Abstract
Evolutionarily young genes that serve essential functions represent a paradox since they must perform a function that either was not required until after their birth or was redundant with another gene. How young genes rapidly acquire essential function is largely unknown. Here, we trace the evolutionary steps by which the Drosophila gene Umbrea acquired an essential role in chromosome segregation in D. melanogaster, since its origin less than 15 million years ago. Umbrea neofunctionalization occurred via loss of an ancestral heterochromatin-localizing domain, followed by alterations that rewired its protein interaction network and led to species-specific centromere localization. Our evolutionary cell biology approach provides temporal and mechanistic detail into how young genes gain essential function. Such innovations may constantly alter the repertoire of centromere proteins in eukaryotes.
Young essential genes (1) challenge long-standing dogmas about the relationship between essentiality and conservation (2). Partitioning of essential, ancestral functions (subfunctionalization) between (old) parental and (young) daughter genes (3, 4) explains one route by which young genes become essential. More difficult to understand is how new genes become essential via the emergence of novel function (neofunctionalization) (5). This could result from partial duplication of ancestral genes, novel gene fusions, or by rapid amino acid changes (6). The contribution of each of these processes to the acquisition of essential function is unknown, as are the underlying molecular changes.
To gain insight into the birth and evolution of essential function, we focused on one newly evolved gene in Drosophila. Umbrea/HP6/CG15636 arose via duplication of the intronless Heterochromatin Protein 1B (HP1B) gene into an intron of the dumpy gene (Fig. 1A)(7). HP1B is a chromosomal protein, which predominantly localizes to heterochromatin in D. melanogaster cells and regulates gene expression(8). HP1B is dispensable for viability (8), yet Umbrea is essential in D. melanogaster (1, 9) based on RNAi knockdown phenotypes. The 100% late larval-pupal lethality upon Umbrea knockdown could be rescued by an Umbrea-GFP fusion (Fig. S1). Genetic knockout experiments (Fig. S1) further confirmed that Umbrea is essential in D. melanogaster.
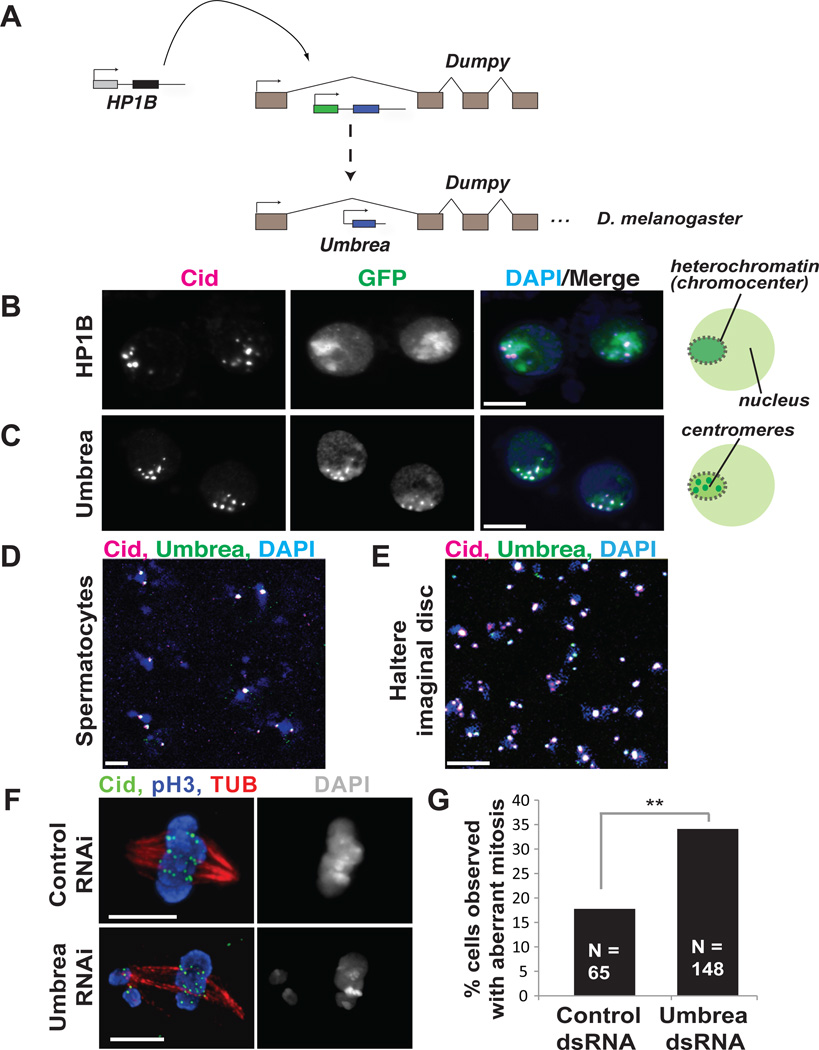
Fig. 1. Neofunctionalization of Umbrea.
(A) Umbrea originated via gene duplication of the intronless HP1B gene into an intron of the dumpy locus. (B) GFP-tagged HP1B localizes to heterochromatin in D. melanogaster Kc cells (GFP in green, anti-Cid in magenta, and DAPI in blue, colocalization appears white). (C) In contrast, Umbrea-GFP localizes to centromeres. (D,E) Endogenous Umbrea colocalizes with centromeres in testes and in larval imaginal discs (anti-Cid in magenta, anti-Umbrea in green, and DAPI in blue, bar = 5 microns). (F,G) S2 cells depleted of Umbrea by RNAi revealed increased mitotic errors (anti-Cid in green, phospho-H3 staining mitotic chromosomes in blue, and anti-tubulin in red), compared to dsRNA control.
We traced Umbrea’s evolutionary path following duplication from HP1B to understand when and how essential function was gained by comparing the localization of HP1B and Umbrea in D. melanogaster Kc cells. GFP-tagged HP1B proteins from both D. melanogaster and D. ananassae (whose divergence predates birth of Umbrea (7)), localized to pericentric heterochromatin and euchromatin (Figs. 1B and S2). In contrast, Umbrea-GFP predominantly localized to interphase centromeres, but not telomeres (Fig. 1C, S3A–B). Specific antibodies raised against Umbrea (Fig S4A) confirmed its centromeric localization in developing spermatocytes and larval imaginal discs (Fig 1D–E and S4B–C).
On the basis of Umbrea’s essentiality and centromere localization, we hypothesized that Umbrea was required for chromosome segregation. Upon depletion of of Umbrea by RNAi knockdown (Fig. S5A), D. melanogaster S2 cells displayed increased mitotic errors, including delayed chromosome alignment, early anaphase onset, lagging anaphase chromosomes, and multipolar configurations, compared to control cells (p<.05) (Fig. 1F–G, S5B, Movies S1-S3). These data suggest that Umbrea promotes proper chromosome segregation, but is not required for the localization of the centromeric histone Cid (Fig. 1F).
To date the birth of Umbrea and subsequent changes, we sequenced the Umbrea locus from 32 Drosophila species (Fig. S6A). While HP1B was preserved (7), we found Umbrea in only 20 of 32 species, dating its monophyletic origin to 12–15 million years ago (Fig. 2A, S6B). Using maximum likelihood methods, we observed evidence of both episodic and recurrent positive selection acting on Umbrea (Fig. S7A–D). These findings, together with the altered localization, lead us to conclude that neofunctionalization, not subfunctionalization, drove the divergence of Umbrea (10). Although Umbrea is essential in D. melanogaster, it was lost at least three independent times, in D. fuyamai, D. eugracilis, and in the suzukii clade (Fig. 2A), suggesting that Umbrea was not essential at or immediately following its birth.
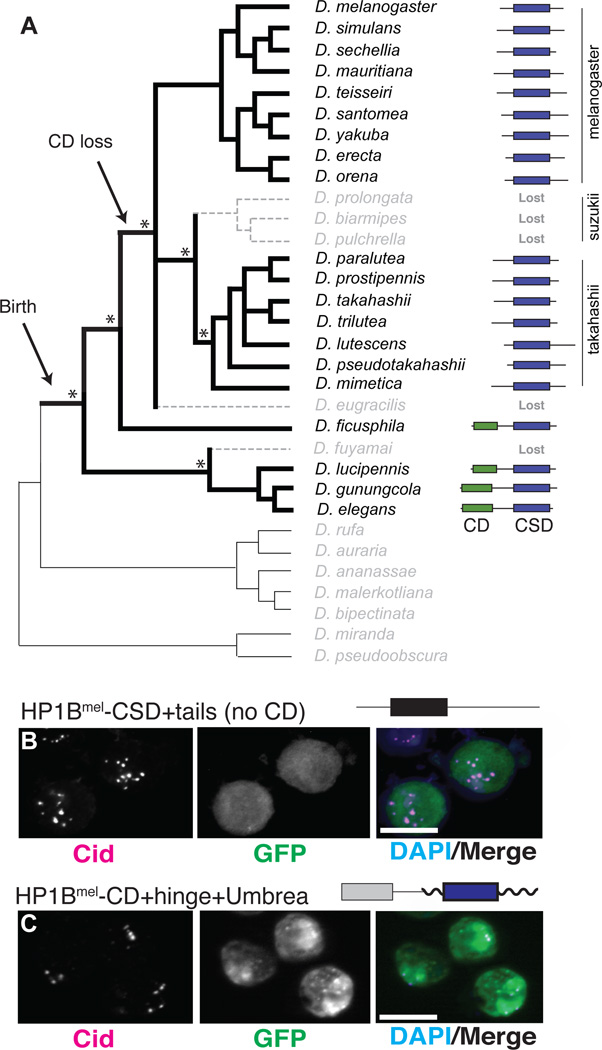
Fig. 2. Dynamic evolution of Umbrea following birth.
(A) PCR to shared syntenic sites followed by sequencing (Fig. S6) revealed the presence and structure of Umbrea genes. Asterisks indicate strong support for key branch points in the phylogeny(25), suggesting that Umbrea was lost at least three times. Umbrea is presented with HP1 canonical domains: chromodomain (CD, green) and chromoshadow domain (CSD, blue). (B) Localization of GFP-tagged HP1B lacking its CD is diffuse in D. melanogaster Kc cell nuclei (GFP is green, anti-Cid is magenta, and DAPI staining of DNA in blue, bar = 5 microns). (C) In contrast, HP1B-CD-hinge fused to Umbreamel delocalizes it from centromeres.
Four lineages retained full-length Umbrea genes, two of which preserved an intact chromodomain (CD) and ancestral residues essential for binding histone H3 tri-methyl lysine 9 (H3K9me) (Fig. S8) (11). However, most extant Umbreas have lost their CDs, and retained only the chromoshadow domain (CSD), which mediates protein-protein interactions (12) (Fig. 2A). We first tested how CD loss affected HP1B function. We found that an HP1B-GFP fusion lacking the CD lost heterochromatin localization (Fig. 2B), consistent with the requirement of HP1-CD for H3K9me binding (13). Furthermore, fusion of the HP1B CD and hinge to Umbrea-GFP reverted localization from centromeres to heterochromatin (Fig. 2C), suggesting that loss of the ancestral CD was necessary for Umbrea to gain new function. Our findings support a model of neofunctionalization that is facilitated via intermediate loss-of-function (14). Although CD loss was necessary, it was not sufficient for Umbrea neofunctionalization; both full-length (D. fuyamai) and CSD-only (D. eugracilis and the suzukii clade) Umbrea genes have been lost in evolution.
We next investigated the consequences of evolution in the Umbrea-CSD. CSDs are only found in HP1-family proteins and mediate interactions with other HP1s or proteins possessing degenerate PxVxL motifs (15). An amino acid alignment of HP1B and Umbrea revealed conservation of residues defining the CSD structural fold (Fig. 3A). In contrast, three of the nine residues that mediate specificity for PxVxL-recognition (16) changed along the branch leading to the melanogaster species subgroup (Fig. 3A, S9). We found that D. melanogaster Umbrea-CSD localized to centromeres (Fig. 3B). This property was not shared amongst HP1B- or even other Umbrea-CSDs, since neither ‘parental’ HP1Bmel-CSD nor Umbreaptak-CSD could localize to centromeric regions in D. melanogaster cells (Fig. S10B, 3C). We conclude that a discrete transition for centromere localization occurred in Umbrea-CSD after divergence of the melanogaster and takahashii subgroups, coincident with changes in the PxVxL-recognition residues. Indeed, reversion of these three residues (C15, I57, F59, Fig 3A, S9) to the ancestral state delocalized Umbreamel-CSD from centromeres (Fig. 3D). Moreover, replacement of the same residues in Umbreaptak-CSD to corresponding residues in Umbreamel resulted in a gain of centromere localization (Fig. 3E). These results suggest that centromeric localization by Umbrea-CSD originated in the common ancestor of the melanogaster species subgroup 5–7 million years ago. Consistent with this, we find that GFP-Umbreatei localizes to centromeres in D. teissieri cells (Fig. 3F). Centromeric localization may have also coincided with gain of essentiality, as Umbrea was lost three times prior to, but not after, CSD modification (Fig. 2A).
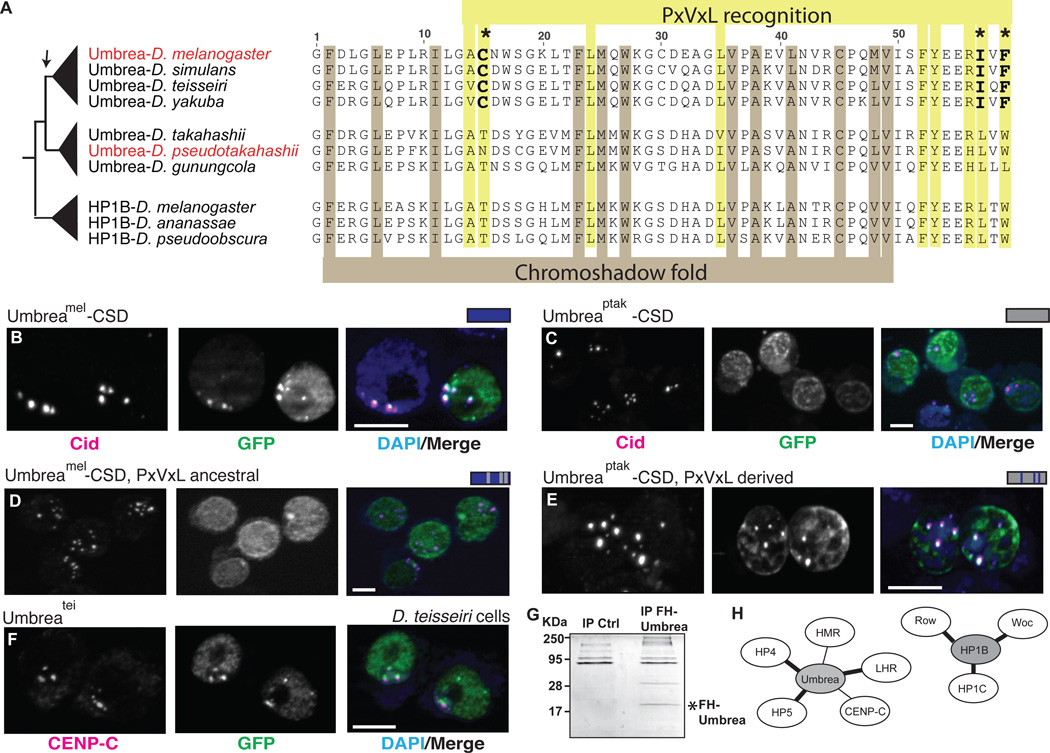
Fig. 3. Chromoshadow changes led to Umbrea centromere localization via altered protein-protein interactions.
(A) An amino acid alignment of HP1B and Umbrea CSDs reveals conservation of fold-defining residues but divergence in PxVxL-recognition residues. In particular, 3 changes (bold) are predicted to affect the binding specificity of Umbrea CSD. (B) GFP-tagged Umbreamel CSD (green) colocalizes with Cid (magenta) at centromeres in D. melanogaster Kc cells (bar = 5 microns, colocalization appears white). (C) However, GFP-tagged Umbreaptak CSD does not localize to centromeres. (D) Reversion of Umbreamel PxVxL-recognition residues (C-I-F) to ancestral states (T-L-W) causes delocalization from centromeres. (E) In contrast, introducing PxVxL-recognition residues (C-I-F) is sufficient to localize Umbreaptak CSD to centromeres (compare to Fig. 3C). (F) Umbreatei colocalizes with centromeric protein CENP-C in D. teissieri cells. (G) Immunoprecipitation of Flag-HA-tagged Umbrea pulls down protein complexes in S2 cells. (H) Analysis of these complexes reveals that Umbrea and HP1B have mutually exclusive protein-protein interactions. Umbrea interacts with centromere and heterochromatin proteins (Table S2, bold lines indicate confirmation of previously reported interactions(9, 17)), but not with the primary targets of HP1B(18).
To test the prediction that mutation of PxVxL recognition resulted in CSD centromere localization by alteration of protein interactions, we performed proteomic analyses to identify proteins that co-immunoprecipitate with Umbrea in S2 cells (Fig. 3G). Many chromatin factors were found in this set (Table S1), including heterochromatin proteins HP4/Hip and HP5 (previously shown to be direct interactors of Umbrea(9, 17)), as well as novel interactions with the H3K9 methyltransferase Su(var)3–9 and the centromeric protein Cenp-C. Importantly, we found no overlap with protein partners of HP1B, which include the euchromatic proteins HP1C, Woc and Row (18) (Fig. 3H), suggesting a rewiring of the protein interaction network of Umbrea.
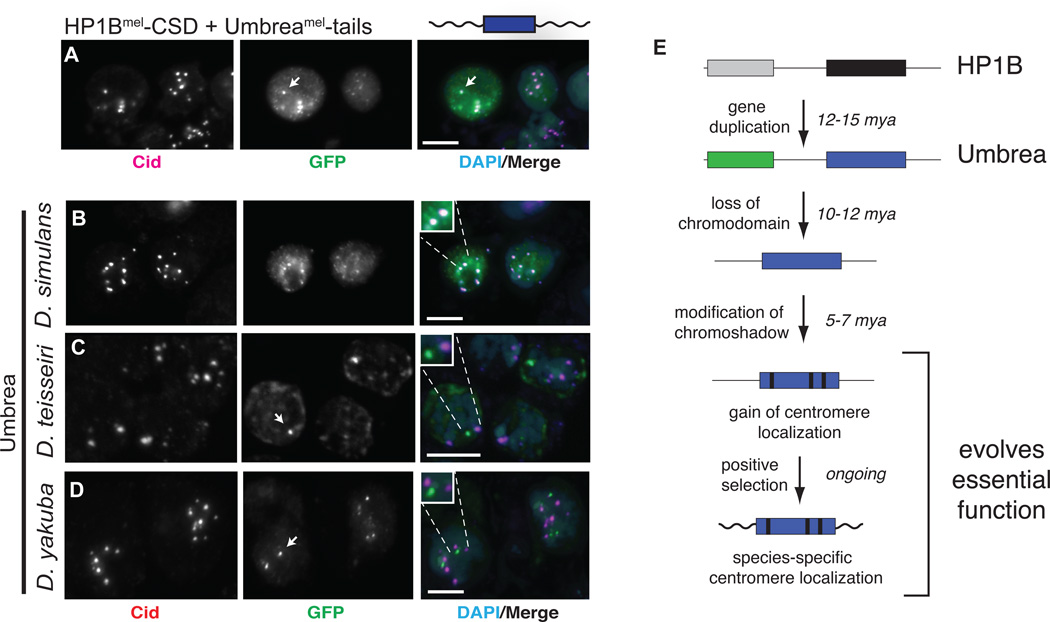
Our evolutionary analyses (Fig. S7A–D) indicated that the most recent innovations in Umbrea occurred in the short tail sequences that flank the CSD. We tested how these changes contributed to Umbrea neofunctionalization. While HP1Bmel-CSD alone showed no discrete localization (Fig. 2B), addition of Umbreamel-tails was sufficient to confer centromere localization (Fig. 4A). These data indicate that Umbrea may target centromeres using both the CSD and the tails. While the CSD likely mediates its localization via protein-protein interactions, Umbrea-tails may bind centromeric nucleic acids, analogous to the hinge region of mammalian HP1alpha, which binds DNA in vitro (19). Since centromeric DNA sequence diverges rapidly (20), we tested whether rapid evolution of the Umbrea-tails resulted in species-specificity. We found that Umbreasim localized (Fig. 4B) to centromeres in D. melanogaster. However, Umbreatei and Umbreayak did not (Fig. 4C–D), localizing instead to distinct foci. Although positive selection of Umbrea preceded its centromeric localization (Fig. S7), these data suggest that positive selection in the melanogaster species subgroup resulted in species-specific centromere targeting, reminiscent of CenH3/Cid in Drosophila (21). For example, despite mislocalizing in D. melanogaster cells, Umbreatei appropriately localized to D. teissieri centromeres (Fig. 3F).
Fig. 4. Species-specific centromere targeting of Umbrea.
(A) GFP-fusion of Umbreamel-tails with HP1B-CD (green) localizes to centromeres (anti-Cid in magenta, bar = 5 microns, colocalization appears white). (B-D) D. melanogaster Kc cell centromeric localization (Cid, red) of Umbrea orthologs (GFP, green) from D. simulans, D. teissieri and D. yakuba worsens with increased divergence. (E) Steps to essential neofunctionalization by Umbrea following gene duplication.
Our analyses suggest that gain of essential function evolved in discrete steps (Fig. 4E) (5) that involved the loss of an ancestral domain (CD), rewiring of protein interaction networks (CSD), and species-specific changes (tails). Umbrea was likely not essential for much of its evolutionary history, since intermediate forms were lost multiple times.
Our finding that Umbrea rapidly became essential for the conserved process of chromosome segregation is unexpected. Drosophila species that never possessed or lost Umbrea still carry out chromosome segregation. This suggests that the essential function of Umbrea might be a result of a lineage-specific requirement. Just as genetic conflicts arising during meiosis may drive rapid evolution of existing centromere proteins (22), we propose that recurrent changes at centromeric DNA satellites could drive the retention of duplicate genes like Umbrea to alleviate selective pressure on essential centromeric proteins. This is analogous to pathogen-driven genetic conflict, which promotes the diversification of existing and new antiviral immune genes (23). This process would result in idiosyncratic retention of centromeric proteins that become essential as they integrate into existing networks. Intriguingly, other HP1B-derived CSD-only genes are found in other Drosophila species that diverged prior to the birth of Umbrea (7), raising the possibility of convergent evolution of Umbrea-like centromere factors. This process may explain the broad diversity and divergence amongst centromere proteins across taxa (24). While a large fraction of the many young, essential genes identified in Drosophila (1) may result from subfunctionalization, others (like Umbrea) may illuminate other essential processes that could require recurrent genetic innovation to mitigate previously unappreciated adaptive challenges within the cell.
Supplementary Material
Acknowledgments
We thank J. Bloom, M. Daugherty, M. Emerman, D. Gottschling, M. Levine, M. Patel, N. Phadnis, K. Peichel and W. Shou for helpful comments and Drosophila colleagues for generous sharing of reagents. This work was supported by NIH Training grant T32HG000035 and an NSF GRFP (to BDR), an EU network grant (EpiGeneSys 257082, to AI), NSF award 1024973 (to BGM), a grant from the Mathers Foundation and grant R01GM074108 from the NIH (to HSM). HSM is an Early Career Scientist of HHMI. Umbrea DNA sequences have been submitted to GenBank under accession numbers KC660086- KC660100. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (PRIDE partner repository dataset identifier PXD000163).
References
- 1.Chen S, Zhang YE, Long M. New genes in Drosophila quickly become essential. Science. 2010;330:1682. doi: 10.1126/science.1196380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miklos GL, Rubin GM. The role of the genome project in determining gene function: insights from model organisms. Cell. 1996;86:521. doi: 10.1016/s0092-8674(00)80126-9. [DOI] [PubMed] [Google Scholar]
- 3.Force A, et al. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Evol. 1999;49:169. doi: 10.1007/pl00006540. [DOI] [PubMed] [Google Scholar]
- 5.Innan H, Kondrashov F. The evolution of gene duplications: classifying and distinguishing between models. Nat Rev Genet. 2010;11:97. doi: 10.1038/nrg2689. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Dean AM, Brunet F, Long M. Evolving protein functional diversity in new genes of Drosophila. Proc Natl Acad Sci U S A. 2004;101:16246. doi: 10.1073/pnas.0407066101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine MT, et al. Phylogenomic Analysis Reveals Dynamic Evolutionary History of the Drosophila Heterochromatin Protein 1 (HP1) Gene Family. PLoS Genet. 2012;8:e1002729. doi: 10.1371/journal.pgen.1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang D, Wang D, Sun F. Drosophila melanogaster heterochromatin protein HP1b plays important roles in transcriptional activation and development. Chromosoma. 2011;120:97. doi: 10.1007/s00412-010-0294-5. [DOI] [PubMed] [Google Scholar]
- 9.Joppich C, Scholz S, Korge G, Schwendemann A. Umbrea, a chromo shadow domain protein in Drosophila melanogaster heterochromatin, interacts with Hip, HP1 and HOAP. Chromosome Res. 2009;17:19. doi: 10.1007/s10577-008-9002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn MW. Distinguishing Among Evolutionary Models for the Maintenance of Gene Duplicates. J Hered. 2009;100:605. doi: 10.1093/jhered/esp047. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs SA, Khorasanizadeh S. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science. 2002;295:2080. doi: 10.1126/science.1069473. [DOI] [PubMed] [Google Scholar]
- 12.Murzina N, Verreault A, Laue E, Stillman B. Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol Cell. 1999;4:529. doi: 10.1016/s1097-2765(00)80204-x. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 14.Ohta T. Evolution by gene duplication and compensatory advantageous mutations. Genetics. 1988;120:841. doi: 10.1093/genetics/120.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smothers JF, Henikoff S. The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr Biol. 2000;10:27. doi: 10.1016/s0960-9822(99)00260-2. [DOI] [PubMed] [Google Scholar]
- 16.Thiru A, et al. Structural basis of HP1/PXVXL motif peptide interactions and HP1 localisation to heterochromatin. EMBO J. 2004;23:489. doi: 10.1038/sj.emboj.7600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giot L, et al. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 18.Abel J, Eskeland R, Raffa GD, Kremmer E, Imhof A. Drosophila HP1c is regulated by an auto-regulatory feedback loop through its binding partner Woc. PLoS One. 2009;4:e5089. doi: 10.1371/journal.pone.0005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meehan RR, Kao CF, Pennings S. HP1 binding to native chromatin in vitro is determined by the hinge region and not by the chromodomain. EMBO J. 2003;22:3164. doi: 10.1093/emboj/cdg306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lohe AR, Brutlag DL. Identical satellite DNA sequences in sibling species of Drosophila. J Mol Biol. 1987;194:161. doi: 10.1016/0022-2836(87)90365-2. [DOI] [PubMed] [Google Scholar]
- 21.Vermaak D, Hayden HS, Henikoff S. Centromere targeting element within the histone fold domain of Cid. Mol Cell Biol. 2002;22:7553. doi: 10.1128/MCB.22.21.7553-7561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malik HS, Henikoff S. Major evolutionary transitions in centromere complexity. Cell. 2009;138:1067. doi: 10.1016/j.cell.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty MD, Malik HS. Rules of engagement: molecular insights from host-virus arms races. Annu Rev Genet. 2012;46:677. doi: 10.1146/annurev-genet-110711-155522. [DOI] [PubMed] [Google Scholar]
- 24.Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol. 2008;9:33. doi: 10.1038/nrm2310. [DOI] [PubMed] [Google Scholar]
- 25.Prud'homme B, et al. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440:1050. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- 26.Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293:1098. doi: 10.1126/science.1062939. [DOI] [PubMed] [Google Scholar]
- 27.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 28.Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006;34:W609. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 30.Cox J, et al. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 31.Voog J, D'Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature. 2008;454:1132. doi: 10.1038/nature07173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong B. Color blindness. Nat Methods. 2011;8:441. doi: 10.1038/nmeth.1618. [DOI] [PubMed] [Google Scholar]
- 33.Blower MD, Karpen GH. The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat Cell Biol. 2001;3:730. doi: 10.1038/35087045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heeger S, et al. Genetic interactions of separase regulatory subunits reveal the diverged Drosophila Cenp-C homolog. Genes Dev. 2005;19:2041. doi: 10.1101/gad.347805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klattenhoff C, et al. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell. 2009;138:1137. doi: 10.1016/j.cell.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao G, et al. HipHop interacts with HOAP and HP1 to protect Drosophila telomeres in a sequence-independent manner. EMBO J. 2010;29:819. doi: 10.1038/emboj.2009.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heun P, et al. Mislocalization of the Drosophila centromere-specific histone CID promotes formation of functional ectopic kinetochores. Dev Cell. 2006;10:303. doi: 10.1016/j.devcel.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vizcaino JA, et al. The PRoteomics IDEntifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res. 2013;41:D1063. doi: 10.1093/nar/gks1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.