USE DISORDERS | METH USE DISORDERS | |||||
|---|---|---|---|---|---|---|
| PSQI > 5 (n=34) |
PSQI ≤ 5 (n=17) |
P | PSQI > 5 (n=56) |
PSQI ≤ 5 (n=29) |
P | |
| 9.9±0.6 | 3.5±0.4 | <0.0001 | PSQIa | 10.8±0.6 | 3.4±0.3 | <0.0001 |
| 11.3±0.9 | 6.3±0.7 | 0.001* | ESS | 10.8±0.6 | 8.1±0.9 | 0.043* |
| 44.8±1.1 | 45.5±1.5 | 0.713 | Age | 37.2±1.2 | 37.4±1.6 | 0.944 |
| 12.2±0.2 | 11.9±0.7 | 0.523 | Education | 12.1±0.3 | 11.2±0.7 | 0.194 |
| Stimulant Use | ||||||
| 18.0±1.4 | 19.6±1.7 | 0.486 | Years of use | 11.7±0.9 | 12.7±1.5 | 0.556 |
| 19.6±1.3 | 14.1±2.4 | 0.032* | Recent useb | 18.2±1.2 | 16.4±1.8 | 0.410 |
| 1.7±0.1 | 2.7±0.5 | 0.026* | Grams/day | 1.1±0.1 | 1.4±0.4 | 0.349 |
P<0.05; Reflect Mean ± SEM
Number reflects global PSQI score
Recent use equals the number of days in which stimulant was used in the 30 days.
Table 3.
Characteristics of participants with elevated daytime sleepiness (as defined by ESS scores >9) versus normal daytime sleepiness (as defined by ESS scores ≤ 9) in individuals with cocaine and METH use disorders
| COCAINE USE DISORDERS | METH USE DISORDERS | |||||
|---|---|---|---|---|---|---|
| ESS > 9 (n=26) |
ESS≤ 9 (n=25) |
P | ESS > 9 (n=43) |
ESS≤ 9 (n=42) |
P | |
| 13.7±0.7 | 5.5±0.5 | <0.0001 | ESS | 14.0±0.5 | 5.4±0.5 | <0.0001 |
| 8.8±0.8 | 6.6±0.9 | 0.071 | PSQIa | 9.4±0.8 | 7.1±0.7 | 0.040* |
| 44.5±1.2 | 45.5±1.4 | 0.573 | Age | 38.2±1.4 | 36.3±1.3 | 0.338 |
| 12.0±0.4 | 12.3±0.3 | 0.619 | Education | 12.3±0.3 | 11.2±0.6 | 0.087 |
| Stimulant Use | ||||||
| 19.3±1.6 | 17.8±1.6 | 0.506 | Years of use | 12.4±1.0 | 11.6±1.2 | 0.607 |
| 18.9±1.7 | 16.6±1.8 | 0.349 | Recent useb | 18.0±1.4 | 17.1±1.5 | 0.669 |
| 1.7±0.2 | 2.4±0.4 | 0.108 | Grams/day | 1.3±0.2 | 1.1±0.2 | 0.524 |
P<0.05; Reflect Mean ± SEM
Number reflects global PSQI score
Recent use equals the number of days in which stimulant was used in the 30 days.
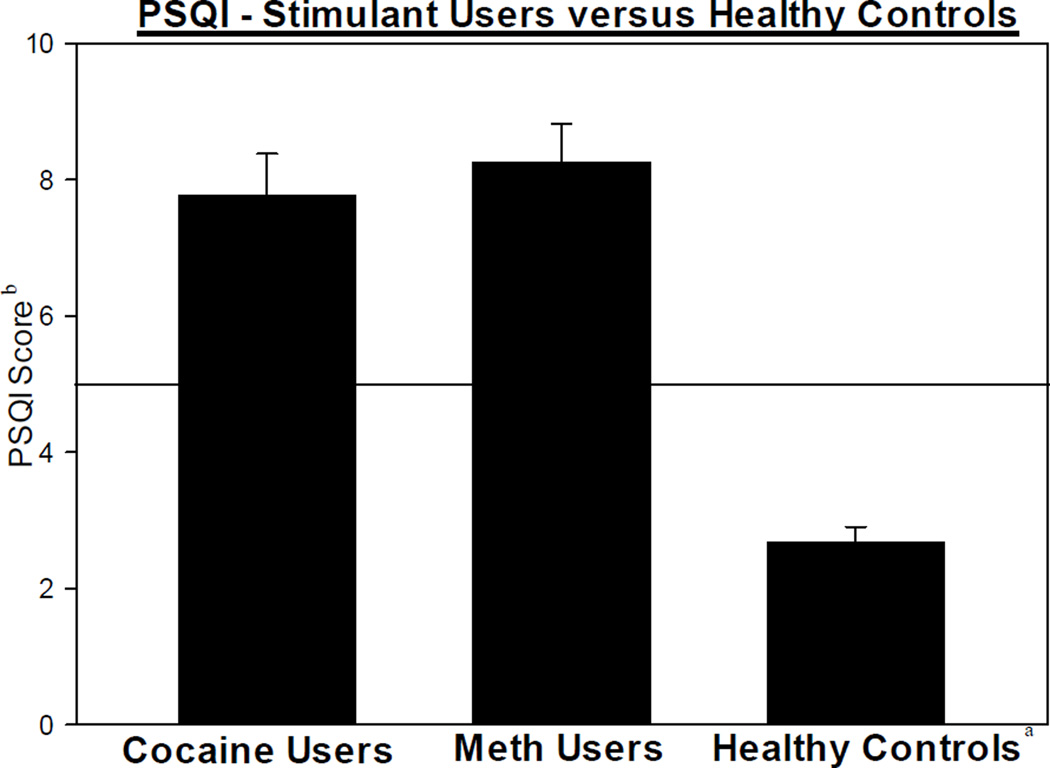
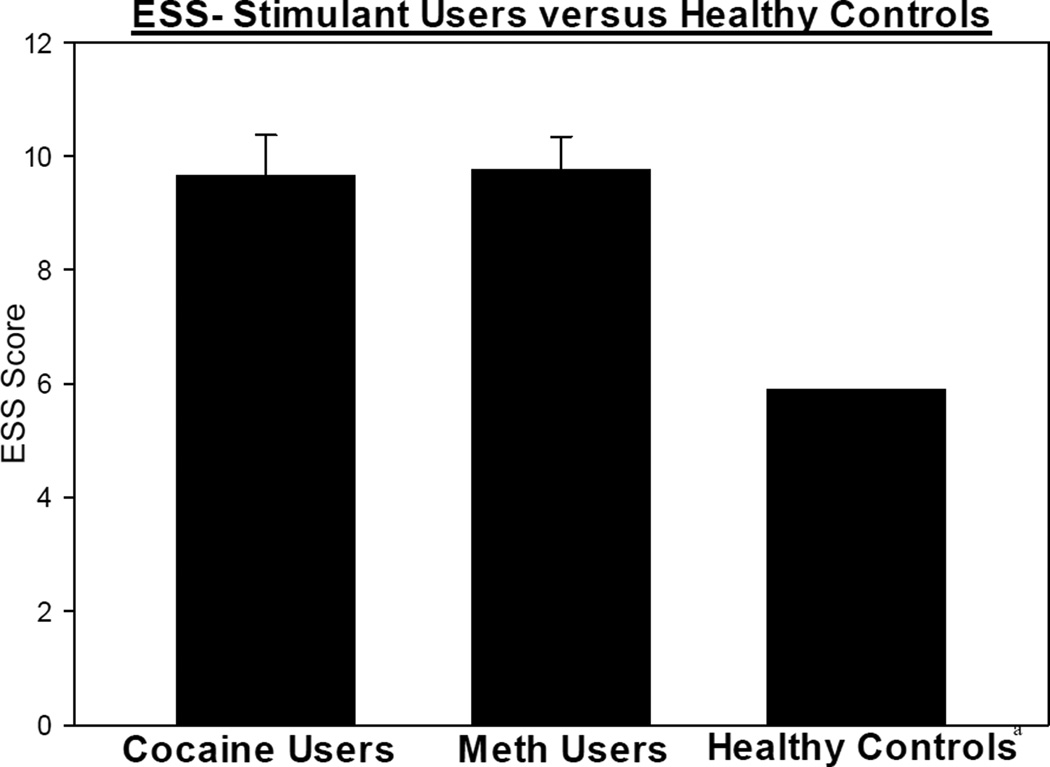
As mentioned earlier, a PSQI score of ≤ 5 indicates normal sleep and, as a comparison point, the mean PSQI score for a healthy adult of similar age is a score 2.7. Thus, participants with cocaine or METH use disorders report poorer sleep quality when compared to controls (Buysse et al., 1989) (Figure 1). Additionally, participants with cocaine or METH use disorders report borderline daytime sleepiness, scoring at the high limit of normal on the ESS. As a comparison point, the mean ESS score for a healthy adult of similar age is 5.9 (Ruggles and Hausman, 2003) (Figure 2). Specifically, in this sample, 67% of participants with a cocaine user disorder were defined as poor sleepers (with a PSQI score > 5) and 51% had elevated daytime sleepiness (with an ESS score > 9) while 66% of participants with a METH use disorder were defined as poor sleepers and 51% had elevated daytime sleepiness.
Figure 1.
PSQI scores in individuals with cocaine and METH use disorders and controlsa
a Control data based off of Buysse et al., 1989 publication
b Number reflects global PSQI score (Mean ± SEM)
Horizontal line distinguishes the cut-off score differentiating good sleep quality (PSQI ≤5) and poor sleep quality (PSQI >5)
Figure 2.
ESS scores in individuals with cocaine and METH use disorders and controlsa
a Control data based off of Ruggles and Hausman, 2003 publication Mean ± SEM
4. Discussion
The results from this study demonstrate that individuals with cocaine or METH use disorders self-reported poor sleep quality for the month preceding the screening interview. These symptoms may have detrimental effects on a number of aspects that are considered crucial for recovery from cocaine or METH use disorders, including detrimental effects on cognitive function. Moreover, there may also be other factors (i.e. comorbid mood disorders, environmental factors, etc.) that may be contributing to or exacerbating these cognitive deficits.
As expected, the results indicate that daytime sleepiness is higher in participants with cocaine or METH use disorders whom also endorse poor sleep quality (this is logical since one of the subscales of the PSQI involves daytime dysfunction, the finding that individuals reporting poorer sleep quality also experience increased daytime sleepiness). A particularly unexpected finding was that cocaine users with fewer sleep deficiencies reported using a greater amount of cocaine per day than those cocaine users with high sleep deficiencies. It was originally hypothesized that individuals who either used more grams per day or used more frequently, would experience greater sleep deficits; however, this was not the case. One potential explanation for this is that the individuals reporting higher or more frequent use may have habituated to these deficits and were not able to accurately self-assess the occurrence of these sleep issues. This would be consistent with potential problems of interoception in these populations often observed through discrepancies between other outcomes like subject-rated and reinforcing effects or self-report and behavioral measures of impulsivity. In addition, the fact that short-term use variables (g/day and days in last month) were associated with PSQI outcomes in cocaine, but not METH users, may be a function of the rapid and short-lived actions of cocaine (T1/2=~ 90min) as compared to METH (T1/2=~11h)".
While the literature on the PSQI and ESS in cocaine and METH users is limited, there have been several reports that other psycho-stimulants, such as MDMA, also have detrimental effects on sleep. In a study of 395 MDMA users, a significant proportion (69.5%) had PSQI scores above the threshold used to identify sleep disturbance. In addition, participants who used larger amounts of ecstasy had poorer sleep (Ogeil et al., 2011). In a separate report of MDMA users, 66.4% of males and 73.5% of females had PSQI scores indicative of poor sleep (>5). However, while females had higher ESS scores as compared to males, both groups had ESS scores below the clinical cut-off of 10 (Ogeil et al., 2013). In a separate study investigating the use of MDMA and sleep medication, the use of MDMA appeared to be associated with the use of sleeping medications; however, it was determined that this association may be accounted for by other factors (Tait et al., 2013). In summary, decreased sleep quality are wide spread across a variety of stimulants, whereas the literature regarding daytime sleepiness is mixed.
In addition, it has long been known that sleep deprivation causes deficits in numerous aspects of cognitive function, including attention/psychomotor vigilance, speech production, language tasks, and decision-making (Kleitman, 1963; Kribbs and Dinges, 1994; Harrison and Horne, 1997, 1998, 1999, 2000). While total sleep deprivation would not be expected in cocaine or METH users, particularly during abstinence, a meta-analysis showed that neurocognitive function was more profoundly affected by partial sleep deprivation than total sleep deprivation (Pilcher and Hufcutt, 1996). Partial sleep deprivation results in elevated daytime sleepiness and may impair response time, short-term recall, working memory, and response inhibition (Durmer and Dinges, 2005). A less studied but equally important mediator of cognitive function, excessive daytime sleepiness, can be the byproduct of poor sleep quality, shortened time spent asleep, or fragmented sleep. Perhaps the best-studied condition of excessive daytime sleepiness is in individuals with sleep apnea (Engelman and Douglas, 2006). Individuals with sleep apnea have average ESS Scores in the range of 13–16 out of 24. Individuals with sleep apnea also experience deficits in attention, learning and memory, and executive function (Kales et al., 1985; Findley et al., 1986). Individuals who abuse cocaine or METH have elevated ESS scores compared to healthy adults, often close to the range of individuals with sleep apnea, and display deficits in the same cognitive domains as those individuals. In addition, mild excessive daytimes sleepiness has been defined as an ESS range of 11–15 while severe excessive daytime sleepiness has been defined as a score of >16. According to this description, of those individuals with a cocaine use disorder, 27% had mild excessive daytime sleepiness while 14% demonstrated severe daytime sleepiness. Of those individuals with a METH use disorder, 34% had mild excessive daytime sleepiness while 13% demonstrated severe daytime sleepiness.
Despite the detailed and informative outcomes presented, some methodological limitations should be noted. First, this research design relied only on self-report measures and did not include any objective assessment of sleep behaviors (i.e. electroencephalogram); notwithstanding, self-reports have demonstrated reliability when confidentiality is ensured (Babor et al., 1990). That being said, there is the possibility that the individual may have not been able to accurately recall the symptoms indicative of poor sleep quality or daytime sleepiness. Furthermore, by obtaining objective measures, we may have been able to determine other factors which may contribute to sleep deficits which we were unable to explore in this manuscript (i.e. how cocaine usage patterns objectively impact the trajectory of TST). Second, while we were able to compared our findings with reference based normative data found in the literature, it would have been beneficial to include a non-drug using control group as well to determine differences between drug using and non-drug using populations. Third, we did not assess whether participants had current or past sleep disorder such as obstructive sleep apnea, restless leg syndrome, narcolepsy or another disorder associated with poor sleep quality which may potentially confound the results. In addition, we did not assess whether any of the participants were rotating shift workers which would have provided more insight as to abnormal sleep schedules. Fourth, as mentioned earlier, cocaine and METH withdrawal can have detrimental effects on sleep including increased awakenings during the night, a reduction in sleep quality, and a reduction in TST (McGregor et al., 2008). Unfortunately, while we do know that the subjects used cocaine within 72 hours of completing the assessments, we cannot confirm whether they were in active withdrawal, thus potentially affecting their responses on the assessments. Despite these limitations, this study demonstrates that there are distinct differences in sleep quality and daytimes sleepiness between individuals with stimulant use disorders and healthy controls. In conclusion, this report confirms that self-reported sleep deficits are present in stimulant users and we postulate that remediation of these deficits may be effective in improving cognitive functioning in these individuals.
Highlights.
-
-
In cocaine users, poor sleepers reported using cocaine for more days out of the past 30
-
-
In cocaine users, good sleepers reported using more grams of cocaine/day
-
-
In METH users, poor sleepers reported higher levels of daytime sleepiness
-
-
Cocaine and METH users had elevated PSQI and ESS scores when compared to controls
Acknowledgments
Funding Source: National Institute on Drug Abuse: DA 018185, DA 014593, DA 017754 and RR 00865
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No author has a financial relationship with commercial interests to report.
References
- Aharonovich E, Amrhein PC, Bisaga A, Nunes EV, Hasin DS. Cognition, commitment language, and behavioral change among cocaine-dependent patients. Psychology of Addictive Behavaviors. 2008;22:557–562. doi: 10.1037/a0012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug and Alcohol Dependence. 2003;71:207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Brown J, Del Boca FK. Validity of self-reports in applied research on addictive behaviors: Fact or fiction? Behavioral Assessment. 1990;12:5–31. [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a New Instrument for Psychiatric Practice and Research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Engelman H, Douglas N. Sleep 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2006;59:618–622. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley LJ, Barth JT, Powers DC, Wilhoit SC, Boyd DG, Suratt PM. Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest. 1986;90:686–690. doi: 10.1378/chest.90.5.686. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Pulvirenti L, Withers N, Golshan S, Koob G. The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: a preliminary report. Biological Psychiatry. 1994;35:843–849. doi: 10.1016/0006-3223(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Sleep deprivation affects speech. Sleep. 1997;20:871–877. doi: 10.1093/sleep/20.10.871. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. Journal of Sleep Research. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. One Night of Sleep Loss Impairs Innovative Thinking and Flexible Decision Making. Organizational Behavior and Human Decision Processes. 1999;78:128–145. doi: 10.1006/obhd.1999.2827. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. Journal of Experimental Psychology. 2000;6:236–249. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Roehrs T, Schuh K, Warbasse L. The effects of cocaine on mood and sleep in cocaine-dependent males. Experimental and Clinical Psychopharmacology. 1999;7:338–346. doi: 10.1037//1064-1297.7.4.338. [DOI] [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. Journal of Clinical and Experimental Neuropsychology. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- Kales A, Caldwell AB, Cadieux RJ, Vela-Bueno A, Ruch LG, Mayes SD. Severe obstructive sleep apnea--II: Associated psychopathology and psychosocial consequences. Journal of Chronic Diseases. 1985;38:427–434. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness. Chicago: The University of Chicago Press; 1963. [Google Scholar]
- Kowatch RA, Schnoll SS, Knisely JS, Green D, Elswick RK. Electroencephalographic sleep and mood during cocaine withdrawal. Journal of Addictive Diseases. 1992;11:21–45. doi: 10.1300/J069v11n04_03. [DOI] [PubMed] [Google Scholar]
- Kribbs N, Dinges D. Vigilance decrement and sleepiness. In: Harsh J, Ogilvie R, editors. Sleep Onset Mechanism. Washington, D.C.: American Psychological Association; 1994. pp. 113–125. [Google Scholar]
- McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM. Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. Journal of Substance Abuse Treatment. 2008;35:334–342. doi: 10.1016/j.jsat.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug and Alcohol Dependence. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Ogeil RP, Rajaratnam SM, Broadbear JH. Male and female ecstasy users: differences in patterns of use, sleep quality and mental health outcomes. Drug and Alcohol Dependence. 2013;132:223–230. doi: 10.1016/j.drugalcdep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Ogeil RP, Rajaratnam SM, Phillips JG, Redman JR, Broadbear JH. Ecstasy use and self-reported disturbances in sleep. Human Psychopharmacology. 2011;26:508–516. doi: 10.1002/hup.1233. [DOI] [PubMed] [Google Scholar]
- Perez AY, Kirkpatrick MG, Gunderson EW, Marrone G, Silver R, Foltin RW, Hart CL. Residual effects of intranasal methamphetamine on sleep, mood, and performance. Drug and Alcohol Dependence. 2008;94:258–262. doi: 10.1016/j.drugalcdep.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher J, Hufcutt A. Effects of sleep deprivation on performanc: a meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Post RM, Gillin JC, Wyatt RJ, Goodwin FK. The effect of orally administered cocaine on sleep of depressed patients. Psychopharmacologia. 1974;37:59–66. doi: 10.1007/BF00426683. [DOI] [PubMed] [Google Scholar]
- Ruggles K, Hausman N. Evaluation of excessive daytime sleepiness. WMJ : official publication of the State Medical Society of Wisconsin. 2003;102:21–24. [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Medicine Reviews. 2008;12:381–389. doi: 10.1016/j.smrv.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecruiber Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonara LI, Keiskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC. Reliability and Validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): According to the SCID-P. European Psychiatry. 1997;12:232–241. [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait RJ, George A, Olesen S. 'Ecstasy' and the use of sleep medications in a general community sample: a 4-year follow-up. Addiction. 2013;108:1640–1648. doi: 10.1111/add.12200. [DOI] [PubMed] [Google Scholar]
- Watson R, Bakos L, Compton P, Gawin F. Cocaine use and withdrawal: the effect on sleep and mood. The American Journal of Drug and Alcohol Abuse. 1992;18:21–28. doi: 10.3109/00952999209001608. [DOI] [PubMed] [Google Scholar]
- Watson R, Hartmann E, Schildkraut JJ. Amphetamine withdrawal: affective state, sleep patterns, and MHPG excretion. American Journal of Psychiatry. 1972;129:263–269. doi: 10.1176/ajp.129.3.263. [DOI] [PubMed] [Google Scholar]