Abstract
Purpose
To assess the microarchitectural changes occurring during surgery for vitreomacular traction (VMT) utilizing intraoperative optical coherence tomography (iOCT).
Methods
A retrospective, consecutive, case series of eyes undergoing pars plana vitrectomy (PPV) for VMT with performance of concurrent iOCT. A custom, microscope-mounted, portable, spectral domain optical coherence tomography (SD-OCT) system was used. Clinical characteristics and iOCT images were analyzed.
Results
Twelve eyes of 12 patients were included with a mean preoperative visual acuity (VA) of 20/78 improving to 20/51 (p = 0.02), post-operatively. iOCT was successfully performed in 100% of cases. Microarchitectural changes were noted on iOCT following surgical release of the VMT particularly in the outer retina with increased subretinal hyporeflectivity (e.g., expansion of the distance between the RPE and photoreceptor layers). In 5/12 (42%) eyes, iOCT findings altered the surgical procedure (e.g., internal limiting membrane peeling, gas tamponade) to address the subclinical findings [e.g., full-thickness macular hole (FTMH) formation, residual membrane].
Conclusions
Intrasurgical imaging utilizing iOCT during VMT surgery may identify subclinical changes (e.g., occult FTMH formation) that may impact surgical decision-making. Architectural changes may occur following surgical maneuvers that are particularly noted in the outer retina. The functional significance of these changes requires further investigation.
Keywords: Optical Coherence Tomography, OCT, Intraoperative OCT, Intrasurgical OCT, iOCT, Vitreomacular Traction Syndrome, VMT, Retinal Surgery, vitreomacular adhesion
Introduction
Anomalous posterior vitreous separation and abnormal vitreomacular adhesion results in vitreomacular traction (VMT).1 When VMT results in visual loss and metamorphopsia, therapeutic intervention may be needed. Until recently, the only treatment for VMT was pars plana vitrectomy (PPV). The recent approval of ocriplasmin (Jetrea, Thrombogenics, NJ) offers a pharmcologic therapeutic alternative for select cases.2 Spectral-domain optical coherence tomography (SD-OCT) has yielded high resolution, in vivo, tomographic views of macular pathology which has resoundingly impacted the understanding in the diagnosis and management of VMT in the clinic setting.3
The application of OCT technology to the operating room has the potential to impact surgical management of VMT. The feasibility of intraoperative OCT (iOCT) has been described in numerous conditions, including macular holes, optic-pit related maculopathy, epiretinal membrane (ERM), retinal detachment, and retinopathy of prematurity.4–10 The objective of VMT surgery is to remove all epiretinal tissues (e.g. posterior hyaloid, ERM) that are imposing traction on the fovea, and in some cases removal of the internal limting membrane (ILM) may be indicated. Surgical elevation of the hyaloid may result in unroofing of foveal cysts or full-thickness MH (FTMH) formation. The ability to discern the microarchitectural structure of the vitreretinal interface and any significant surgical alterations (e.g., FTMH formation) could result in alterations to surgical procedures that might improve surgical outcomes and patient management. In this study, we describe the iOCT findings in the intrasurgical management of VMT using a microscope-mounted iOCT system, and we delineate the significant microarchitectual alterations noted following surgical manipulation. Additionally, we outline the role that iOCT may have in influencing surgical decision-making and management in VMT cases.
Methods
A retrospective consecutive multi-surgeon case series was performed for all eyes undergoing pars plana vitrectomy (PPV) for VMT with concurrent iOCT imaging. All eyes underwent preoperative OCT scanning with Cirrus SD-OCT system (Carl Zeiss Meditec, Dublin, CA) with verified VMT in the clinic prior to surgery. No cases were noted to have FTMH on the preoperative OCT performed in clinic. Twelve eyes from 12 patients were identified. This study was approved by the Cleveland Clinic Foundation (CCF) Institutional Review Board (IRB) and all tenets of the Declaration of Helsinki were followed.
Surgical Procedure
All patients underwent standard 3-port PPV (23 or 25-gauge) repair of VMT. Following completion of the core PPV, the hyaloid was carefully elevated using the vitreous cutter. Based on surgeon preference, dilute triamcinolone acetonide was utilized to stain the hyaloid for enhanced visualization in some cases. The internal limiting membrane (ILM) was also peeled in cases with an epiretinal membrane that was noted on OCT either preoperatively (in the clinic) or on the first iOCT scan prior to initiating peeling. Additionally, ILM peeling was performed if a FTMH was noted on iOCT scanning. If the ILM was peeled, indocyanine green (ICG) was applied to stain the inner limiting membrane (ILM) to aid in visualization. When performed, membrane peeling technique was performed with either vitreoretinal forceps or a diamond-dusted membrane scraper combined with vitreoretinal forceps for peel completion. A partial or complete air-fluid was performed based on surgeon preference. After air-fluid exchange, gas selection was chosen based on surgeon preference and surgical indication. Any specific post-operative positioning was also based on surgeon preference and surgical indication.
iOCT Scanning System
Utilizing a custom, microscope-mounted system, the Bioptigen Envisu SDOIS (Bioptigen, Research Triangle Park, NC) handheld probe was attached to the ophthalmic microscope, as previously described (FIGURE 1).4, 5 iOCT was performed at various surgical milestones as determined by the surgeon, including preincision, post-hyaloid elevation, and following ILM peeling. A consistent image acquisition protocol was utilized, including cubic 10 × 10 mm volume scans (at 0 and 90 degrees) and 10 mm radial volume scans. Each scan consisted of 100 B-scans distributed across the area with 1000 A-scans per B-scan. For the 10 × 10 mm cube scans, this translated to a scan density of one B-scan every 0.1 mm.
Figure 1.
Microscope mounted SD-OCT system (Bioptigen SDOIS) utilized for intraoperative scanning.
iOCT Image Analysis
All scanning sequences were exported for image analysis. Qualitative and quantitative analysis was performed by two independent reviewers. Quantitative analysis was performed using ImageJ (NIH Freeware, Bethesda, MD) software. Two independent reviewers reviewed each scan for two quantitative variables prior to and following hyaloid elevation: subretinal hyporeflectivity [i.e., ellipsoid zone (i.e., inner segment/outer segment (IS/OS)) to RPE distance] and central foveal thickness (CFT). The outer boundary utilized for measurement was the middle of the RPE. The inner boundary varied based on location of interest (e.g., ILM, middle of ellipsoid zone). The pre-incision and post-hyaloid measurements were compared using paired t-test.
Results
Clinical Characteristics and Demographics
Twelve eyes of 12 patients were identified that underwent surgical repair for a preoperative diagnosis of VMT who had concurrent iOCT imaging. The median age was 73.5 years (range 56 to 83 years). There were five males (42%) and seven females (58%). The mean preoperative visual acuity (VA) was 20/72 (range: 20/30 to 20/200). Four eyes (33%) were phakic and 3 of those 4 eyes underwent cataract extraction with intraocular lens placement at time of PPV. All other eyes were pseudophakic at the time of PPV. One eye (8%) underwent 25-gauge PPV and 11 eyes (92%) underwent 23-gauge PPV. Six of 12 eyes (50%) had associated ERM that was noted in the preoperative OCT scan in clinic and the preincision scan on iOCT. No postoperative surgical complications were noted. The mean post-operative VA improved to 20/51 (p = 0.02).
Qualitative iOCT Analysis and iOCT Impact on Surgical Procedures
Eleven of 12 eyes had iOCT scans completed immediately prior to beginning the PPV. One eye was not imaged prior to PPV due to poor signal strength and surgeon preference, but was subsequently imaged following hyaloid elevation. Twelve of 12 eyes showed complete clearance of tractional forces surrounding the fovea on iOCT prior to completing the surgical procedure. Following removal of the foveal traction (e.g., release of the posterior hyaloid, membrane peeling), there appeared to be an immediate reduction in foveal height. Additionally, an increase in the ellipsoid zone-RPE distance was also visualized in the subfoveal space as well as in the areas of membrane peeling (FIGURE 2).
Figure 2.
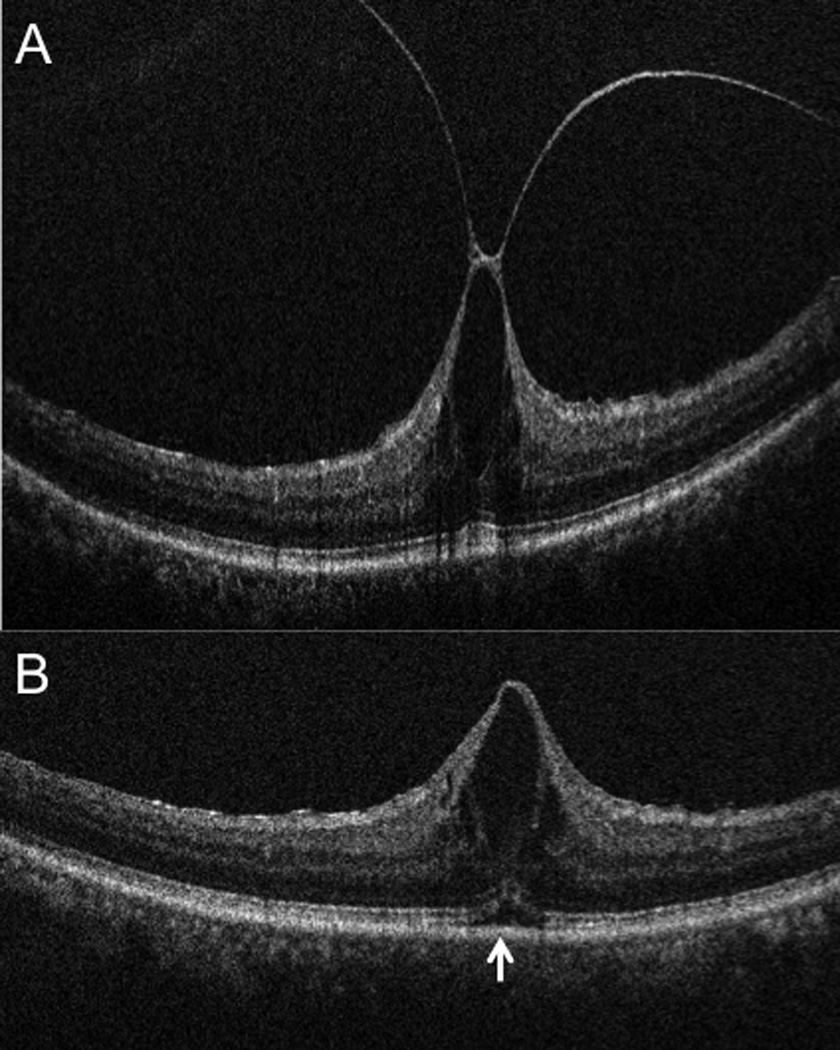
Intraoperative ophthalmic coherence tomography (iOCT) 10-mm B-scan showing (A) vitreomacular traction (arrowhead) prominent foveal traction prior to elevated the hyaloid. (B) iOCT 10-mm B-scan revealing complete release of traction with reduction in central foveal thickness. Increased subretinal hyporeflectance is noted (arrow). The slight variation in RPE curvature is due to subtle changes in scanner alignment that occur in the operating room environment.
For 2 of 11 eyes (18%), the pre-incision iOCT scans immediately prior to vitrectomy revealed conversion from VMT without MH (confirmed on the preoperative scan obtained in clinic) to a FTMH (Figure 3). When the PPV was commenced in both cases, the FTMH was subclinical due to its small size. In both of these cases, the surgeon altered the surgical plan based on the iOCT findings to include ILM peeling, long-term gas tamponade, and postoperative facedown positioning to address the FTMH. Both eyes showed successful closure of the FTMH in the postoperative period. These two eyes were excluded from additional quantitative analysis given the change in pathology from VMT to FTMH.
Figure 3.
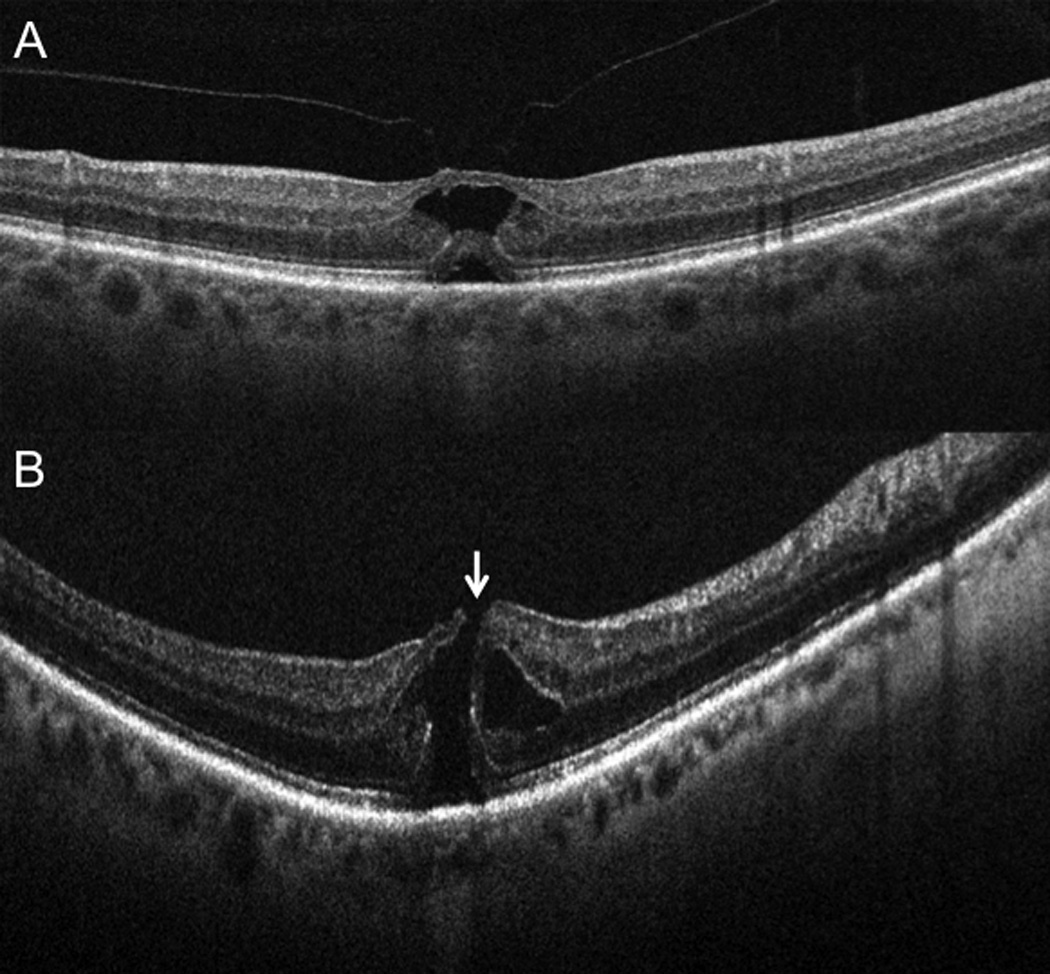
Subclinical interval development of a full-thickness macular hole. (A) Preoperative 6-mm B-scan (Cirrus) at time of surgical scheduling in clinic revealing vitreomacular traction without full-thickness defect (B) Intraoperative OCT 10-mm B-scan (Bioptigen) showing interval development of subclinical full-thickness macular hole immediately prior to vitrectomy (arrow). This altered surgical approach with gas tamponade and internal limiting membrane peeling.
Of the remaining eyes, 10 of 10 eyes had iOCT imaging performed following hyaloid elevation. Following release of the hyaloid, two eyes showed loss of inner retinal integrity with possible FTMH formation (FIGURE 4). These changes were not apparent clinically to the surgeon and were only definitively visualized with iOCT. In both of these cases, the surgeon altered the surgical plan to include ILM peeling, gas tamponade, and postoperative facedown positioning based on the iOCT findings. Both eyes showed successful closure of the FTMH.
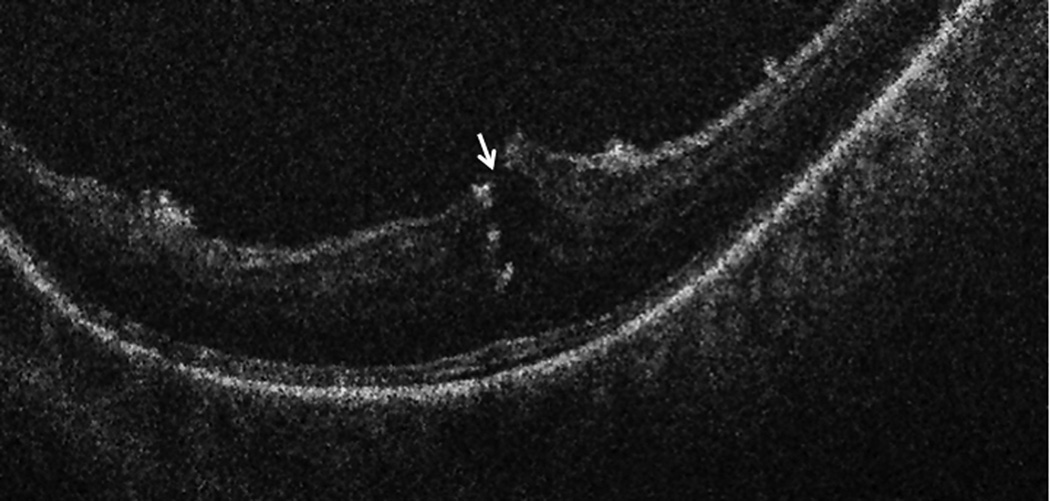
Figure 4.
Intraoperative OCT B-scan following posterior hyaloid release revealing loss of integrity of the inner retina. Hyperreflective areas within the retinal substance represent intraretinal triamcinolone particles following loss of inner retinal integrity.
In the seven eyes with underlying ERM, all seven had ILM/ERM peeling and iOCT imaging was performed following this peel. Following peeling, 1/7 (14%) eyes with ERM were noted on iOCT to have a residual membrane within the macular arcade that the surgeon determined required additional membrane peeling due to its proximity to the fovea (FIGURE 5).
Figure 5.
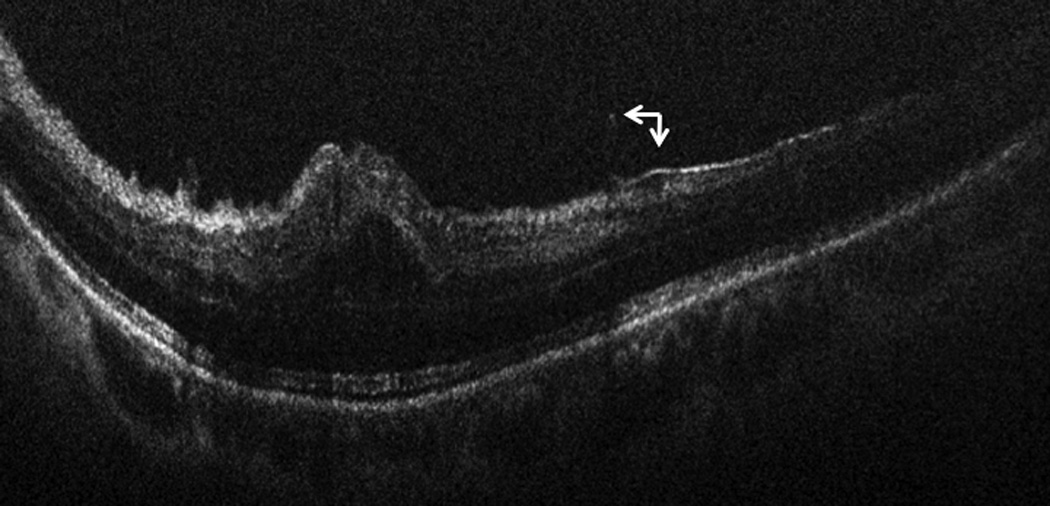
Intraoperative OCT B-scan following membrane peeling and release of hyaloid traction, revealing residual occult membrane (arrow) in close proximity to the fovea requiring additional membrane peeling.
Overall, iOCT impacted the surgical plan in 5 of 12 (42%) eyes (e.g., ILM peeling, gas tamponade, positioning, additional peeling). Two eyes were noted to have subclinical FTMH prior to initiating the procedure, 2 eyes were noted to have a possible/definitive FTMH following hyaloid elevation, and 1 eye was noted to have residual ERM following an initial peel that required additional membrane peeling.
Quantitative iOCT Analysis
Nine of 9 eyes where both a pre-incision and post hyaloid scan were obtained of sufficient quality to measure exhibited a decrease in CFT. Mean preoperative CFT was 792 microns (range: 488 to 1546 microns). Following hyaloid release, the mean CFT was 694 microns (range: 428 to 1354 microns). This represented a mean decrease of 98 microns (−12%) following release of the hyaloid traction (p = 0.002) (Figure 6). Of these nine eyes, all appeared to have subfoveal expansion of hyporeflectivity (e.g., increased RPE to ellipsoid zone distance). Only five eyes had both preincision and post-hyaloid scans of sufficient quality to accurately measure the distance between the RPE and ellipsoid zone. Four of 5 (80%) eyes showed increased distance between the RPE and ellipsoid zone lines with expansion of subretinal hyporeflectivity. The mean change in RPE to ellipsoid zone distance was + 28 microns (25%), p = 0.05 (Figure 6).
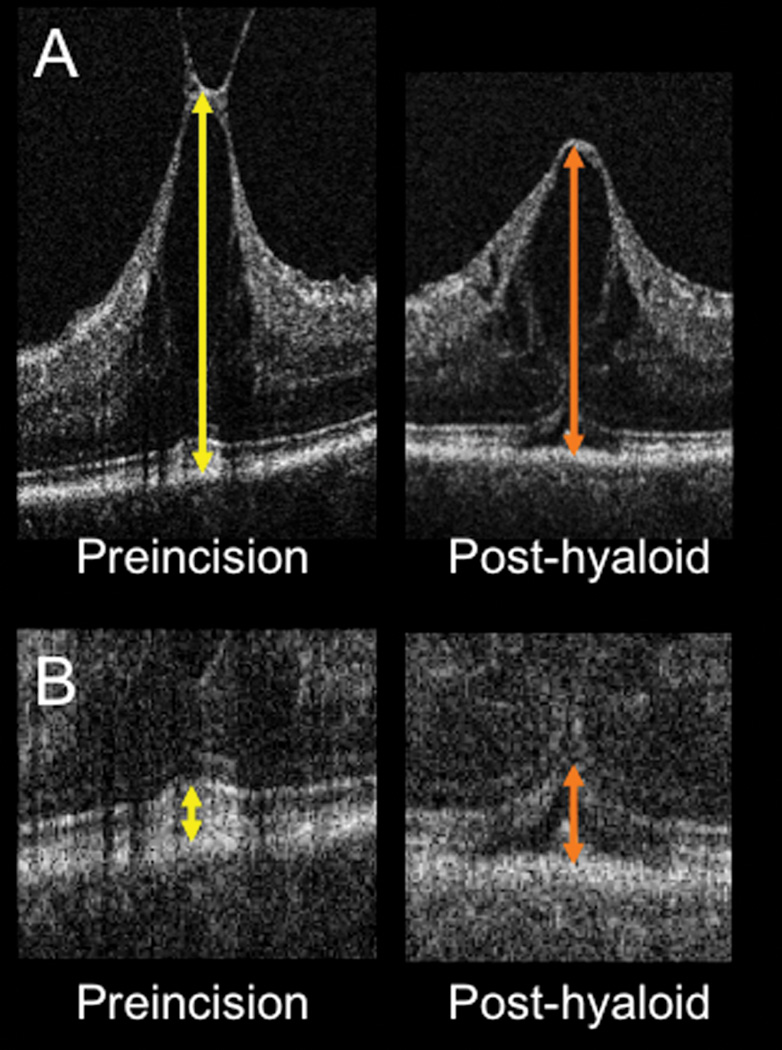
Figure 6.
(A) Intraoperative OCT B-scan showing rapid change in contour and decreased central foveal thickness (arrows) following release of the posterior hyaloid. (B) Intraoperative OCT B-scan revealing increased subretinal hyporeflectivity (arrows) following elevation of the posterior hyaloid.
Discussion
Optimal management for VMT continues to evolve. The use of iOCT during VMT surgery may provide an important adjunct to verify the achievement of surgical objectives and identify alterations in foveal anatomy that may require an altered surgical approach. In this study, we find that significant alterations occur in foveal architecture and outer retinal structure following surgical release of the posterior hyaloid. Additionally, we find that in a large percentage of cases (42%) the iOCT appeared to have a direct impact on surgical-decision making and provided important information to the surgeon that altered the surgical approach. In one example, iOCT revealed a residual membrane that was not otherwise visualized by the surgeon through the surgical microscope. Currently, adjuncts (e.g., triamcinolone, indocyanine green) are typically used to maximize membrane visualization and removal. In this case re-staining was not performed since the iOCT revealed the membrane. Re-staining may have identified the membrane without iOCT. Further research is needed to better assess the role of iOCT in membrane visualization and the relative utility of contrast dyes and iOCT. The combined use of contrast dyes and iOCT may also have value in enhanced visualization of anatomic structures (e.g., posterior hyaloid relationships).11
To our knowledge, only a single case report has been published regarding the use of iOCT and VMT. In that report by Dayani and colleagues, tractional release was confirmed following hyaloid elevation utilizing iOCT visualization. There was a suggestion of improved contour, however, no quantitative analysis was performed. In the current study, several qualitative features were noted on iOCT following release of VMT. Following elevation, a high percentage of eyes appeared to show outer retinal alterations with increased height of the hyporeflective band between the ellipsoid zone and the RPE. This may represent photoreceptor stretching secondary to induced traction during hyaloid elevation. Alternatively, this may reflect subclinical neurosensory retinal detachment with photoreceptor disinsertion. The functional implications of these alterations are unknown and further research is needed to better correlate the outer retinal alterations with visual function/recovery. Interestingly, changes in the outer retina have been reported on SD-OCT following pharmacologic vitreolysis with ocriplasmin including alterations to the ellipsoid zone and increased subfoveal hyporeflectivity (e.g., subfoveal fluid).12 Whether these changes are similar to the changes noted intrasurgically deserve further investigation.
Subclinical anatomic alterations may occur between preoperative imaging and surgical intervention that would otherwise alter the surgeon’s plan. This study revealed that in 2 of 11 of cases of VMT progression occurred to a subclinical FTMH prior to surgical intervention. Two weeks and 6 weeks had passed in these cases, respectively, between the preoperative scan in clinic and the preincision iOCT scan. Same-day standard SD-OCT would have also revealed the change, but this is not usually standard clinical practice. In addition to changes that occur between the clinical visit and the surgical intervention, retinal anatomy may also change during surgical intervention. In this study, two additional cases were noted to have potential FTMH on iOCT during surgical intervention. This resulted in 33% of cases being treated surgically as a FTMH (e.g., ILM peeling, gas tamponade) rather than with routine VMT surgical management which may not have occurred without the assistance of iOCT.
Additional information regarding intrasurgical dynamics during VMT surgery could be potentially obtained using a microscope integrated OCT system (in contrast to the microscope-mounted system in this study). Though currently not commercially available in the United States, these predominantly research systems have been utilized to describe real-time surgical maneuvers, visualization of microsurgical instrumentaiton, and dynamic imaging of instrument motion and tissue interaction.13–16
This study has several limitations including its retrospective nature and relatively small sample size. The current OCT systems that are utilized for intraoperative imaging, including the Bioptigen system used in this study, lack image registration and tracking. This greatly hampers comparative measurements. To account for some of these limitations, scans were taken from multiple orientation (e.g., 0 degrees, 90 degrees, radial) to maximize visualization of the area of interest. Due the retrospective nature of the study, functional correlation with the anatomic changes was unable to be assessed in an optimal manner. In addition, specfic staining or re-staining with dyes, such as indocyanine green, was not mandated. Some of the alterations visualized with iOCT may have also been able to be visualized with re-staining, though not clinically apparent through the surgical microscope.
This study continues to build on the body of evidence that iOCT may provide important insights to the pathophysiology of surgical ophthalmic diseases while acting as a unique surgeon feedback tool that may impact surgical decision-making.4–10, 16 The novel outer retinal architectural changes identified need additional study, particularly in relation to the impact on functional outcomes. Additionally, the direct impact of iOCT on surgical-decision making in VMT surgery needs to be studied on a larger prospective scale to better delineate its role. In order to answer some of these questions, we have initiated a prospective multi-surgeon iOCT study, PIONEER, examining many of these issues as they relate to iOCT in ophthalmic surgery, including VMT.
Summary Statement.
Intraoperative optical coherence tomography (iOCT) can assess the microarchitectural changes that occur during surgical interventions for vitreomacular traction syndrome. Significant microstructural alterations may be noted utilizing iOCT, including changes that may impact surgical decision-making.
Acknowledgments
Financial Support: Research to Prevent Blindness (PKK); NIH/NEI K23EY022947 (JPE)
Footnotes
Financial Disclosures:
JPE: Bioptigen (P), Thrombogenics (C,S), Regeneron (S)
TT: None
PKK: Research to Prevent Blindness (R), Carl Zeiss Meditec (C), Topcon (C), Alcon (C), Novartis (C), Bausch and Lomb (C)
DFM: None
GSM: None
SKS: Bausch and Lomb (C, R); Bioptigen (P); Allergan (R)
References
- 1.Johnson MW. Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149(3):371–382. doi: 10.1016/j.ajo.2009.11.022. e1. [DOI] [PubMed] [Google Scholar]
- 2.Stalmans P, Benz MS, Gandorfer A, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012;367(7):606–615. doi: 10.1056/NEJMoa1110823. [DOI] [PubMed] [Google Scholar]
- 3.Chen TC, Cense B, Pierce MC, et al. Spectral domain optical coherence tomography: ultra-high speed, ultra-high resolution ophthalmic imaging. Arch Ophthalmol. 2005;123(12):1715–1720. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 4.Ehlers JP, Ohr MP, Kaiser PK, Srivastava SK. Novel microarchitectural dynamics in rhegmatogenous retinal detachments identified with intraoperative optical coherence tomography. Retina. 2013;33(7):1428–1434. doi: 10.1097/IAE.0b013e31828396b7. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers JP, Xu D, Kaiser PK, et al. INTRASURGICAL DYNAMICS OF MACULAR HOLE SURGERY: An Assessment of Surgery-Induced Ultrastructural Alterations with Intraoperative Optical Coherence Tomography. Retina. 2013 doi: 10.1097/IAE.0b013e318297daf3. [DOI] [PubMed] [Google Scholar]
- 6.Ehlers JP, Kernstine K, Farsiu S, et al. Analysis of pars plana vitrectomy for optic pit-related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011;129(11):1483–1486. doi: 10.1001/archophthalmol.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavala SH, Farsiu S, Maldonado R, et al. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee LB, Srivastava SK. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011;42:e71–e74. doi: 10.3928/15428877-20110804-05. Online. [DOI] [PubMed] [Google Scholar]
- 10.Ray R, Baranano DE, Fortun JA, et al. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology. 2011;118(11):2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers JP, McNutt SA, Kaiser PK, Srivastava SK. Contrast-enhanced intraoperative optical coherence tomography. Br J Ophthalmol. 2013 doi: 10.1136/bjophthalmol-2012-303048. E-pub. [DOI] [PubMed] [Google Scholar]
- 12.Freund KB, Shah SA, Shah VP. Correlation of transient vision loss with outer retinal disruption following intravitreal ocriplasmin. Eye (Lond) 2013;27(6):773–774. doi: 10.1038/eye.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehlers JP, Tao YK, Farsiu S, et al. Integration of a spectral domain optical coherence tomography system into a surgical microscope for intraoperative imaging. Invest Ophthalmol Vis Sci. 2011;52(6):3153–3159. doi: 10.1167/iovs.10-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehlers JP, Tao YK, Farsiu S, et al. Visualization of real-time intraoperative maneuvers with a microscope-mounted spectral domain optical coherence tomography system. Retina. 2013;33(1):232–236. doi: 10.1097/IAE.0b013e31826e86f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao YK, Ehlers JP, Toth CA, Izatt JA. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery. Opt Lett. 2010;35(20):3315–3317. doi: 10.1364/OL.35.003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binder S, Falkner-Radler CI, Hauger C, et al. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31(7):1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]