Abstract
Using transition metals such as manganese(II), iron(II), cobalt(II), nickel(II), copper(II), and zinc(II), several new metal complexes of cross-bridged tetraazamacrocyclic chelators namely, cyclen- and cyclam-analogs with benzyl groups, were synthesized and screened for in vitro antimalarial activity against chloroquine-resistant (W2) and chloroquine-sensitive (D6) strains of Plasmodium falciparum. The metal-free chelators tested showed little or no antimalarial activity. All the metal complexes of the dibenzyl cross-bridged cyclam ligand exhibited potent antimalarial activity. The Mn2+ complex of this ligand was the most potent with IC50s of 0.127 and 0.157 µM against the chloroquine-sensitive (D6) and chloroquine-resistant (W2) P. falciparum strains, respectively. In general, the dibenzyl hydrophobic ligands showed better antimalarial activity compared to the activity of monobenzyl ligands, potentially because of their higher lipophilicity and thus better cell penetration ability. The higher antimalarial activity displayed by the manganese complex for the cyclam ligand in comparison to that of the cyclen, correlates with the larger pocket of cyclam compared to that of cyclen which produces a more stable complex with the Mn2+. Few of the Cu2+ and Fe2+ complexes also showed improvement in activity but Ni2+, Co2+ and Zn2+ complexes did not show any improvement in activity upon the metal-free ligands for anti-malarial development.
Keywords: Antimalarial agents, metal complexes, tetraazamacrocycles, cross-bridged tetraazamacrocycles, synthesis, biological activity
1. Introduction
Malaria is a major health problem, especially in the developing countries1 of South Saharan Africa, Southeast Asia and in South America. Today, almost all the existing antimalarial drugs, particularly the 4-aminoquinoline based drugs, such as chloroquine (CQ) that have historically been used to treat the disease effectively, have been rendered less effective due to chemo-resistance problems.2 Artemisinin and its derivatives offer promising curative alternatives but due to their thermal instability3,4 and high cost of therapy5 in the economically disadvantaged regions of the world where the disease is prevalent, access to them is difficult, and their therapeutic use has been severely limited.
Due to the prevalence of Plasmodium falciparum strains resistant to chloroquine and other antimalarial drugs, the search for new antimalarial drugs has been of high priority for the control of malaria. Over the past two decades, a number of antimalarial agents, particularly the 4-aminoquinoline-based drugs, have been developed and tested against chloroquine-resistant parasites.6, 7 Although there has been substantial improvement in the conventional organic synthetic strategies used for the development of antimalarial agents, researchers have sought out ways to develop more innovative approaches in order to develop more efficacious drugs to cure the disease. One of the most promising new approaches involves the use of transition metal ion complexes to produce novel antimalarial drugs.8
Metal-based chemotherapies have existed for centuries, but in recent years there has been an increasing interest in the application of transition metal complexes or organometallic complexes in medicine and in other areas of biological sciences.9–12 Metal complexes have been used as drugs in a variety of diseases, as exemplified by the continued success of the platinum complex, cis-PtCl2(NH3)2 (cisplatin), as an anticancer drug.13–15 This important breakthrough has indeed stimulated a renewed interest in metal complex based chemotherapy. Today, other metal-containing drugs have been developed in a variety of therapeutic areas including malaria. To ensure that effective metal containing antimalarial agents are produced, the present study exploited the metal-drug synergism approach.16–19
Thus far, several reports have shown that incorporation of transition metal ions into organic pharmacophores offer new opportunities to design unique metal-containing compounds which compliment the molecular diversity created by purely organic scaffolds.20,21 These reports show that the incorporation of transition metal ions into rationally designed ligands can result in enhancement of the biological activity.22 There are also several reports of enhancement of the efficacy of existing drugs, e.g. chloroquine, when transition metal ions were coordinated to the parent drug structures.23 A thorough literature review revealed that several transition metal complexes exhibit high antimalarial activity against chloroquine-sensitive and –resistant strains of P. falciparum and, consequently, have become antimalarial drug candidates. It is also well documented that many metal complexes of chloroquine or other 4-aminoquinoline based antimalarials have activities superior to that of chloroquine, which is one of the most successful drugs currently being used for antimalarial chemotherapy. The consistent enhancement of these drugs when coordinated to metal ions reinforces the fact that metal complexes are important resources for the generation of structural or chemical diversity in the area of antimalarial drug development.20,23
In a recent report, a gold-chloroquine antimalarial agent was obtained by coordinating chloroquine (CQ) to a [Au(PPh3)]+ fragments to give a new compound, [Au(PPh3)(CQ)]PF6, which is more active than CQ alone in vitro against cultures of chloroquine resistant strains of P. falciparum and also against P. berghei both in vitro and in vivo.23 Also, it has been reported that the coordination complexes of gold(I) and thiosemicarbazone exhibited enhanced antiplasmodial activity against two P. falciparum strains (D10 and W2) in vitro when compared to the activity of the ligand alone.24
Many other enhancements of antimalarial activity by metal complexation of a variety of ligands compared to the antimalarial activity of the free ligands themselves have been extensively documented. For example, a reaction of the chloroquine (CQ) free base with [Rh(COD)Cl]2 (COD = 1,5-cyclooctadiene) yielded Rh(COD)(CQ)Cl, which has comparable in vitro antimalarial activity to that of chloroquine diphosphate and reduced parasitemia 1.33 times more than chloroquine in vivo, without any sign of acute toxicity observed up to 30 days.25,26
It has been documented that because of the avidity of Plasmodium parasites for free iron, one way of using iron in antimalarial drug design is by adding iron to an existing drug such as chloroquine to effectively remove the chloroquine resistance.27–29 Several studies have shown that organometallic complex based drug molecules are very promising in overcoming various types of drug resistance and allow improved specificity and drug targeting, thus minimizing side effects associated with chemotherapy.30 For example, ferrocene serves as a reliable organometallic scaffold for the construction of an ordered structure via hydrogen bonding.31,32 It is worth noting that as a consequence of this drug development effort, the novel antimalarial drug, ferroquine (FQ), in which ferrocene group is incorporated into chloroquine structure, is being developed at Sanofi-Avantis.33
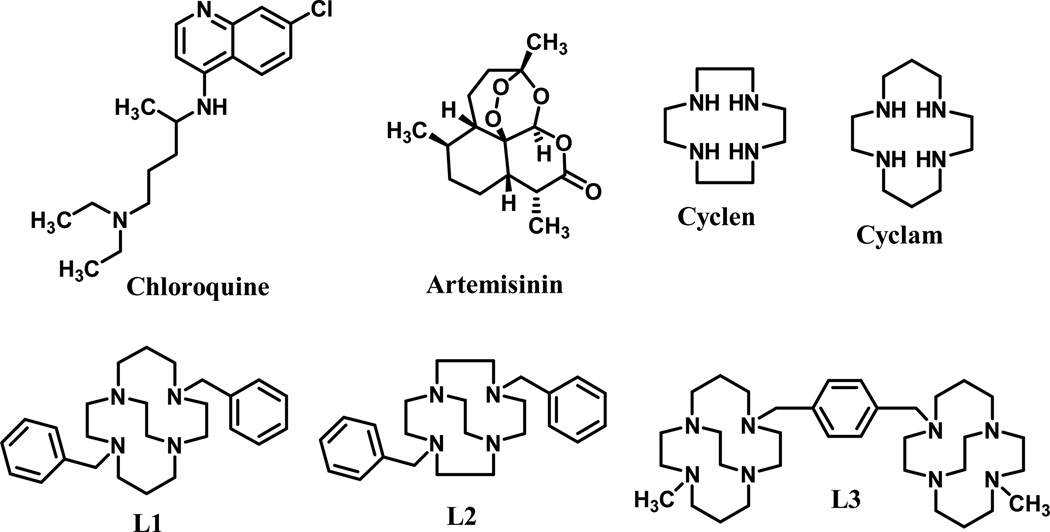
In order to circumvent drug resistance caused by the P. falciparum parasites, we have employed the metal coordination approach to prepare series of metal complexes using crossbridged and side-bridged tetraazamacrocyclic ligands L1, L2, and L3 (Fig. 1). The effect of metal coordination on the ligands was investigated for antimalarial activity.
Figure 1.
Structures of ligands used for the preparation of complexes and standard drugs (chloroquine and artemisinin) used as controls.
Even though the synthetic tetraazamacrocyclic compounds, particularly, cyclen and cyclam, and their analogs or their metal complexes have been extensively utilized in applications of a variety of diagnostic and magnetic resonance imaging (MRI) contrast agents,34 their metal based derivatives are still relatively unexplored in antimalarial drug development. A recent report, in which the 7-chloro-4-(1,4,7,10-tetraaza-cyclododec-1-yl)-quinoline-Zn2+ complex was found to demonstrate a high antimalarial activity has motivated us to pursue the present investigation.35 We hypothesized that the particularly kinetically inert transition metal complexes of cross-bridged tetraazamacrocycles36–49 (Fig. 1, L1–L3) would be stable, potentially active antimalarials against drug-sensitive and –resistant strains of malarial parasites.35 These complexes have several properties that suggested this activity to us: (1) tight-binding metal complexation that will prevent loss of the metal ion even in vivo; (2) aromatic functional groups reproducing the aromaticity of chloroquine; and (3) multiple amine functional groups, as also present in chloroquine.
The ligands L1–L3 in Fig. 1 represent different combinations of aromatic and tetraazamacrocycle units for screening to determine which combination of groups maximizes antimalarial efficacy. Ligands L1 and L2 both contain two aromatic groups and only one tetraazamacrocycle. Ligand L3 contains only one aromatic group and two tetraazamacrocycles. L3 was also chosen because it has proven to be biologically active against other disease states. Bis-tetraazamacrocycles are potent CXCR4 antagonists, as exemplified by the FDA approved drug Plerixafor. Cross-bridged analogues, particularly in their transition metal complexes, have demonstrated CXCR4 binding efficiency even superior to Plerixafor.50–54 Development of this route for biological activity has encouraged us to explore other potential disease states which may benefit from these cross-bridged bis-tetraazamacrocycle complexes. Finally, we also screened six different metal ions in their complexes with each ligand. This approach was taken to allow us to draw a conclusion about which metal ion is most appropriate for inclusion in metal complex antimalarials.
2. Results and Discussion
2.1. Chemistry
The ligands L1, L2 and L3 have all been published previously.55 Transition metal complexes of these ligands were generally formed from anhydrous metal salts in nonprotic solvents in an inert atmosphere glove box. Mn2+, Fe2+, and Co2+ divalent complexes are often air sensitive and must be protected from oxygen. Additionally, it is known that protection of cross-bridged tetraazamacrocycle ligands from sources of water, or even protic solvents, is helpful in complexation reactions as they are strongly basic and protonation can defeat complexation.
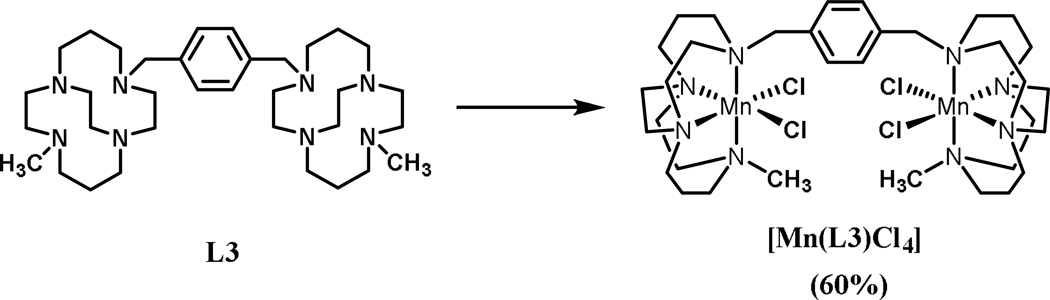
2.1.1. Manganese Complex [Mn2(L3)Cl4]
This complexation reaction is representative of all of the L3 complexes. Complexation of the ligand was carried out using the anhydrous metal chloride salt in anhydrous DMF in an inert atmosphere glove box (Scheme 1). The anhydrous MnCl2 is not very soluble at room temperature in DMF, so the reaction was heated to ~50 ºC and it proceeded smoothly at this temperature with stirring overnight. This complex is air sensitive, as indicated by formation of a brown solution when the reaction solution was exposed to air.
Scheme 1.
Synthetic procedure of metal complex [Mn(L3)Cl4]. Reagents and conditions: MnCl2, anhydrous DMF, 50 °C, 18 h, 60%.
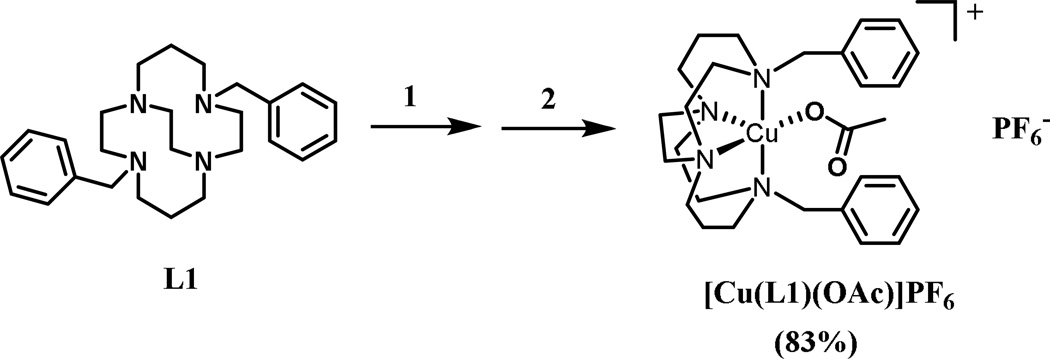
2.1.2. Copper Complex [Cu(L1)(OAc)]PF6
This complexation is representative of all of the dibenzyl macrocyle complexes of L1 and L2. Complexation of the ligand was carried out using the anhydrous metal acetate salt in anhydrous DMF in an inert atmosphere glove box and proceeded smoothly at room temperature with overnight stirring (Scheme 2). Once the complexation had occurred, the reaction solution was removed from the glove box and concentrated to dryness, which yielded a viscous oil product. In order to produce a more easily handled solid complex, as well as to purify the product, an anion metathesis reaction with NH4PF6 in dry methanol was carried out. With only two cis coordination sites available, only one acetate anion was able to coordinate to the metal ion, leaving an uncoordinated acetate anion that could be replaced with PF6−. Hexafluorophosphate anions precipitated the complex cation from methanol as a microcrystalline powder.
Scheme 2.
Synthetic procedure for [Cu(L1)(OAc)]PF6. Reagents and conditions: (1) Cu(OAc)2, anhydrous DMF, rt,18 h. (2) NH4PF6, MeOH.
2.2. Biological Evaluation
2.2.1. In Vitro Antimalarial Activity
Both the free ligands and their metal complexes were tested for in vitro antimalarial activity against the D6 (chloroquine sensitive) and W2 (chloroquine resistance) strains of P. falciparum. The test performed was based on the determination of plasmodial lactate dehydrogenase (LDH) activity. Varying concentrations of the test samples of either the complexes or the free ligands were added to a suspension of red blood cells infected with P. falciparum (D6 or W2) strains containing 2% parasitemia and 2% hematocrit in RPMI-1640 medium supplemented with 10% human serum. Inhibition of the uptake of [3H] hypoxanthine served as a measure of parasite viability56,57 and the potencies of all the test compounds against chloroquine resistant strains of P. falciparum were also confirmed by [3H] hypoxanthine incorporation. Artemisinin and chloroquine were used as drug controls and DMSO was included as a vehicle control. The selectivity index (SI) and cytotoxicity to mammalian cells were also determined.
As shown in Table 1, while the Mn2+ and Co2+ complexes of the dibenzyl cross-bridged cyclam ligand L1 demonstrated a higher antimalarial activity against the chloroquine resistant (W2) strain of the parasite than the D6 strain, the Fe2+, Ni2+, Cu2+, and Zn2+ complexes of the same ligand all showed lower antimalarial activity towards W2 than towards D6. The manganese complex is clearly the most active complex against both strains of the malarial parasites, with nearly an order of magnitude greater SI than some of the other metals. In fact, this manganese complex was the most active of all of the compounds tested.
Table 1.
In Vitro Antimalarial Activities of Ligands and Complexes Against D6 and W2 strains of Plasmodium falciparum.
| Compound | D6 IC50 (µM) |
D6 SI | W2 IC50 (µM) | W2 SI | VERO IC50 ng/mL |
|---|---|---|---|---|---|
| L1 | 2.679 | >4.4 | 4.358 | >2.7 | NC |
| Mn(L1)Cl2 | 0.157 | 38.45 | 0.127 | 47.793 | 3222.8 |
| Fe(L1)Cl2 | 0.324 | >27.6 | 0.426 | >21 | NC |
| [Co(L1)(OAc)]PF6 | NA | - | 5.162 | >1.4 | NC |
| [Ni(L1)(OAc)]PF6 | 0.825 | >8.6 | 1.326 | >5.4 | NC |
| [Cu(L1)(OAc)]PF6 | 0.423 | >16.7 | 0.809 | >8.7 | NC |
| [Zn(L1)(OAc)]PF6 | 0.342 | >20.6 | 0.593 | >11.9 | NC |
| L2 | 1.350 | >9.3 | 2.008 | >6.3 | NC |
| Mn(L2)Cl2 | 0.265 | >35.6 | 0.545 | >17.3 | NC |
| Fe(L2)Cl2 | 1.384 | >6.8 | 2.139 | >4.4 | NC |
| [Co(L2)(OAc)]PF6 | NA | - | NA | - | NC |
| [Ni(L2)(OAc)]PF6 | 1.110 | >6.7 | 1.538 | >4.8 | NC |
| [Cu(L2)(OAc)]PF6 | 0.147 | >50.3 | 0.312 | >23.6 | NC |
| [Zn(L2)(OAc)]PF6 | 2.057 | >3.6 | 3.814 | >1.9 | NC |
| L3 | 1.278 | >6.4 | 1.414 | >5.8 | NC |
| [Mn2(L3)Cl4] | 0.957 | >6 | 0.959 | >5.9 | NC |
| Fe(L3)2Cl4 | NA | - | NA | - | NC |
| [Co2(L3)(OAc)2](PF6)2 | NA | - | NA | - | NC |
| Ni2(L3)(OAc)2](PF6)2 | NA | - | NA | - | NC |
| [Cu2(L3)(OAc)2](PF6)2 | NA | - | NA | - | NC |
| [Zn2(L3)(OAc)2](PF6)2 | NA | - | NA | - | NC |
| Artemisinin (ART) | 0.024 | >>35.5 | 0.004 | >211.4 | NC |
| Chloroquine (CQ) | 0.037 | 20.4 | 0.679 | >1.1 | NC |
NC, no cytotoxicity up to about 10 µM; NA, no activity; SI, selectivity index (IC50 for Vero cells/IC50 for P. falciparum)
For the metal complexes of the dibenzyl cross-bridged cyclen ligand L2, the Cu2+ complex showed a higher antimalarial activity against the chloroquine resistant (W2) strain of the parasite, while the Mn2+ complex demonstrated a lower, but comparable antimalarial activity against the parasites. The Fe2+, Co2+, Ni2+, and Zn2+ complexes were generally less effective against the chloroquine resistant parasite, and less active overall than either Mn2+ or Cu2+. All of the metal complexes showed low to moderate antimalarial activity against the chloroquine sensitive (D6) strain of the parasite.
Bis-macrocyclic ligand L3 demonstrated an activity towards both malaria strains similar to that of the monocyclic dibenzyl ligands L1 and L2. Interestingly, the ligand made from the combination of two tetraazamacrocycles and one aromatic group (L3) exhibits nearly the same activity as the combination of two aromatic groups and one tetraazamacrocycle, at least for the free ligands themselves. However, a striking difference is seen when comparing the transition metal complexes of L3. Only the manganese complex of L3 was active against either malarial strain. Why the linking of two such macrocycle complexes together greatly reduces the activity is not readily apparent, although the higher positive charge carried by these bimetallic complexes may prevent them from readily crossing the parasite’s membranes. It is also worth emphasizing, once again, the superior activity of the manganese complex, compared to the other metal ions. This appears to be a general trend of these tetraazamacrocyclic complexes, and is one that we hope to test further in future studies on other manganese analogues.
With regard to the cyclam L1 analogs, the activity ratio of metal complex: free ligand, with the ligand value normalized to 1.00 as calculated by dividing the IC50 (free ligand)/IC50 (metal complex) for the antimalarial activity against chloroquine sensitivity (D6) strains of P. falciparum was in the range of 3.25–17.1. For the antimalarial activity against chloroquineresistant (W2) strains of P. falciparum, the ratio was in the range of 3.29–34.3. The increasing order of potency of antimalarial activity (W2 and D6) for these complexes is Co2+<Ni2+< Cu2+ < Zn2+< Fe2+ ≤ Mn2+. In contrast, for the cyclen L2 analogs, the same ratio for the D6 strains of the P. falciparum, was in the range of 0.656– 9.18 and for the W2 strains of the P. falciparum, the ratio was in the range of 0.526–6.44; with the copper complex exhibiting the most potency followed by the manganese complex.
In comparison of these complexes to the positive controls, chloroquine and artemisinin, the cyclam based manganese complex which is the most potent, is 4.24 times less potent than chloroquine and 6.54 times less potent than Artimisinin against the chloroquinesensitive (D6) strains of P. falciparum. However, it also shows signs of toxicity as indicated by the Vero IC50 value. It is also worth noting that this complex is superior to chloroquine and it is 5.35 times more potent than chloroquine against the chloroquine-resistant (W2) strain of P. falciparum although it is 32 times less potent than artimisinin.
These results suggest that the higher antimalarial activity displayed by the manganese complex for the cyclam ligand compared to that of the cyclen may be due to the larger pocket of the cyclam which makes it form more stable complexes with manganese.40 The cavity size difference also results in adjustments in the redox properties of the two complexes,45 which may also be important, depending on the mechanism of action. We should be able to tune multiple properties of these complexes by adjusting the cavity size and changing the benzyl substituents to other groups. Interestingly, the manganese complex of L1 exhibits superior antimalarial activity against the chloroquine-resistant strains of P. falciparum in comparison to chloroquine.
The fact that all the free ligands tested showed fairly low antimalarial activity, suggests that the metal complexation may have played a critical role in making the complexes exhibit antimalarial activity, rather than the roles played by the free ligand or the metal alone.22 Additionally, the generally low cytotoxicity of these ligands against mammalian cells, also suggests that the ligands are attractive for further development of new metal complex based antimalarial drugs.22
3. Conclusions
The screening of the antimalarial activity metal complexes of the tetraazamacrocyclic compounds generated by reactions of cyclam or cyclen cross-bridged and side-bridged ligands with the respective transition metal ions of Mn2+, Fe2+, Co2+, Ni2+, Cu2+, and Zn2+, was carried out. The results revealed that coordination of a transition metal ion to a biologically active molecule may result in an improvement of the activity of the resulting molecule depending on the identity of the metal ion chosen. As shown in Table 1, this trend is illustrated by the coordination of Mn2+, Fe2+, Co2+, Ni2+, Cu2+, and Zn2+ ions to the dibenzyl cross-bridged cyclam ligand L1, which produced complexes with increased potency, whereas only the Co2+ complex of the same ligand L1 did not result in an enhancement of activity compared to the free ligand. This data demonstrates that although the biological activity of a molecule can be enhanced through metal ion coordination, the improvement occurs only when a suitable metal-ligand pairing is made. Interestingly, only Cu2+ and Mn2+ enhanced the activity of L2, emphasizing that a suitable metal-ligand pairing is required. The same metals (Fe2+, Ni2+, Zn2+) which enhance the activity of L1 do not enhance potency when incorporated into a very similar analogue, L2. Apparently, there is a fine balance between the properties of the organic ligand and the metal ion that must be optimized to produce enhancement in biological activity. Finally, the metal complexes generated using ligand L3, are mostly inactive. The use of two tetraazamacrocycle pharmacophores and only one aromatic group does not appear to lead to improved antimalarial activity for the resulting metal complexes, even though the ligand itself has activity similar to L1 and L2, and L3 complexes are known to have other biological activity.50–54 Perhaps the additional positive charge and polarity accompanying the second metal ion are too much to overcome in order to get the drug molecule into the parasite. It should be noted, however, that the Mn2+ complex of L3 does in fact increase its activity. In all three ligand cases, Mn2+ improves the activity. This property of Mn2+ will be explored further with other tetraazamacrocyclic ligands.
Based on the findings of this investigation, a significant insight into the design of new transition metal based antimalarial drugs was obtained. These results will support the further development of this promising new class of compounds with potential for the development of new drug(s) for malaria chemotherapy that may contribute to improving future therapeutic potentials. Furthermore, although certainly not an exhaustive survey, certain metal ions which were found to be suitable for the design of the new metal complexes of tetraazamacrocyclic derivatives for malaria chemotherapy have been identified. Depending on the ligand used, manganese(II), copper(II), and iron(II) have shown to be the most suitable metals that can form the basis for rational antimalarial drug design.
4. Experimental Section
4.1. Chemistry
General
All the materials were reagent grade, and used as supplied. The reactions were performed in anhydrous solvents under inert atmosphere unless otherwise indicated. Anhydrous solvents (acetonitrile and DMF) as well as all other reagents were used as received from a commercial source. Elemental analysis was carried out by Quantitative Technologies, Inc. in Whitehouse, NJ. Electrospray MS was obtained using a Shimadzu LCMS-2020 in 1:1 methanol/water mixture. 1H and 13C NMR spectra were recorded at 300 MHz and 75 MHz, respectively on Bruker 300 spectrometer with TMS as internal standard.
4.2. Synthesis of ligands
Ligand L1, 4,11-dibenzyl-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane and L2, 4,10-dibenzyl-1,4,7,10-tetraazabicyclo[5.5.2]tetradecane were prepared as outlined in reference.55 Ligand L3, 4-methyl-11-[4-(4-methyl-1,4,8,11-tetraaza-bicyclo[6.6.2]hexadec-11-ylmethyl)-benzyl]-1,4,8,11-tetraaza-bicyclo[6.6.2]hexadecane was prepared according to the literature procedure.58
4.3. General synthetic procedure for complexes M(L)Cl2 and [M(L)(OAc)]PF6
Reactions of MnCl2 or FeCl2 with ligands L1 and L2 in a 1:1 ratio under mild conditions in a glove box gave the complexes Mn(L)Cl2 and Fe(L)Cl2, respectively.45 The complexes were isolated in good yields as air stable white solid Mn(L)Cl2 and/or pale brown Fe(L)Cl2. Complexes [M(L)(OAc)]PF6, where M= Cu, Zn, Ni, and Co, were also synthesized in two steps by first reacting the appropriate metal acetates with the ligands L1, L2, and L3 in CH3CN or DMF at room temperature for 24 h, followed by solvent removal to give an oily product which was subsequently dissolved in methanol and reacted with 5 equivalents of NH4PF6 to give the desired complexes.58 All these new complexes were characterized by electrospray, MS, and elemental analyses. Complexes Mn(L1)Cl2, Fe(L1)Cl2, Mn(L2)Cl2 and Fe(L1)Cl2 were synthesized45,58 and complex [Ni(L1)OAc]PF6 was synthesized according to the literature procedure.59
4.3.1. Synthesis of Tetrachloro(4-methyl-11-[4-(4-methyl-1,4,8,11-tetraaza-bicyclo [6.6.2]hexadec-11-ylmethyl)-benzyl]-1,4,8,11-tetraaza-bicyclo [6.6.2]hexadecane) manganese(II) [Mn2(L3)Cl4]
The ligand, L3 (0.300 g, 0.511 mmol) and anhydrous manganese(II) chloride salt (0.129 g, 1.02 mmol) were added to 20 ml of dry DMF in an inert atmosphere glove box. Upon stirring and heating (~50 °C) both reactants dissolved to give a colorless solution. Overnight stirring at this temperature resulted in the formation of a white precipitate. The reaction was stopped after stirring for ~18 h and cooled to room temperature. Inside the glove box, the white solid product was collected by filtration on a fine glass frit and washed with DMF followed by ether, then dried under vacuum to give the product (0.256 g, 60%). FAB+ mass spectral analysis yielded peaks at m/z = 797, consistent with Mn2LCl3+, and m/z = 379, consistent with Mn2L3Cl22+. Elemental analysis (%) calcd. [Mn2C34H62N8Cl4)] (834.599 g/mol): C 48.93, H 7.49, N 13.43; Found C 48.54, H 7.59, N 13.24.
4.3.2. Synthesis of Acetato(4,11-dibenzyl-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane)copper(II)hexafluorophosphate[Cu(L1)(OAc)]PF6
Ligand L1, (0.406 g, 1.00 mmol) and anhydrous copper(II) acetate salt (0.182 g, 1.00 mmol) were added to 25 ml of dry DMF in an inert atmosphere glove box. The reaction was stirred at room temperature for 18 h. The crude blue-green [Cu(L1)(OAc)][(OAc)] solution was removed from the glove box, filtered to remove any trace solids, and evaporated to dryness, giving a blue-green oil. The crude product was dissolved in 10 ml of dry methanol, to which was added dropwise, a 5 ml dry methanol solution of 5 equivalents (0.815 g, 5.00 mmol) of NH4PF6. A blue-green powder of the [Cu(L1)(OAc)]PF6 salt precipitated immediately, and was stored overnight in a freezer at −5 °C to complete the precipitation. The solid was collected on a fine glass frit, washed with cold methanol and ether, and dried under vacuum to give the final product (0.573 g, 83%). Elemental analysis (%) calcd. [CuC26H38N4(C2H3O2)]PF6 • H2O (692.185 g/mol): C 48.59, H 6.26, N 8.09; Found C 48.65, H 6.18, N 8.21. MS (ES) m/z 528.3 and 530.3 [CuL(OAc)]+.
4.4. Biological evaluation
4.4.1. In vitro antimalarial activity
The in vitro antimalarial activity was measured by a colorimetric assay that determines the parasitic lactate dehydrogenase (pLDH) activity. The assay was performed in a 96-well microplate using two P. falciparum clones, [Sierra Leone D6 (chloroquine-sensitive) and Indochina W2 (chloroquine-resistant)]. For the assay, a suspension of red blood cells infected with P. falciparum (D6 or W2) strains (200 µL, with 2% parasitemia and 2% hematocrit in RPMI-1640 medium supplemented with 10% human serum and 60 µg/mL amikacin) was added to the wells of a 96-well plate containing 10 µL of test samples at various concentrations. The plate was flushed with a gas mixture of 90% N2, 5% O2, and 5% CO2 in a modular incubation chamber (Billups-Rothenberg, 4464 M) and incubated at 37 °C for 72 h. Plasmodial LDH activity was determined by using Malstat reagent (Flow Inc., Portland, OR) as described earlier.56,57 The IC50 values were computed from the dose-response curves generated by plotting percent growth against test concentrations. DMSO (0.25%), artemisinin, and chloroquine were included in each assay as vehicle and drug controls, respectively. The selectivity indices (SI) were determined by measuring the cytotoxicity of samples toward mammalian cells (Vero; monkey kidney fibroblast).
4.4.2. Cytotoxicity assay
The in vitro cytotoxic activity was determined against noncancerous Vero cell lines (monkey kidney fibroblast), obtained from the American Type Culture Collection (ATCC, Rockville, MD). The assay was performed in 96-well tissue culture treated microplates. Cells (25 000 cells/well) were seeded in the wells of the plate and incubated for 24 h. Samples were added and plates were again incubated for 48 h. The number of viable cells was determined using Neutral Red according to a modification of the procedure of Borenfreund et al.60,61
Acknowledgements
This project as well as the publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health through Grant Number 8P20GM103447 (TJH and MOFK). TJH acknowledges the Research Corporation (CC6505), the Oklahoma Center for the Advancement of Science and Technology (HR13-157), and the Henry Dreyfus Teacher-Scholar Awards Program for support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chellan P, Land KM, Shokar A, Au A, An SH, Taylor D, Smith PJ, Chibale K, Smith GS. Organometallics. 2013;32:4793–4804. [Google Scholar]
- 2.Le Bras J, Musset L, Clain J. Med. Mal. Infect. 2006;36:401–405. doi: 10.1016/j.medmal.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Tang Y, Dong Y, Vennerstrom JL. Med. Res. Rev. 2004;24:425–448. doi: 10.1002/med.10066. [DOI] [PubMed] [Google Scholar]
- 4.Mutabingwa TK. Acta. Trop. 2005;95:305–315. doi: 10.1016/j.actatropica.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Yeung S, Van Damme W, Socheat D, White NJ, Mills A. Malar. J. 2008;7:87. doi: 10.1186/1475-2875-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, Biagini GA, Bray PG, Gibbons P, Berry N, Winstanley PA, Mukhtar A, Bonar-Law R, Hindley S, Bambal RB, Davis CB, Bates M, Hart TK, Gresham SL, Lawrence RM, Brigandi RA, Gomez-delas-Heras FM, Gargallo DV, Ward SA. J. Med. Chem. 2009;52:1408–1415. doi: 10.1021/jm8012618. [DOI] [PubMed] [Google Scholar]
- 7.O’Neil PM, Ward SA, Berry NG, Jeyadevan JP, Biagini GA, Asadollaly E, Park BK, Bray PG. Curr. Top. Med. Chem. 2006;6:479–507. doi: 10.2174/156802606776743147. [DOI] [PubMed] [Google Scholar]
- 8.Sekhon BS, Bimal N. J. Pharm. Educ. Res. 2012;3:52–63. [Google Scholar]
- 9.Gasser G, Metzler-Nolte N. In: Bioinorganic Medicinal Chemistry. Alessio E, editor. Weinheim: Wiley-VCH Verlag; 2011. pp. 351–382. [Google Scholar]
- 10.Hernandes MZ, Pontes FJS, Coelho LCD, Moreira DRM, Pereira VRA, Leite ACL. Curr. Med. Chem. 2010;17:3739–3750. doi: 10.2174/092986710793213779. [DOI] [PubMed] [Google Scholar]
- 11.Arrowsmith RL, Pascu SI, Smugowski H. Organomet. Chem. 2012;38:1–35. [Google Scholar]
- 12.Hartinger CG, Metzler-Nolte N, Dyson PJ. Organometallics. 2012;31:5677–5685. [Google Scholar]
- 13.Sun M. Science. 1983;222:145. doi: 10.1126/science.222.4620.145. [DOI] [PubMed] [Google Scholar]
- 14.Farrell N. In: Catalysis by Metal Complexes. James BR, Ugo R, editors. Vol. 11. Kluwer: Dordrecht; 1989. pp. 46–62. [Google Scholar]
- 15.Kopf-Maier P, Kopf P. Chem. Rev. 1987;87:1137–1152. [Google Scholar]
- 16.Navarro M, Gabbiani C, Messori L, Gambino D. Drug Disc. Today. 2010;15:1070–1078. doi: 10.1016/j.drudis.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Delgado RA, Anzellotti A. Mini Rev. Med. Chem. 2004;4:23–30. doi: 10.2174/1389557043487493. [DOI] [PubMed] [Google Scholar]
- 18.Sharma V. Mini Rev. Med. Chem. 2005;5:337–351. doi: 10.2174/1389557053544029. [DOI] [PubMed] [Google Scholar]
- 19.Navarro M. Coord. Chem. Rev. 2009;253:1619–1626. [Google Scholar]
- 20.Jones CJ, Thornback JR. Medicinal Chemistry of Coordination Chemistry. Chapter 4. Cambridge: Royal Society of Chemistry; 2007. [Google Scholar]
- 21.Aird RE, Cummings J, Ritchie AA, Muir M, Morris RE, Chen H, Sadler PJ, Jodrell DI. Br. J. Cancer. 2002;86:1652–1657. doi: 10.1038/sj.bjc.6600290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahl D, Athar F, Soares MBP, Santos de Sa M, Moreira DRM, Srivastava RM, Leite ACL, Azam A. Bioorg. Med. Chem. 2010;18:6857–6864. doi: 10.1016/j.bmc.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Navarro M, Perez H, Sanchez-Delgado RA. J. Med. Chem. 1997;40:1937–1937. doi: 10.1021/jm9607358. [DOI] [PubMed] [Google Scholar]
- 24.Khanye SD, Smith GS, Lategan C, Smith PJ, Gut J, Rosenthal PJ, Chibale K. J. Inorg. Biochem. 2010;104:1079–1083. doi: 10.1016/j.jinorgbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Delgado RA, Navarro M, Perez H, Urbina JA. J. Med. Chem. 1996;36:1095–1099. doi: 10.1021/jm950729w. [DOI] [PubMed] [Google Scholar]
- 26.Gambiano D, Otero L. Inorg. Chim. Acta. 2012;393:103–114. [Google Scholar]
- 27.Domarle O, Blampain G, Agnaniet H, Nzadiyabi T, Lebibi J, Brocard J, Maciejewski L, Biot C, Georges AJ, Millet P. Antimicrob. Agents Chemother. 1998;42:540–544. doi: 10.1128/aac.42.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halder KC, Henderson CL, Cross GAM. Proc. Natl. Acad. Sci. USA. 1986;83:8565–8569. doi: 10.1073/pnas.83.22.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez MH, Jungery M. Nature. 1986;324:388–391. doi: 10.1038/324388a0. [DOI] [PubMed] [Google Scholar]
- 30.Biot C, Castro W, Botte CY, Navarro M. Dalton Trans. 2012;41:6335. doi: 10.1039/c2dt12247b. [DOI] [PubMed] [Google Scholar]
- 31.Allardyce CS, Butler PA, Dyson PJ, Fontecilla-Camps JC, Krautler B, Hirao T, Le Maux P, Moriuchi T, Severin K, Simonneaux G, Volbeda A. In: Bioorganometallic Chemistry. Simonneaux G, editor. Vol. 17. Berlin, Heidelberg, New York: Springer; 2006. pp. 177–210. [Google Scholar]
- 32.Navarro M, Castro W, Biot C. Organometallics. 2012;31:5715–5727. [Google Scholar]
- 33.Supan C, Mombo-Ngoma G, Dal-Bianco MP, Salazar CLO, Issifou S, Mazuir F, Filali-Ansary A, Biot C, Ter-Minassian D, Ramharter M, Kremsner PG, Lella B. Antimicrob. Agents Chemother. 2012;56:3165–3173. doi: 10.1128/AAC.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker WC, Choi MJ, Hill DC, Thompson JL, Petillo PA. J. Org. Chem. 1999;64:2683–2689. doi: 10.1021/jo981920r. [DOI] [PubMed] [Google Scholar]
- 35.Khan MOF, Levi MS, Tekwani BL, Khan SI, Kimura E, Borne RF. Antimicrob. Agents Chemother. 2009;53:1320–1324. doi: 10.1128/AAC.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubin TJ, McCormick JM, Collinson SR, Alcock NW, Busch DH. J. Chem. Soc. Chem. Commun. 1998:1675–1676. [Google Scholar]
- 37.Hubin TJ, McCormick JM, Alcock NW, Clase HJ, Busch DH. Inorg. Chem. 1999;38:4435. doi: 10.1021/ic990491s. [DOI] [PubMed] [Google Scholar]
- 38.Hubin TJ, Alcock NW, Busch DH. Acta Cryst. C. 1999;55:1404. [Google Scholar]
- 39.Hubin TJ, Tyryshkyn N, Alcock NW, Busch DH. Acta Cryst. C. 1999;55:1888. [Google Scholar]
- 40.Hubin TJ, McCormick JM, Collinson SR, Perkins CM, Alcock NW, Kahol PK, Raghunathan A, Busch DH. J. Am. Chem. Soc. 2000;122:2512. [Google Scholar]
- 41.Hubin TJ, Alcock NW, Busch DH. Acta Cryst. C. 2000;56:37. doi: 10.1107/s010827019901255x. [DOI] [PubMed] [Google Scholar]
- 42.Hubin TJ, McCormick JM, Alcock NW, Busch DH. Inorg. Chem. 2001;40:435. doi: 10.1021/ic9912225. [DOI] [PubMed] [Google Scholar]
- 43.Hubin TJ, Alcock NW, Clase HJ, Busch DH. Supramol. Chem. 2001;13:261. [Google Scholar]
- 44.Hubin TJ, Alcock NW, Clase HJ, Seib L, Busch DH. Inorg. Chim. Acta. 2002;337:91. [Google Scholar]
- 45.Hubin TJ, McCormick JM, Collinson SR, Alcock NW, Clase HJ, Busch DH. Inorg. Chim. Acta. 2003;346:76–86. [Google Scholar]
- 46.Hubin TJ, Alcock NW, Morton MD, Busch DH. Inorg. Chim. Acta. 2003;348:33–40. [Google Scholar]
- 47.Hubin TJ. Coord. Chem. Rev. 2003;241:27. [Google Scholar]
- 48.Lichty J, Allen SM, Grillo AI, Archibald SJ, Hubin TJ. Inorg. Chim. Acta. 2004;357:615. [Google Scholar]
- 49.Maples DL, Maples RD, Hoffert WA, Parsell TH, van Asselt A, Silversides JD, Archibald SJ, Hubin TJ. Inorg. Chim. Acta. 2009;362:2084–2088. doi: 10.1016/j.ica.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan A, McRobbie G, Madden LA, Empson CJ, Bridgeman AJ, Ullom R, Hubin TJ, Greenman J, Archibald SJ. Immunol. 2005;116:75. [Google Scholar]
- 51.Valks GC, McRobbie G, Lewis EA, Hubin TJ, Hunter TM, Sadler PJ, Pannecouque C, De Clerq E, Archibald SJ. J. Med. Chem. 2006;49:6162–6165. doi: 10.1021/jm0607810. [DOI] [PubMed] [Google Scholar]
- 52.Archibald SJ, Daelemans D, Hubin TJ, Huskens D, Schols D, Van Laethem K, De Clercq E, Pannecouque C. Antiviral Res. 2009;82:A45–A45. [Google Scholar]
- 53.Khan A, Nicholson G, McRobbie G, Greenman J, Pannecouque C, Daelemans D, Schols D, De Clercq E, Hubin TJ, Archibald SJ. Antiviral Res. 2009;82:A59–A60. [Google Scholar]
- 54.Timmons JC, Hubin TJ. Coord. Chem. Rev. 2010;254:1661–1685. [Google Scholar]
- 55.Weisman GR, Wong EH, Hill DC, Rogers ME, Reed DP, Calabrese JC. Chem. Commun. 1996:947–948. [Google Scholar]
- 56.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinrichs DJ. Am. J. Trop. Med. Hyg. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 57.Makler MT, Hinrichs DJ. Am. J. Trop. Med. Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 58.Khan A, Nicholson G, Greenman J, Madden L, McRobbie G, Pannecouque C, De Clercq E, Silversides JD, Ullom R, Maples DL, Maples RD, Hubin TJ, Archibald SJ. J. Am. Chem. Soc. 2009;131:3416–3417. doi: 10.1021/ja807921k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith R, Huskens D, Daelemans D, Mewis RE, Garcia CD, Cain AN, Carder Freeman TN, Pannecouque C, De Clercq E, Schols D, Hubin TJ, Archibald SJ. Dalton Trans. 2012;41:11369–11377. doi: 10.1039/c2dt31137b. [DOI] [PubMed] [Google Scholar]
- 60.Borenfreund E, Babich H, Martin-Alguacil N. In Vitro Cell Dev. Biol. 1990;26:1030–1034. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 61.Kamiyama T, Matsubara J. Int. J. Parasitol. 1992;22:1137–1142. doi: 10.1016/0020-7519(92)90032-g. [DOI] [PubMed] [Google Scholar]