Abstract
This study investigates the outcome of children <10 years old with newly-diagnosed ependymoma treated on the prospective multinational “Head Start” III clinical trial. Between April 2004 and July 2009, 19 children with newly-diagnosed ependymoma were enrolled. All children were to receive five induction chemotherapy cycles followed by one consolidation cycle of myelo-ablative chemotherapy and autologous hematopoietic cell rescue. Children between 6 and 10 years of age or with residual tumor prior to consolidation were to receive irradiation thereafter. Median age of 19 children (8 female) was 20 months at diagnosis. Median follow up was 44 months. The primary site was infratentorial in 11 and supratentorial in 8 patients. Gross total resection was achieved in 10 patients. After induction chemotherapy, all three supratentorial ependymoma patients with residual disease achieved a complete response (CR), while only one of six infratentorial patients with residual disease achieved CR. Three infratentorial patients developed progressive disease during induction chemotherapy. All four infratentorial patients with residual disease who underwent autologous hematopoietic cell transplant, failed to achieve CR. Four patients received focal irradiation following chemotherapy. The 3-year event free survival (EFS) and overall survival (OS) for supratentorial ependymoma were 86 ± 13 % and 100 % respectively. The 3-year EFS and OS for infratentorial ependymoma were 27 ± 13 % and 73 ± 13 % respectively. The role of intensive induction and consolidation chemotherapy in deferring irradiation should be investigated further in children with supratentorial ependymoma with residual disease following surgery. This approach appears ineffective in children with infratentorial ependymoma in the absence of irradiation.
Keywords: Ependymoma, Supratentorial, Infratentorial, Irradiation, Surgery
Introduction
Ependymomas account for about 6–10 % of childhood brain tumors. They are predominantly intracranial, although spinal ependymomas do occur and are more common in adults [1]. The treatment strategy showing the best survival rates in childhood ependymomas has been radical surgery followed by post-operative irradiation [2]. Concerns about the long-term consequences of cranial irradiation have led several groups to evaluate the use of post-operative chemotherapy to delay or eliminate the need for irradiation especially in the younger children [3–5]. Unfortunately, the results are largely disappointing, and patients with incompletely resected tumors, metastatic disease, and the youngest patients continue to fare poorly.
The majority of pediatric ependymomas are infratentorial (60–70 %) and these tend to be more difficult to resect completely and also have an increased likelihood of presenting with metastatic disease. There is a trend among most studies suggesting a worse prognosis for infratentorial tumors versus supratentorial tumors [3, 6, 7]. Several groups have identified genetic differences between supratentorial and infratentorial ependymomas that may play a role in their behavior [8–10]. Furthermore, completely resected supratentorial ependymomas have an excellent prognosis irrespective of histology and several studies have suggested that resection followed by observation alone may be sufficient therapy for this subset [11–13].
The “Head Start” III is a multi-national, multi-institutional prospective clinical trial that enrolled young children (<10 years old) with malignant brain tumors, with the intent of reducing or eliminating irradiation using age and response-based criteria. In this report, we present the outcomes of young children with newly diagnosed ependymoma enrolled on the “Head Start” III protocol.
Methods
Eligibility
Children younger than 36 months of age with localized infratentorial ependymoma, and less than 10 years of age with metastatic infratentorial ependymoma were eligible for this study. In addition, children with supratentorial ependymoma under 10 years of age were eligible. Children with localized supratentorial ependymoma with WHO grade II histology, who underwent complete surgical resection were excluded from the study.
Staging
All patients underwent maximal possible surgical resection at diagnosis. After surgical resection, extent of disease evaluation was performed using brain and whole spine magnetic resonance imaging (MRI) with and without gadolinium, and lumbar cerebrospinal fluid (CSF) analysis on or after postoperative day 14. Gross total resection (GTR) was defined as no visible tumor on postoperative imaging studies. Metastatic disease was classified as M0, M1, M2, and M3, based on previously published criteria [5]. Central review of imaging reports was performed to determine the degree of resection. Central review of pathology was performed retrospectively by two pathologists (A. J and S. Z).
Treatment
Induction chemotherapy consisted of five cycles administered at 21–28 day intervals. Cisplatin (3.5 mg/kg) on day 0, etoposide (4 mg/kg/day) followed by cyclophosphamide (55 mg/kg/day) with mesna on days 1 and 2, methotrexate 270 mg/kg on day 3 followed by leucovorin rescue, were administered during cycles 1, 3, and 5. Oral etoposide (1.65 mg/kg/day) once daily from day 0 to day 9, oral temozolomide (6.5 mg/kg/day) once daily from day 0 to day 4, and intravenous cyclophosphamide (55 mg/kg/day) with mesna on days 10 and 11 were administered during cycles two and four. Vincristine (0.05 mg/kg) was administered on days 0, 7, 14 during cycles 1, 2, and 3 only. Hematopoietic progenitor cell collection by peripheral blood or bone marrow harvest was undertaken following cycles 1 or 3. Disease evaluation was performed at the end of induction. Those with evidence of progressive disease were removed from protocol therapy. Patients with radiographic evidence of residual tumor were strongly considered for second look surgery.
Consolidation chemotherapy consisted of carboplatin (500 mg/m2/day) on days −8 to −6, thiotepa (300 mg/m2/day) and etoposide (250 mg/m2/day) on days −5 to −3. The dose of carboplatin was adjusted using the Calvert formula to achieve an area under the curve of 7 mg/ml/min. Autologous hematopoietic progenitor cells were reinfused on day 0. Recombinant granulocyte-colony stimulating factor (G-CSF) 5 mcg/kg was started on day 1 following progenitor cell infusion.
Radiation therapy
Patients less than 6 years of age received irradiation only if they had evidence of residual disease at the end of induction. All patients 6–10 years of age received irradiation after recovery from consolidation chemotherapy. Irradiation was administered after day 42 following consolidation and consisted of conformal focal irradiation to 59 Gy. In addition to focal irradiation, patients with disseminated disease at diagnosis received 24 Gy craniospinal irradiation if there was no residual disease at the end of induction, and 36 Gy craniospinal irradiation if there was evidence of residual disease at the end of induction.
Response criteria
Criteria for response by radiological studies (MRI with and without gadolinium) were similar to previously published studies [5], and patients were classified as having a complete response (CR), continuing complete response (CCR), partial response (PR), minor response (MR), stable disease (SD), or progressive disease (PD).
Follow-up imaging
Magnetic resonance imaging of the brain with and without gadolinium contrast was performed every 3 months until 2 years following the end of treatment; then every 4 months until 3 years, then every 6 months until 4 years, and annually thereafter.
Informed consent
Signed informed consent was obtained from parents/legal guardians and assent from the child where appropriate as per institutional guidelines. Prior institutional review board approval was required at treating institution before enrolling any child on study. The trial was registered with the ClinicalTrials.gov as NCT00392886.
Statistical considerations
The Kaplan–Meier method was used to estimate the survival probability. Event-free survival (EFS) was defined as the time from the date of diagnosis to progression, relapse, or death from any cause. Overall survival (OS) was defined as time from the date of diagnosis to death.
Results
Patient demographics
Nineteen children less than 10 years of age with ependymoma were enrolled between 2004 and 2009 from 11 institutions (Table 1). Almost three quarters of the patients were less than 3 years of age. The primary tumor location was infratentorial in 11 patients. Four patients with infratentorial tumor had metastatic lesion(s) at diagnosis; spinal cord in three, and intracranial in one. Central review of pathology was completed in 18 patients. Tumor from one patient with infratentorial ependymoma originally diagnosed as anaplastic (WHO grade III) was reclassified as cellular (WHO grade II) pathology after central review. Overall, seven of eight patients with supratentorial tumors and 5 out of 11 infratentorial tumors had anaplastic histology. Lumbar CSF was negative for tumor cells in all patients.
Table 1.
Patient characteristics (n = 19)
| n (%) | |
|---|---|
| Age at diagnosis | |
| ≤ 35 months | 14 (73) |
| 36–71 months | 3 (16) |
| > 72 months | 2 (11) |
| Sex | |
| Female | 8 (42) |
| Male | 11 (58) |
| Site | |
| Infratentorial | 11 (58) |
| Supratentorial | 8 (42) |
| M stage at diagnosis | |
| M0 | 15 (79) |
| M3 | 4 (21) |
| Extent of resection | |
| Gross total resection | 10 (53) |
| Partial or subtotal resection | 9 (47) |
| Histology | |
| Anaplastic | 12 (63) |
| Cellular | 7 (37) |
Outcome
Supratentorial ependymoma
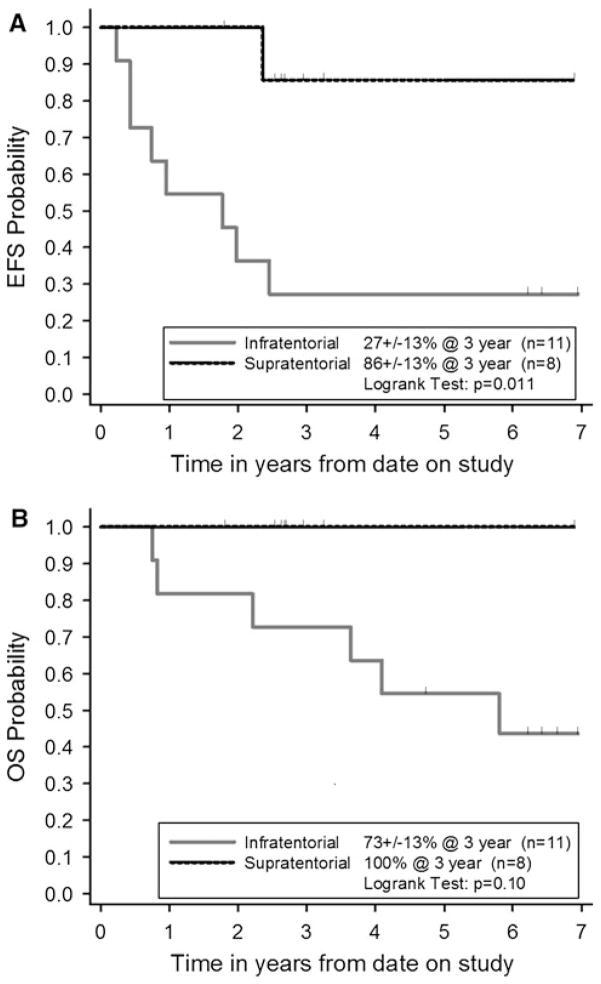
Of the eight supratentorial ependymoma patients, three had residual tumor after surgery (Table 2). All of them achieved a CR by the end of induction. One patient who was greater than 6 years of age at diagnosis received focal irradiation after consolidation. The tumor recurred in another patient 29 months after diagnosis and was treated by surgical resection followed by focal irradiation. The median follow-up of supratentorial ependymoma patients was 33 months (range 16–77 months). The 3-year EFS and OS for patients with supratentorial ependymomas were 86 ± 13 % and 100 % respectively (Fig. 1).
Table 2.
Treatment and Outcome of Supratentorial Ependymoma
| Patient | Age (months) | M | Extent of resection | Status following induction | Status following AHPCR | RT | Relapse | Time to relapse (months) | Outcome | Time to death or last follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | M0 | GTR | CCR | CCR | – | N | – | NED | 31+ |
| 2 | 18 | M0 | GTR | CCR | CCR | – | N | – | NED | 39+ |
| 3 | 19 | M0 | GTR | CCR | CCR | – | N | – | NED | 16+ |
| 4 | 28 | M0 | GTR | CCR | CCR | – | N | – | NED | 22+ |
| 5 | 46 | M0 | GTR | CCR | CCR | F(r) | Y | 29 | NED | 35+ |
| 6 | 18 | M0 | STR | CR | CCR | – | N | – | NED | 24+ |
| 7 | 64 | M0 | STR | CR | CCR | – | N | – | NED | 40+ |
| 8 | 75 | M0 | STR | CR | CCR | F(c) | N | – | NED | 74+ |
AHPCR autologous hematopoietic progenitor cell rescue, RT radiotherapy, GTR gross total resection, CCR continuing complete response, N no, NED no evidence of disease, F(r) focal irradiation following relapse/progression, Y yes, STR subtotal resection, F(c) focal irradiation following recovery from consolidation therapy, CR complete response
Fig. 1.
Kaplan–Meier estimates of a Event- free survival (EFS) and b Overall survival (OS)
Infratentorial ependymoma
Of the seven patients with localized infratentorial tumors, GTR was achieved in four patients, and three patients had residual tumor after the initial surgery (Table 3). Of the four patients with GTR, one developed a new lesion at the end of induction while the other three had CCR. Of the three patients with residual tumor after initial surgery, one CR, one PR and one PD were observed at the end of induction chemotherapy. Overall five of seven patients with localized infratentorial tumors developed progressive or recurrent disease either during or after completion of treatment. The two patients who did not have any events had both received focal irradiation off-protocol following consolidation as part of initial therapy.
Table 3.
Treatment and Outcome of Infratentorial Ependymoma
| Patient | Age (months) | M | Extent of resection | Status following induction | Status following AHPCR | RT | Relapse | Time to relapse (months) | Outcome | Time to death or last follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | M0 | GTR | CCR | – | F (c) | N | – | NED | 82+ |
| 2 | 30 | M0 | GTR | CCR | CCR | F (c) | N | – | NED | 78+ |
| 3 | 9 | M0 | GTR | CCR | CCR | F (r) | Y | 31 | NED | 82+ |
| 4 | 2 | M0 | GTR | PD | – | – | Y | 6 | DOD | 10 |
| 5 | 20 | M0 | STR | CR | CCR | F (r) | Y | 22 | DOD | 71 |
| 6 | 19 | M0 | STR | PR | PR | – | Y | 9 | DOD | 11 |
| 7 | 20 | M0 | Partial | PD | – | F (r) | Y | 4 | A, SD | 59+ |
| 8 | 41 | M3 | GTRa | PD | – | F (r) | Y | 6 | DOD | 46 |
| 9 | 20 | M3 | STRa | PR | PR | CSI (r) | Y | 12 | DOD | 51 |
| 10 | 23 | M3 | STRa | PR | PR | – | Y | 24 | DOD | 28 |
| 11 | 85 | M3 | Partiala | SD | SD | CSI (c) | N | – | NED | 66+ |
AHPCR autologous hematopoietic progenitor cell rescue, RT radiotherapy, GTR gross total resection, CCR continuing complete response, N no, NED no evidence of disease, F(r) focal irradiation following relapse/progression, Y yes, F(c) focal irradiation following recovery from consolidation therapy, CR complete response, DOD dead of disease, PD progressive disease, PR partial response, STR subtotal resection, SD stable disease, A alive, CSI (r) craniospinal irradiation following relapse/progression, CSI (c) craniospinal irradiation following recovery from consolidation therapy
Extent of resection of the primary
Of the four patients with metastatic infratentorial tumors, two PR, one SD and one PD were observed in response to chemotherapy. One patient who was greater than 6 years of age at diagnosis received craniospinal irradiation, and was reported alive 4 years following diagnosis. The other three patients died due to progressive disease. The median follow-up of infratentorial ependymoma patients was 59 months (range 10–82 months). The 3-year EFS and OS for children with infratentorial tumors were 27 ± 13 % and 73 ± 13 % respectively (Fig. 1). The 5-year EFS and OS for children with infratentorial tumors were 27 ± 13 % and 55 ± 15 % respectively. All children who were alive at last follow up had received radiation therapy either as part of initial therapy prior to relapse or at the time of tumor relapse or progression.
Toxicity
There were no toxic deaths. The most common grade 3 or 4 toxicities were neutropenia with or without associated fever, anemia, leucopenia, thrombocytopenia and oropharyngeal mucositis. There were no unexpected toxicities or undue delays in administering chemotherapy due to toxicity.
Discussion
We have presented the results of 19 children with intracranial ependymomas treated on the “Head Start” III prospective clinical trial. Survival differences among supratentorial and infratentorial tumors suggest that the clinical and biological behavior of ependymoma is determined by its location. This is consistent with other published results whereby patients with ependymomas arising in supratentorial locations have demonstrated a better prognosis when compared to patients with infratentorial ependymomas [3, 6, 7]. However, some studies have failed to show a difference [2, 14]. This clinical difference is likely not fully explained by the increased resectability of supratentorial tumors [15]. Even though ependymomas arising in both locations are morphologically similar, recent studies have shown that they have significant biological differences. Supratentorial ependymoma express more neuronal markers when compared to infratentorial ependymoma; in particular, neurofilament protein, light polypeptide expression is associated with better prognosis in supratentorial tumors [8]. Supratentorial ependymomas also demonstrate more frequent deletions on chromosome 9 when compared to infratentorial tumors [9].
Results for supratentorial ependymoma patients in the current study are significantly better when compared to prior “Head Start” studies even though children with completely resected parenchymally based WHO grade II supratentorial ependymoma, a subset with known good prognosis, were excluded from this, but not prior “Head Start” studies [5]. We did include similar patients with anaplastic histology, based on studies which suggested anaplastic histology to be a poor prognostic factor [2, 16]. However, it should be noted that the reproducibility of histopathological grading of ependymoma is poor, and there is wide variation among the reported distribution of anaplastic and WHO grade II tumors between different institutions [15, 17]. Major differences in chemotherapy in this trial, when compared to previous “Head Start” studies, were inclusion of high-dose methotrexate for all patients, and the use of oral temozolomide and oral etoposide within two relatively less dose intensive courses alternating with the three methotrexate-containing induction courses. There were no toxic deaths in this study as compared to previous studies [5]. Our results are consistent with the almost 54 % response rate seen overall in the Children’s Cancer Group 9942 study [18], although it was not reported how many of the responders had supratentorial tumors. Patients with completely resected anaplastic supratentorial ependymoma who were observed without further therapy have done well in other studies [11, 13], and as such we do not know how much chemotherapy played a role in the favorable outcome of the five patients with completely resected supratentorial ependymoma in the current study. Although three patients with residual supratentorial tumors achieved a CR to induction therapy, this was not centrally reviewed, and one of them went on to receive radiation therapy. Therefore, it is difficult to draw any definitive conclusion about chemotherapy efficacy. In addition, improvement in neurosurgical techniques and supportive care may have played a role in achieving a better outcome.
The 3-year EFS and OS for children with infratentorial ependymoma on “Head Start” I and II studies were 5 ± 5 % and 42 ± 12 % respectively [5]. These results are not significantly different from the current study. The better EFS for infratentorial patients in this study likely reflects the fact that three times as many patients with infratentorial tumors received irradiation upfront on the current study compared with the previous “Head Start” studies. Our current study reinforces the importance of irradiation in children with infratentorial ependymoma. The question of whether irradiation can be delayed in very young patients by administration of initial chemotherapy remains unanswered. Three of 11 patients with infratentorial ependymoma progressed during induction chemotherapy. Duffner et al. [19] after analyzing the long term results of the “Baby POG I” study concluded that in children with ependymoma, delay of irradiation of more than 1 year adversely affected survival. Best results using chemotherapy to delay or avoid irradiation were reported from CNS9204 study [4]. In that study, children younger than 3 years of age with ependymoma received alternative blocks of myelosuppressive and non-myelosuppressive chemotherapy every 14 days for 1 year. The majority of patients had infratentorial Ependymoma. EFS of 41.8 % and OS of 63.4 % were achieved at 5 years; 42 % of the survivors did not receive radiation therapy. Other groups have questioned the need for delaying radiation therapy with chemotherapy. EFS of 71 % and OS of 84 % at 5 years was reported using a strategy of maximal surgical resection followed by immediate postoperative conformal radiation therapy in all children regardless of age by Merchant et al. [2]. Excellent long term functional outcomes have been reported in these children recently [20, 21].
In conclusion, the role of dose intensive induction and consolidation chemotherapy should be further investigated in patients with supratentorial ependymoma with evidence of residual disease following initial surgery. Six of eight patients with supratentorial ependymoma avoided irradiation. Unfortunately, the results were not encouraging for patients with infratentorial Ependymoma. New therapies must be developed in order to improve the outcome and potentially spare the need for irradiation in young patients with infratentorial ependymoma especially those with residual or metastatic disease.
Acknowledgments
Funding Alex’s Lemonade Stand Foundation; Pediatric Cancer Research Foundation; Soccer for Hope Foundation; Isabella Grace Jordan Fund.
Footnotes
Conflict of interest None.
Contributor Information
Rajkumar Venkatramani, Email: rvenkatramani@chla.usc.edu, Division of Hematology/Oncology, Children’s Hospital Los Angeles, 4650, Sunset Boulevard, Mailstop 54, Los Angeles, CA 90027, USA.
Lingyun Ji, University of Southern California, Los Angeles, CA, USA.
Joseph Lasky, Harbor-UCLA Medical Center, Torrance, CA, USA.
Kelley Haley, Division of Hematology/Oncology, Children’s Hospital Los Angeles, 4650, Sunset Boulevard, Mailstop 54, Los Angeles, CA 90027, USA.
Alexander Judkins, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Shengmei Zhou, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Richard Sposto, Division of Hematology/Oncology, Children’s Hospital Los Angeles, 4650, Sunset Boulevard, Mailstop 54, Los Angeles, CA 90027, USA.
Randal Olshefski, Nationwide Children’s Hospital, Columbus, OH, USA.
James Garvin, Columbia-Presbyterian, New York, NY, USA.
Tanya Tekautz, Cleveland Clinic, Cleveland, OH, USA.
Gloria Kennedy, SUNY Upstate, Syracuse, NY, USA.
Shahrad Rod Rassekh, British Columbia’s Children’s Hospital, Vancouver, BC, Canada.
Theodore Moore, Mattel Children’s Hospital at UCLA, Los Angeles, CA, USA.
Sharon Gardner, New York Langone Medical Center, New York, NY, USA.
Jeffrey Allen, New York Langone Medical Center, New York, NY, USA.
Richard Shore, Children’s Mercy Hospital, Kansas City, MI, USA.
Christopher Moertel, University of Minnesota, Minneapolis, MN, USA.
Mark Atlas, Schneider Children’s, New Hyde Park, NY, USA.
Girish Dhall, Division of Hematology/Oncology, Children’s Hospital Los Angeles, 4650, Sunset Boulevard, Mailstop 54, Los Angeles, CA 90027, USA.
Jonathan Finlay, Division of Hematology/Oncology, Children’s Hospital Los Angeles, 4650, Sunset Boulevard, Mailstop 54, Los Angeles, CA 90027, USA.
References
- 1.Zacharoulis S, Moreno L. Ependymoma: an update. J of Child Neurol. 2009;24:1431–1438. doi: 10.1177/0883073809339212. [DOI] [PubMed] [Google Scholar]
- 2.Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol. 2009;10:258–266. doi: 10.1016/S1470-2045(08)70342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grill J, Le Deley M-C, Gambarelli D, et al. Postoperative chemotherapy without irradiation for ependymoma in children under 5 years of Age: a multicenter trial of the French Society of Pediatric Oncology. J Clin Oncol. 2001;19:1288–1296. doi: 10.1200/JCO.2001.19.5.1288. [DOI] [PubMed] [Google Scholar]
- 4.Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol. 2007;8:696–705. doi: 10.1016/S1470-2045(07)70208-5. [DOI] [PubMed] [Google Scholar]
- 5.Zacharoulis S, Levy A, Chi SN, et al. Outcome for young children newly diagnosed with ependymoma, treated with intensive induction chemotherapy followed by myeloablative chemotherapy and autologous stem cell rescue. Pediatr Blood Cancer. 2007;49:34–40. doi: 10.1002/pbc.20935. [DOI] [PubMed] [Google Scholar]
- 6.Perilongo G, Massimino M, Sotti G, et al. Analyses of prognostic factors in a retrospective review of 92 children with ependymoma: Italian Pediatric Neuro-oncology Group. Med Pediatr Oncol. 1997;29:79–85. doi: 10.1002/(sici)1096-911x(199708)29:2<79::aid-mpo3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 7.Pollack IF, Gerszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery. 1995;37:655–666. doi: 10.1227/00006123-199510000-00008. (Discussion 666–667) [DOI] [PubMed] [Google Scholar]
- 8.Andreiuolo F, Puget S, Peyre M, et al. Neuronal differentiation distinguishes supratentorial and infratentorial childhood ependymomas. Neuro Oncol. 2010;12:1126–1134. doi: 10.1093/neuonc/noq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider D, Monoranu C-M, Huang B, et al. Pediatric supratentorial ependymomas show more frequent deletions on chromosome 9 than infratentorial ependymomas: a microsatellite analysis. Cancer Genet Cytogenet. 2009;191:90–96. doi: 10.1016/j.cancergencyto.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Hukin J, Epstein F, Lefton D, Allen J. Treatment of intracranial ependymoma by surgery alone. Pediatr Neurosurg. 1998;29:40–45. doi: 10.1159/000028683. [DOI] [PubMed] [Google Scholar]
- 12.Palma L, Celli P, Mariottini A, Zalaffi A, Schettini G. The importance of surgery in supratentorial ependymomas. Child’s Nerv Syst. 2000;16:170–175. doi: 10.1007/s003810050487. [DOI] [PubMed] [Google Scholar]
- 13.Venkatramani R, Dhall G, Patel M, et al. Supratentorial ependymoma in children: to observe or to treat following gross total resection? Pediatr Blood Cancer. 2012;58:380–383. doi: 10.1002/pbc.23086. [DOI] [PubMed] [Google Scholar]
- 14.McGuire CS, Sainani KL, Fisher PG. Both location and age predict survival in ependymoma: a SEER study. Pediatr Blood Cancer. 2009;52:65–69. doi: 10.1002/pbc.21806. [DOI] [PubMed] [Google Scholar]
- 15.Robertson PL, Zeltzer PM, Boyett JM, et al. Survival and prognostic factors following radiation therapy and chemotherapy for ependymomas in children: a report of the Children’s Cancer Group. J Neurosurg. 1998;88:695–703. doi: 10.3171/jns.1998.88.4.0695. [DOI] [PubMed] [Google Scholar]
- 16.Horn B, Heideman R, Geyer R, et al. A multi-institutional retrospective study of intracranial ependymoma in children: identification of risk factors. J Pediatr Hematol Oncol. 1999;21:203–211. doi: 10.1097/00043426-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. doi: 10.1186/1477-5751-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garvin JH, Jr, Selch mt, Holmes E, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children’s Cancer Group protocol 9942: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012;59:1183–1189. doi: 10.1002/pbc.24274. [DOI] [PubMed] [Google Scholar]
- 19.Duffner PK, Horowitz ME, Krischer JP, et al. The treatment of malignant brain tumors in infants and very young children: an update of the Pediatric Oncology Group experience. Neuro oncol. 1999;1:152–161. doi: 10.1093/neuonc/1.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Pinto M, Conklin HM, Li C, Xiong X, Merchant TE. Investigating verbal and visual auditory learning after conformal radiation therapy for childhood ependymoma. Int J Radiat Oncol Bio Phys. 2010;77:1002–1008. doi: 10.1016/j.ijrobp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int J Radiat Oncol Bio Phys. 2012;84:217–223. doi: 10.1016/j.ijrobp.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]