Abstract
Objective
To describe patterns of care for pediatric patients with advanced heart disease who experience inhospital death.
Design
Retrospective single-institution medical record review.
Setting
A tertiary care pediatric hospital.
Participants
All patients younger than 21 years who died in the inpatient setting between January 1, 2007, and December 31, 2009, with primary cardiac diagnoses or who had ever received a cardiology consult (N = 468). After excluding patients with significant noncardiac primary diagnoses, 111 children formed the analytic sample.
Main Outcome Measure
In-hospital deaths of children with heart disease during a 3-year period.
Results
Median age at death was 4.8 months (age range, 1 day to 20.5 years), with 84 deaths (75.7%) occurring before age 1 year. Median length of terminal hospital stay was 22 days (range, 1–199 days). Diagnoses included 84 patients (75.7%) with congenital heart disease, 10 (9.0%) with cardiomyopathy/myocarditis, 9 (8.1%) with pulmonary hypertension, and 8 (7.2%) with heart transplants. Sixty-two patients (55.9%) had received cardiopulmonary resuscitation during their last hospital admission. At the end of life, 21 children (18.9%) had gastrostomy tubes and 26 (23.4%) had peritoneal drains. Most patients (91.9%) received ventilation, with half also receiving mechanical circulatory support. Eighty-three patients (74.8%) experienced additional end-organ failure. Classified by mode of death, 76 patients (68.5%) had disease-directed support withdrawn, 28 (25.2%) died during resuscitation, and 7 (6.3%) died while receiving comfort care after birth. Eighty-three percent of parents were present at the time of death.
Conclusion
Infants and children who die of advanced heart disease frequently succumb in the intensive care setting with multisystem organ failure and exposure to highly technical care.
Significant advances in the medical and surgical care of infants and children with complex cardiac disease have led to increased survival rates. Overall mortality from congenital heart disease declined 40% between 1979 and 1997 and 25% between 1999 and 2006.1,2
Despite dramatically improved short- and long-term outcomes, however, advanced heart disease (AHD) remains one of the leading causes of nonaccidental death in childhood in the United States.3 Progression to AHD in children from either cardiomyopathy or palliated congenital heart disease often results in long-term intensive medical management, recurrent hospitalization, or both. The mortality associated with pediatric AHD stems from its sequelae, including low cardiac output, respiratory failure, malignant arrhythmias, stroke, thromboembolism, multiorgan dysfunction, and infection.4 Close to half of all mortality due to congenital heart disease occurs in infancy.2 Scant medical literature exists regarding end-of-life care for these children. We therefore sought to describe patterns of care at the end of life for children with AHD who experienced inhospital death.
METHODS
PATIENT POPULATION
After approval by the Children’s Hospital Boston Office of Clinical Investigation institutional review board, the medical records of all patients with AHD who died between January 1, 2007, and December 31, 2009, on the Cardiology Service at Children’s Hospital Boston were retrospectively reviewed. In addition, the medical records of all patients who died elsewhere in the hospital and had received a cardiology consultation were reviewed to ascertain whether AHD was the primary cause of death (N = 468). Patients were excluded if they (1) died outside the hospital, (2) were older than 21 years at the time of death, or (3) had a primary diagnosis of severe prematurity, active cancer, sepsis, congenital diaphragmatic hernia, or sudden infant death syndrome. The final sample size for record review was 111 patients.
DATA EXTRACTION AND STATISTICAL ANALYSIS
Patient medical records were reviewed for demographic information, including age at death, sex, state of residence, and diagnosis. Descriptions of the cardiac diagnosis and genetic disorders were recorded.
Several variables were designed to assess lifetime burden of disease. All procedures billed as operations were reviewed and categorized as definitive operations (conducted in an operating room and disease directed) or as bedside operations (eg, mediastinal washout, chest closure, thoracotomy, and extracorporeal membrane oxygenation cannulation). In addition to the standard disease-directed surgical procedures, the following procedures were defined as definitive if they were conducted in an operating room: pacemaker insertion, diaphragm plication, and shunt revision. Total numbers of tracheostomy, gastrostomy, and peritoneal drain placements and catheterizations were recorded; biopsy procedures were not counted as catheterizations unless they were accompanied by additional procedures. End-of-life care indicators are described as follows. Location of death, length of last hospital stay, and presence of family at the bedside at the time of death were obtained from final hospital discharge summaries. In addition, documentation of end-of-life discussions with family and palliative care consults were identified by the use of any of the following terms in the medical record in the context of communication with family members: resuscitation status, redirection of care, withdrawal of care/intervention, withholding of care/intervention, and comfort care. Types of mechanical ventilation, medications, feeding, mental status, and mechanical cardiac support (extracorporeal membrane oxygenation, ventricular assist device, and cardiopulmonary bypass) within 24 hours of death were recorded.
Mode of death was defined based on the following criteria: (1) withdrawal of disease-directed care: the patient was declared brain dead or was receiving life support that was discontinued; (2) died during resuscitation: the patient was undergoing cardiopulmonary resuscitation when a decision was made to cease; and (3) comfort care after birth: the patient never received any cardiac-directed therapy except prostaglandin E2. Primary and secondary causes of death were coded by a single cardiologist (E.D.B.) based on organ systems affected and on conditions such as sepsis, capillary leak syndrome, and graft rejection. Multisystem organ failure was defined as evidence of end-organ failure of at least 2 organ systems, excluding the heart. Continuous variables are summarized using the median and range, and categorical variables using frequencies and percentages.
RESULTS
PATIENT DEMOGRAPHICS
The median age at death of the 111 patients in the study group was 4.8 months (age range, 1 day to 20.5 years), with 84 deaths (75.7%) occurring in patients younger than 1 year, 15 (13.5%) in those aged 2 to 10 years, 8 (7.2%) in those aged 11 to 17 years, and 4 (3.6%) in those aged 18 to 20 years. Patient characteristics of the study group are summarized in the Table. Patients had a median duration of last hospital stay of 22 days, with a wide range (1–199 days). A little more than half of the deaths occurred in patients from families that resided in state, and another 23% were regional. Seventeen percent of the families were from outside the region. Most patients died in the cardiac intensive care unit setting.
Table.
Characteristics of 111 Pediatric Patients With Advanced Heart Disease
| Characteristic | Value |
|---|---|
| Age at death, median (range) | 4.8 mo (1 d–20.5 y) |
| Length of last hospital stay, median (range), d | 22 (1–199) |
| Sex, No. (%) | |
| Male | 57 (51.4) |
| Female | 54 (48.6) |
| Cardiac diagnosis, No. (%) | |
| Congenital heart disease | 84 (75.7) |
| Cardiomyopathy | 10 (9.0) |
| Transplant | 8 (7.2) |
| Pulmonary hypertension | 9 (8.1) |
| Location of death, No. (%) | |
| Cardiac ICU | 97 (87.4) |
| Medical ICU | 6 (5.4) |
| Hospital floor | 6 (5.4) |
| OR/catheterization laboratory | 2 (1.8) |
| Referral, No. (%) | |
| Massachusetts | 63 (56.8) |
| Other northeasta | 26 (23.4) |
| Other United Statesb | 19 (17.1) |
| International | 3 (2.7) |
Abbreviations: ICU, intensive care unit; OR, operating room.
Other northeast consists of Connecticut, Maine, New Hampshire, Rhode Island, and Vermont.
Other United States indicates all other states except the northeast.
Eighty-four patients (75.7%) had diagnoses of congenital heart disease, and almost one-quarter of all patients had confirmed genetic diagnoses. Of the 84 patients who died of congenital heart disease, almost half (40.5%) had single-ventricle physiologic features, with half of those being hypoplastic left heart syndrome. The remaining patients had complete atrioventricular canal (11.9%), Tetralogy of Fallot/double outlet right ventricle (11.9%), truncus arteriosus (7.1%), pulmonary vein stenosis (7.1%), pulmonary atresia with an intact ventricular septum (3.6%), transposition of the great vessels (3.6%), and other anomalies (14.3%). Of the 24% of patients who did not have congenital heart disease, 10 had cardiomyopathy, 9 had pulmonary hypertension, and 8 had undergone heart transplantation.
BURDEN OF DISEASE AND END-OF-LIFE EXPERIENCE
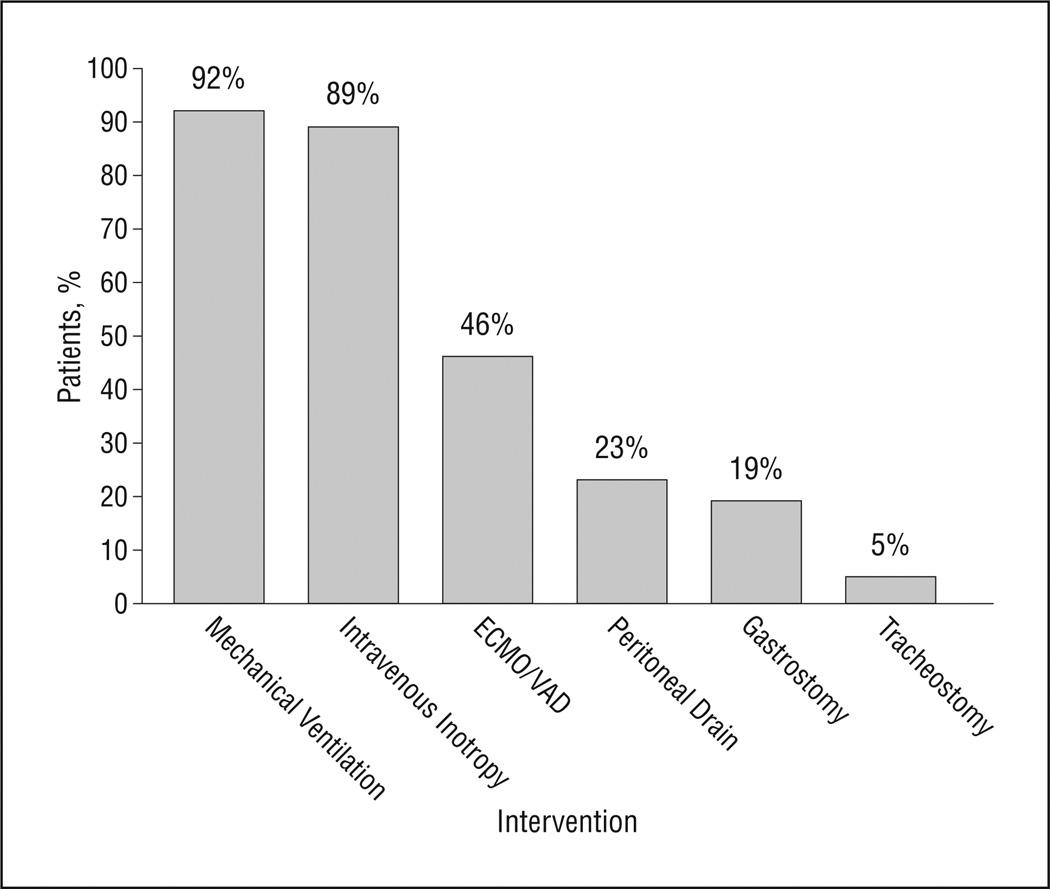
The median lifetime number of catheterizations was 2 (range, 0–13), surgical procedures conducted in an operating room was 1 (range, 0–6), and bedside procedures was 1 (range, 0–16). Sixty-two patients (55.9%) experienced cardiopulmonary resuscitation at some point during their last hospital admission. At the end of life, 5 children (4.5%) had tracheostomies, 21 (18.9%) had gastrostomy tubes, and 26 (23.4%) had peritoneal drains. Figure 1 summarizes the intensity of support at the end of life for the study population. Almost all the patients were intubated and sedated, with almost half receiving mechanical circulatory support (extracorporeal membrane oxygenation /ventricular assist device). One hundred patients (90.1%) were completely sedated, and most (102 [91.9%]) received analgesic medications. In addition to their cardiac disease, during the end-of-life care period, 83 patients (74.8%) had evidence of additional end-organ failure at the time of death (18.9% neurologic, 16.2% renal, 16.2% pulmonary, and 6.3% hepatic); 44.6% of patients had multiorgan failure.
Figure 1.
Interventions performed within 24 hours of death. Most patients received highly technological and invasive measures within 1 day of death. ECMO indicates extracorporeal membrane oxygenation; VAD, ventricular assist device.
CAUSE AND TYPE OF DEATH
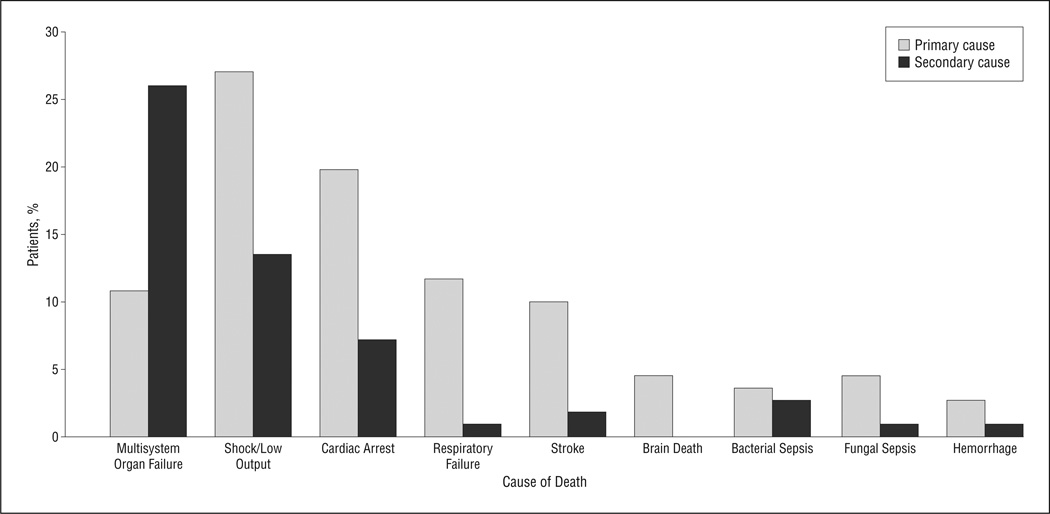
Primary and secondary causes of death are shown in Figure 2. More than half of the patients died of multiorgan system failure. Cardiogenic shock, cardiac arrest, and respiratory failure were the predominant primary causes of death. Primary neurologic causes of death, including stroke and brain death, occurred in an additional 15% of patients. As for mode of death, 76 patients (68.5%) died after discontinuation of disease-directed interventions, 28 (25.2%) died during resuscitation, and 7 (6.3%) died while receiving comfort care after birth. Eighty-three percent of medical records documented parent presence at the bedside at the end of life, and three-quarters demonstrated that an end-of-life care discussion with the family had taken place before death. Sixteen percent of children had received a formal consultation from the pediatric palliative care team.
Figure 2.
Primary and secondary causes of death. Shock/low output is the most common primary cause of death, and multisystem organ failure represents most secondary causes of death.
COMMENT
This study provides novel insights into the patterns of care at the end of life for children with AHD. First, most pediatric deaths associated with AHD occur in the first year of life after a prolonged hospital stay. Second, most children with AHD who die in the hospital do so in an intensive care setting, with nearly half undergoing mechanical circulatory support, almost all (92%) receiving mechanical ventilation, and three-quarters experiencing end-organ failure. Third, in this large tertiary care institution, almost a quarter of the children died a great distance from home. Fourth, this study demonstrates that most patients die after discontinuation of disease-directed care and that another quarter die during active resuscitation. These findings suggest that children with AHD tend to die while receiving highly intensive care.
This population is different from the oncology population, the most common group of children studied at the end of life. Studies5,6 in the oncology population suggest that high-intensity care is associated with significant child suffering. Because most children with cancer do not die in the intensive care unit setting, one hypothesis may be that children with AHD experience even greater suffering at the end of life. Research in children with cancer7,8 has also begun to identify specific ways to improve quality of life and communication surrounding end-of-life care. For example, investigators6 have linked the presence of a primary oncologist who actively coordinates the patient’s care team with improved pain management and promotion of the child’s experience of peacefulness. Another study9 demonstrated that involvement of a pediatric palliative care service is associated with improvements in communication, symptom management, and other important palliative care outcomes.
Several phenomena may account for the limited investigation to date regarding targeted interventions to improve end-of-life care for the pediatric AHD population. First, there is a seemingly common perception that most children with heart disease die a “sudden death” and, therefore, that the “end-of-life” period is short and may be considered not amenable to intervention or improvement. However, based on the present results, children with AHD spend a median of 22 days in the hospital before death, and most patients (69%) die after withdrawal of disease-directed care. Thus, this study suggests that most cardiac deaths in children are not sudden but rather could be anticipated and that advanced care planning may, in fact, be possible in children with AHD. Another difficulty is that the clinical “progression” of heart failure in children is highly variable, with periods of decompensations followed by periods of improvement. This makes the point at which there is “no possibility for long-term survival” often uncertain. This unpredictability may discourage advanced care discussions for children and their families until the time of death is imminent.
The limitations of this study include its retrospective design, its single-center nature, and its variability in documentation at the end of life. In addition, these data do not elucidate the end-of-life experience from the perspective of the patient or family, and, therefore, any inference into the amount of suffering in this population is speculative.
This study demonstrates that infants and children who die of AHD in the current era frequently succumb in the intensive care setting with multisystem organ failure and exposure to highly technical care. Further prospective research is required to address the complex factors that contribute to the care of children with AHD once survival becomes unlikely. Parents of children with AHD will also be an invaluable resource in our endeavor to build a collaborative community to address the palliative care needs, symptom management, and complex decision making of children with AHD and their families and care teams.
Acknowledgments
Funding/Support: This study was supported by the Heart Failure and Transplant Educational Fund (Dr Blume), the Kara Duclos Fund, and the Ghiso Fellowship for Compassionate Medical Care, Harvard Medical School (Ms Morell).
Footnotes
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Morell, Wolfe, Scheurer, Thiagarajan, Morin, Beke, Smoot, and Blume. Acquisition of data: Morell, Morin, Beke, and Blume. Analysis and interpretation of data: Morell, Wolfe, Scheurer, Smoot, Cheng, Gauvreau, and Blume. Drafting of the manuscript: Morell, Scheurer, Thiagarajan, Morin, Beke, and Blume. Critical revision of the manuscript for important intellectual content: Morell, Wolfe, Thiagarajan, Beke, Smoot, Cheng, Gauvreau, and Blume. Statistical analysis: Gauvreau. Obtained funding: Morell and Blume. Administrative, technical, and material support: Blume. Study supervision: Wolfe, Scheurer, Thiagarajan, Beke, Cheng, and Blume.
Financial Disclosure: None reported.
REFERENCES
- 1.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103(19):2376–2381. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 2.Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A. Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation. 2010;122(22):2254–2263. doi: 10.1161/CIRCULATIONAHA.110.947002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125(1):4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal D, Chrisant MR, Edens E, et al. International Society for Heart and Lung Transplantation: practice guidelines for management of heart failure in children. J Heart Lung Transplant. 2004;23(12):1313–1333. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Ullrich CK, Dussel V, Hilden JM, Sheaffer JW, Lehmann L, Wolfe J. End-of-life experience of children undergoing stem cell transplantation for malignancy: parent and provider perspectives and patterns of care. Blood. 2010;115(19):3879–3885. doi: 10.1182/blood-2009-10-250225. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. N Engl J Med. 2000;342(5):326–333. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- 7.Kreicbergs U, Valdimarsdóttir U, Onelöv E, Henter JI, Steineck G. Talking about death with children who have severe malignant disease. N Engl J Med. 2004;351(12):1175–1186. doi: 10.1056/NEJMoa040366. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe J, Klar N, Grier HE, et al. Understanding of prognosis among parents of children who died of cancer: impact on treatment goals and integration of palliative care. JAMA. 2000;284(19):2469–2475. doi: 10.1001/jama.284.19.2469. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe J, Hammel JF, Edwards KE, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol. 2008;26(10):1717–1723. doi: 10.1200/JCO.2007.14.0277. [DOI] [PubMed] [Google Scholar]