Abstract
Introduction
Recently serum soluble urokinase receptor (suPAR) has been proposed as a cause of two thirds of cases of focal segmental glomerulosclerosis (FSGS). It was noted to be uniquely elevated in cases of primary FSGS with higher levels noted in cases that recurred post-transplant. It is also suggested as a possible target and marker of therapy.
Methods
We studied serum and urine suPAR from pre-transplant banked samples from 86 well characterized kidney transplant recipients and 10 healthy controls to determine its prognostic utility.
Causes of native kidney disease were; primary focal segmental glomerulosclerosis, diabetic nephropathy, membranous nephropathy, immunoglobulin A nephropathy, and autosomal dominant polycystic kidney disease. suPAR was measured using a commercially available enzyme-linked immunosorbent assay kit. Urinary suPAR was indexed to creatinine.
Results
Both serum and urine suPAR correlated with proteinuria and albuminuria. Serum suPAR was found to be elevated in all transplant candidates with advanced renal disease compared to healthy controls and could not differentiate disease diagnosis. Urine suPAR was elevated in cases of recurrent focal segmental glomerulosclerosis compared to all other causes of end stage renal disease. Recurrent focal segmental glomerulosclerosis cases had substantially higher proteinuria compared to all other cases. However, elevated urinary suPAR showed a trend in providing additional prognostic information beyond proteinuria in the small cohort of recurrent FSGS cases.
Conclusion
In advanced renal disease, elevated serum suPAR is not unique to FSGS cases. Urinary suPAR appears to be higher in cases of focal segmental glomerulosclerosis destined for recurrence and merits further evaluation.
Keywords: Recurrent FSGS, serum suPAR, urine suPAR, Membranous Nephropathy, IgA Nephropathy, Focal Segmental Glomerulosclerosis, Polycystic Kidney Disease, Diabetic Nephropathy
Introduction
One of the aims of the evaluation of a kidney transplant candidate is determining the risk of native kidney disease recurrence(1). Frequently, patients presenting for kidney transplant evaluation carry a diagnoses based on clinical history and did not undergo a biopsy(1). Some of these cases may be due to primary Focal Segmental Glomerulosclerosis (FSGS). In some cases the correct diagnosis is made only after recurrence in the allograft (2).
FSGS accounts for up to 20% of glomerular disease and is the cause of end stage renal disease in 11 % of children and 1 to 5% of adults (3, 4). Recurrence post-transplant has been reported to be 86% in children and 36% in adults (2). These recurrences are frequently early and can be catastrophic resulting in early loss of the allograft.
Pre and post-transplant therapy in the form of plasma exchange and the use of the anti CD20 monoclonal antibody rituximab have been reported to be successful (2, 5–7). Thus, correct identification of cases of primary FSGS and identifying which ones can recur is of paramount importance given the need for more aggressive monitoring and possible initiation of pre and/or post-transplant therapeutic interventions.
Recently the soluble urokinase receptor suPAR was identified as a potential cause of two thirds of cases of primary FSGS and levels where higher in cases of recurrent FSGS (8, 9). The urokinase receptor uPAR is a glycoprotein membrane bound receptor with three domains (DI, DII, and DIII), no transmembrane domains, and is bound to cell membranes by a glycosylphosphatidylinositol (GPI) anchor. The whole receptor and various segments of it are found free in the serum (suPAR). Low levels of this free receptor are believed to be physiologic and play a role in cell-cell interaction (10).
We hypothesized that serum and/or urine suPAR could differentiate primary FSGS from other causes of kidney disease. We also hypothesized that it will identify cases destined for recurrence. Thus, it can be used in risk stratifying kidney transplant candidates with FSGS or patients whose native kidney disease is not known with certainty.
Results
Study Cohort
Table 1 shows the pre-transplant characteristics of the study patients.
Table 1.
Characteristics of the Study Cohort at Baseline:
| Donors (N=10) |
All Candidates (N=86) |
R-FSGS (N=13) |
NR-FSGS (N=15) |
IgAN (N=15) |
MN (N=13) |
DN (N=15) |
ADPKD (N=15) |
p* | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 42.6 (9.6) | 51.5 (11.2) | 42.5 (18) | 51.2 (14.8) | 45.3 (10.5) | 53.7 (10..8) | 61.2 (10.3) | 54.5 (11.1) | 0.005# |
| Males N (%) | 4 (40) | 57 (66) | 7 (54) | 12 (80) | 11 (73) | 7 (54) | 13 (87) | 7 (47) | 0.121^ |
| eGFR ml/min/SA | 86.0 (14.9) | 11.2 (4.9) | 8.3 (4.1) | 12.7 (4.8) | 10.0 (3.9) | 10.8 (4.1) | 12.6 (6.9) | 12.2 (3.8) | 0.14# |
| Dialysis N(%) | NA | 34 (40) | 8 (62) | 3 (20) | 8 (53) | 8 (62) | 5 (33) | 2 (13) | 0.018^ |
| Urine Available N (%) | 10 (100%) | 61 (71) | 5 (38) | 10 (67) | 15 (100) | 4 (31) | 12 (80) | 15 (100) | <0.0001^ |
| Albuminuria mg/g | 6 (2,11) | 1087 (293, 2587) | 7576 (5383, 8333) | 1674 (852, 2547) | 1022 (712, 1735) | 4928 (2806, 9659) | 1118 (188, 2568) | 105 (46, 388) | 0.0001# |
| Proteinuria mg/g | 19 (14, 30) | 1836 (648, 4435) | 16193 (14797, 20460) | 2478 (1523, 3689) | 1836 (859, 2576) | 8093 (4757, 16057) | 1713 (573, 4128) | 318 (187, 915) | 0.0001# |
p is for trend across the candidate groups excluding donors
Kruskal Wallis test
Fisher’s exact test
Serum suPAR in Health and Disease
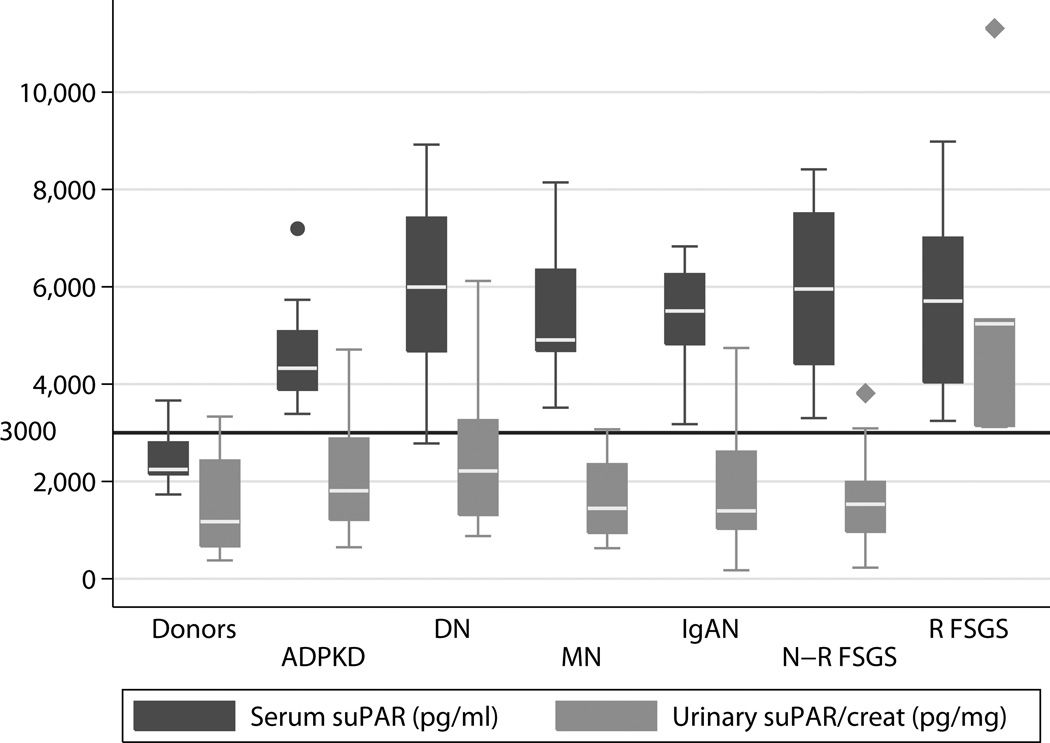
Serum suPAR was elevated in kidney transplant candidates compared to healthy living kidney donors (5164 [4326, 6367] vs. 2246 [2146, 2804] pg/ml, p <0.0001). Figure 1 depicts serum and urinary suPAR in the different patient groups as well as the living kidney donor controls. Combined, the two FSGS groups had elevated serum suPAR compared to donors (5831 [4297, 7258] vs. 2246 [2146, 2804] pg/ml p <0.0001). We did not, however, observe a difference in serum suPAR between FSGS patients compared to the other renal disease states (5831 [4297, 7258] 5107 [4326, 6261] p= 0.37).
Fig 1.
Serum and Urinary suPAR in the Various Subject Groups (N=96 Serum, N=71 Urine)
Urinary suPAR in Health and Disease
Fig 1 depicts serum and urinary suPAR in the various patient groups. Taken together we did not observe a difference between the entire cohort of FSGS patients and controls (1990 [1470, 3813] vs. 1178 [667, 2431] pg/mg p= 0.12). Similarly there was no difference detected between all FSGS cases (recurrent and non-recurrent) and other causes of ESRD. Looking at the subset of FSGS patients that did have a recurrence there was clearly an elevation in the urinary suPAR compared to controls (5239 [3142, 5331] vs. 1178 [667, 2431] p =0.0048) and other causes of ESRD (1598 [1192, 2628] p=0.0016).
suPAR and Renal Function
In the entire cohort there was a negative correlation between renal function and serum suPAR −0.36 p=0.003 but not urinary suPAR −0.118 p= 0.33. The correlation with serum suPAR however was confounded by disease state, as no such correlation was found in the controls alone or the candidates alone.
We did not observe a difference in serum suPAR between patients on dialysis and those who were not (5130 [4374, 6350] vs 5382 [4254, 6386] pg/ml, respectively p = 0.78).
Urinary suPAR was higher in those on dialysis (2574 [1385, 3718] vs 1551 [1130, 2582] pg/mg, respectively p=0.046).
Focusing on transplant candidates not on dialysis there was no correlation between serum or urinary suPAR and renal function: p=0.40 and 0.16 respectively.
Serum and Urinary suPAR Relationship to Proteinuria
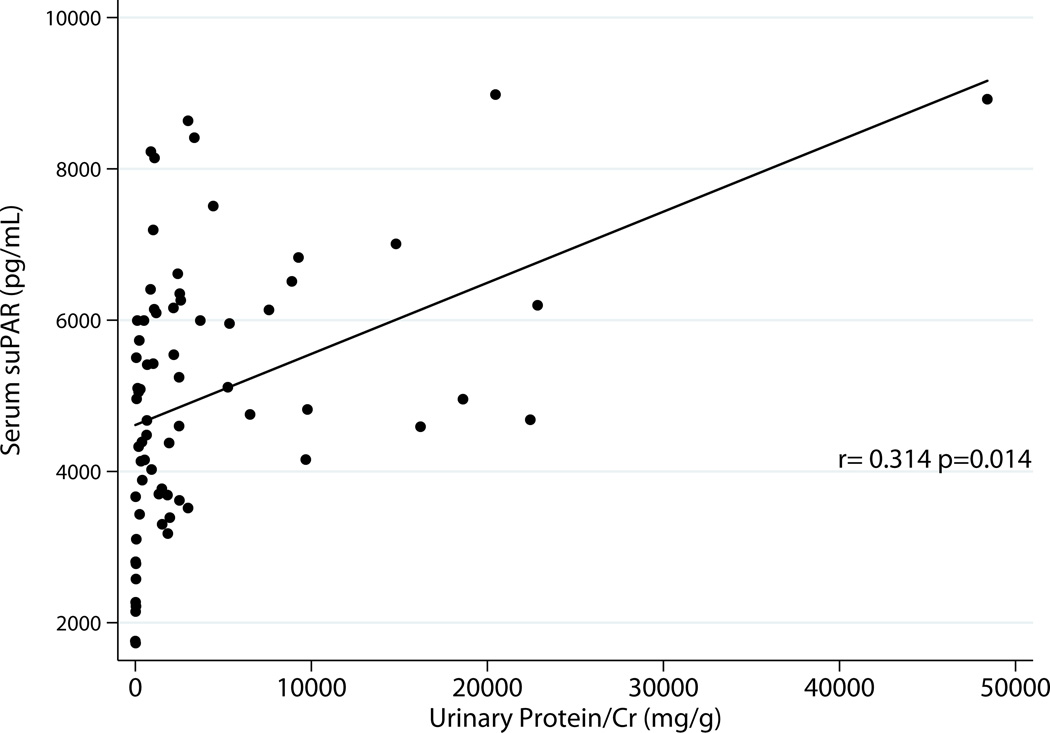
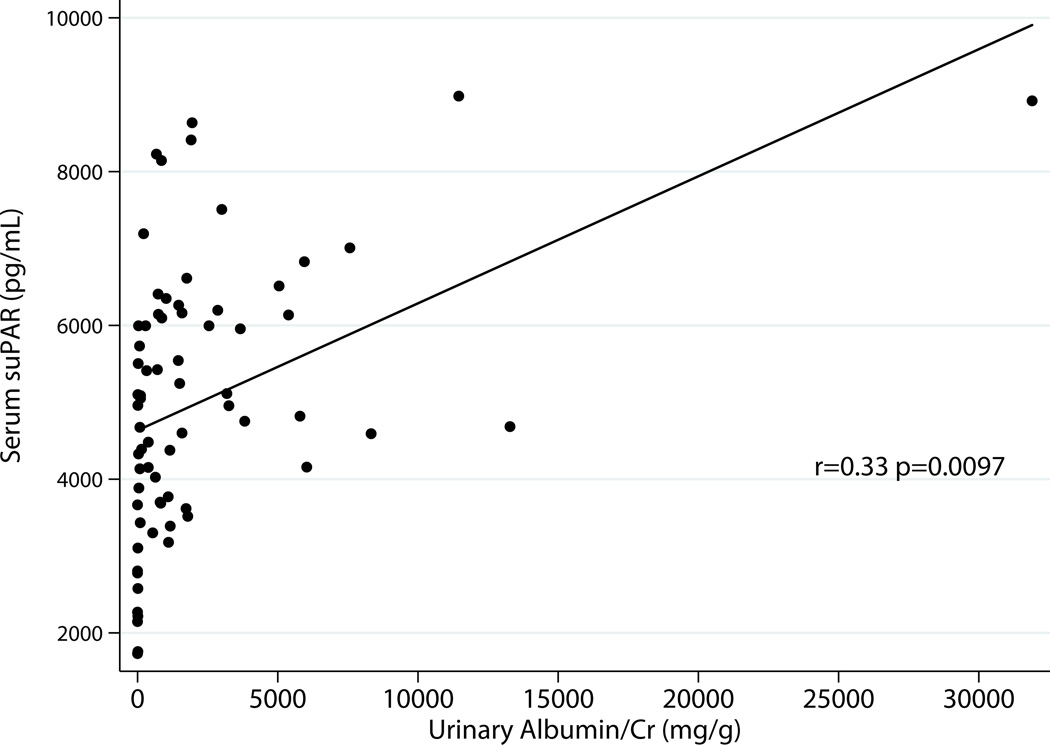
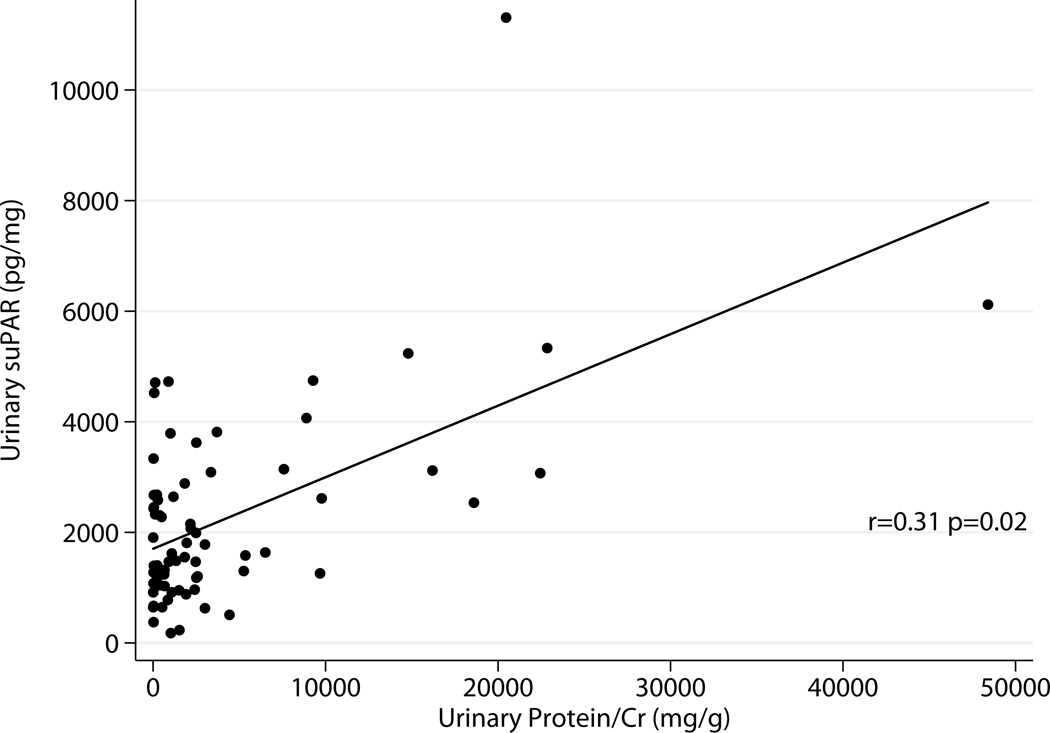
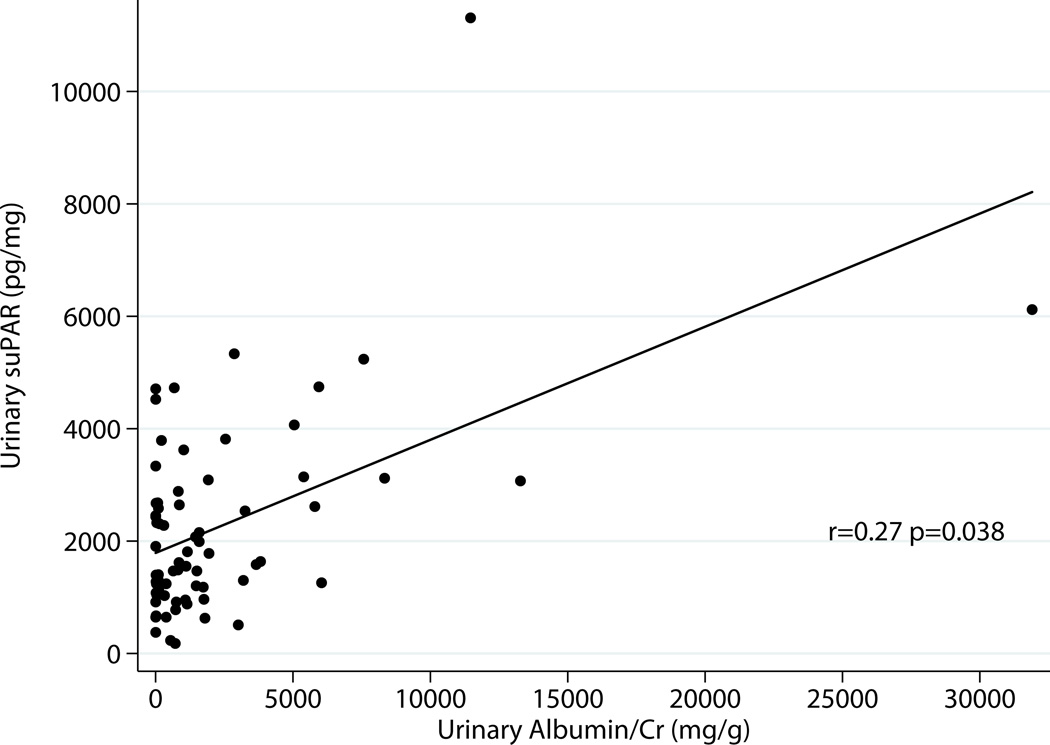
Excluding donors, there was correlation between serum suPAR and total urinary protein r=0.31 p=0.014 as well as albumin excretion r=0.33 p=0.0097 (Fig 2a & 2b). Urinary suPAR also correlated with total urinary protein r=0.31 p=0.02 and albumin excretion r=0.27 p=0.038 (Fig 3a & 3 b).
Fig 2.
a: Relationship between Serum suPAR and Total Proteinuria Excluding Donors (N=61)
b: Relationship between Serum suPAR and Albuminuria Excluding Donors (N=61)
Fig 3.
a: Relationship between Urine suPAR and Total Proteinuria Excluding Donors (N=61)
b: Relationship between Urine suPAR and Albuminuria Excluding Donors (N=61)
Relationship between Serum and Urine suPAR
Serum and urinary suPAR correlated with each other for the entire cohort r=0.41 p= 0.01. This correlation was also present in the combined disease groups r=0.41 p=0.0009 but not in the healthy control population r= −0.13 p=0.72.
Serum suPAR and FSGS
Among the transplant candidates serum suPAR was unable to predict FSGS cases OR 1.0 [95%CI 0.99–1.00, p=0.23] or cases of recurrent FSGS OR 1.0 [95%CI 0.99–1.00, p=0.54].
Urine suPAR, Proteinuria and FSGS
In our cohort, the recurrent FSGS group had elevated levels of urinary suPAR, albumin and total protein excretion compared to other disease groups (Table 1, Fig 1). As Table 2 depicts urinary protein and suPAR, but not albumin excretion were predictors of FSGS recurrence in univariate models, while in a multivariate model urinary suPAR but not total protein displayed a trend (p=0.065).
Table 2.
Univariate and Multivariate Models of Urinary suPAR, Total Proteinuria, and Albuminuria to FSGS
| Any FSGS (All Candidates) |
Recurrent FSGS (All Candidates) |
Recurrent FSGS (FSGS cases only) |
|
|---|---|---|---|
| Univariate | |||
| suPAR (ng/mg) | 1.27 [0.93–1.75, 0.138] | 2.67 [1.23–5.77, 0.013] | 6.36 [0.77–52.16, 0.085] |
| Total Proteinuria (g/g) | 1.05 [0.98–1.12, 0.183] | 1.12 [1.01–1.24, 0.026] | # |
| Albuminuria (g/g) | 1.06 [0.94–1.18, 0.353] | 1.12 [0.98–1.28, 0.089] | 5.29 [0.60–46.34, 0.133] |
| Multivariate | |||
| suPAR (ng/mg) | 2.37 [0.95–5.91, 0.065] | ||
| Total Proteinuria (g/g) | 1.02 [0.92–1.14, 0.674] | ||
OR (Odds ratio) [95% confidence interval (CI), p value]
Total proteinuria >5.35g/g predicts data perfectly
Discussion
In this study we examined pre-transplant urine and serum suPAR in 86 kidney transplant candidates divided into 6 diagnostic groups; Primary FSGS that did not recur post-transplant, FSGS that did recur, IgA nephropathy, membranous nephropathy, autosomal dominant polycystic kidney disease and diabetic nephropathy. We also studied serum and urine obtained from healthy living kidney donors prior to donation as controls.
We found that serum suPAR was elevated in all transplant candidates compared to controls. The elevated serum levels in cases of primary FSGS is in keeping with the previous findings of Wei and colleagues in their seminal work (8). We could not, however, detect a difference in serum suPAR between cases of FSGS that recurred vs. those that did not. We also found elevated pre-transplant levels of serum suPAR in cases of membranous nephropathy that was not previously reported (8) as well as in cases of IgA nephropathy, ADPKD, and diabetic nephropathy, all of which were not previously studied (8). Pre-transplant levels of suPAR were no different between the disease categories. It should be noted, however, that our patient cohort included only patients with advanced renal disease about to receive a renal transplant. Previous work has shown that suPAR is elevated in conditions associated with inflammation, infection and a heightened immune response (11–14). There is also the possibility of increased retention of suPAR in advanced cases of chronic/endstage renal disease in addition to the increased production in these conditions associated with increased inflammation (15). This is one potential reason we did not detect a difference between cases of FSGS and other glomerular and non-glomerular cases of ESRD in this cohort as compared to previous studies reporting such a difference (8, 9).
In our cohort we did not detect a correlation between serum suPAR and estimated GFR in either donors or candidates. It should be noted however that in the candidates the range of eGFR was very limited with all patients having very low GFRs and as such this could have limited our ability to detect a correlation. In healthy subjects the spread of both serum suPAR and eGFR was also limited. Combining donors and candidates there was a correlation between suPAR and GFR but this is likely confounded by the disease state. Donors had high GFR and low suPAR while candidates had low GFR and high suPAR.
Serum suPAR did correlate with the level of proteinuria and albuminuria pre-transplant. Previously it was reported to correlate with proteinuria as a categorical variable (present/absent) but not level of proteinuria (8). This again could be the result of this cohort being so advanced in the renal dysfunction.
This study is one of the first to examine urinary suPAR. Although suPAR is likely freely filtered given its molecular weight of <55, 000 daltons, local production and or processing for the filtered suPAR in the renal tubules could affect urinary levels. Indeed, our data support this possibility since the correlation between serum and urinary suPAR was very modest, Spearman’s ρ 0.41(p=0.0009).
As with serum suPAR, urinary suPAR correlated with both proteinuria and albuminuria (Fig 3). Unlike serum suPAR however, we noted that urinary suPAR was significantly elevated in cases of FSGS that recurred compared to all other causes of ESRD including non-recurrent FSGS (Fig 1). This small group of recurrent FSGS also had elevated levels of albuminuria and total proteinuria. On univariate analysis, total protein and suPAR predicted recurrent FSGS and albuminuria did not. In a model containing suPAR and total protein to predict recurrent FSGS among all transplant candidates, total proteinuria lost its predictive value (p=0.67) while urinary suPAR had a close trend (p=0.065). This finding needs to be confirmed in larger groups of patients with recurrent FSGS, and end stage renal disease with marked proteinuria from other causes.
We believe that the better performance of urinary suPAR over serum suPAR could be because the urinary levels better reflect the intrarenal milieu encompassing systemic levels as well as intrarenal production of suPAR. Another possibility is that intratubular processing of the various suPAR fragments may overcome the limitation of the current assay in differentiating the different fragments of suPAR that has been described (10). If tubular processing of filtered suPAR results in differential removal of nonpathogenic fragments of suPAR then it will allow the identification of elevated levels of suPAR in the recurrent FSGS group vs. all other.
This study has several strengths including a well-characterized patient cohort, a group of patients with similar renal function, post-transplant protocol biopsies that would have excluded unforeseen misdiagnosed native kidney disease, and well documented FSGS disease recurrence by both increasing proteinuria and histology. Limitations of the study include the single time point of measuring serum and urine suPAR at the time of end stage renal disease, and the fact that some patients did not have urine samples present or were anuric. We also studied limited numbers of patients with marked proteinuria outside of the recurrent FSGS group. It should be noted however that compiling a well characterized cohort with urine and serum samples obtained prior to therapy is rare. The findings of this study should be taken as ground work for larger prospective studies. The design of these studies will be challenging given the inclination to possibly modify therapy following the identification of elevated serum and or urine suPAR levels.
In conclusion, we found that in advanced renal disease due to various etiologies serum suPAR was elevated. It could not differentiate FSGS cases from other causes of ESRD nor differentiate cases of FSGS that recur from those that do not. Urinary suPAR was higher in cases of primary FSGS that recurred after kidney transplantation, as were urinary albumin and total protein excretion. Urinary suPAR may provide additional information beyond protein excretion. Further study of the role of urinary suPAR in identifying cases of FSGS at risk for recurrence is warranted.
Methods
Subject Selection
The Mayo Clinic transplant center banks serum and urine samples on all consenting kidney donors and recipients. Patients included in this study had pre transplant serum with or without urine collected and stored in the bio-specimen bank. Patients listed with primary renal disease were identified from the centers transplant data base. These were cross-referenced with available serum and urine samples that were collected just prior to transplant. Each case was reviewed by one investigator (CRF) to comply with the criteria for each diagnostic group below.
Patients were grouped, based on the primary cause of their native kidney disease into six groups as defined below. For controls, we identified 10 living kidney donors with pre donation serum and urine available in the bio-specimens bank. The samples collected closest to the transplant event were used for the analyses.
Primary FSGS was defined by (a) Nephrotic range proteinuria with a clearly documented native renal biopsy revealing focal and segmental glomerulosclerosis in the absence of any other diagnostic lesion. (b) No family history of proteinuric renal disease. (c) Absence of causes of secondary FSGS (including severe obesity, congenital urologic disease, HIV infection, or prior nephrectomy).
Primary cases of FSGS were sub classified into
Recurrent FSGS (N= 13)
Patient who had native kidney disease attributed to primary FSGS and who experienced recurrence within the first post-transplant year. Recurrence was defined as increasing proteinuria post-transplant with a biopsy showing diffuse foot process effacement.
Non-recurrent FSGS (N=15)
Patients with biopsy proven FSGS of the native kidneys who were deemed to have primary FSGS and did not have a post-transplant recurrence. Lack of recurrence was defined as decreasing proteinuria post-transplant and one-year post-transplant had a protocol biopsy without evidence of FSGS or diffuse foot process effacement.
IgA nephropathy (N=15)
Kidney transplant recipients who were diagnosed with IgA nephropathy prior to transplant by renal biopsy or suspected of having IgA nephropathy and on post-transplant biopsy found to have IgA deposition in the renal allografts.
Membranous Nephropathy (N=13)
Patients who had biopsy proven membranous nephropathy as the cause of native kidney disease. Patients with causes for secondary membranous nephropathy were excluded.
Diabetic Nephropathy (N=15)
Patients with type 2 diabetes mellitus and biopsy proven diabetic nephropathy prior to transplant.
Autosomal Dominant Polycystic Kidney Disease (N=15)
Patients with imaging studies revealing extensive cystic kidney disease with or without genetic testing.
Sample processing
All subjects included in this study had consented to collection of their samples (urine and serum) as part of an ongoing bio-specimens bank. All samples used where collected and stored at −70 °C before renal transplant or donor nephrectomy. In the case of FSGS, serum and urine samples were collected prior to plasmapheresis or anti CD20 therapy.. One investigator performed the assays (NV).
Measurement of suPAR
A commercially available kit, Quantikine® Human uPAR Immunoassay (R& D Inc. Minneapolis, MN) was used for quantifying suPAR in the urine and serum. This is a dry phase sandwich ELISA assay. Samples were measured in duplicates after kit validation in the renal function laboratory. Urine suPAR was corrected for creatinine to account for variation in urine concentration.
Estimation of Glomerular Filtration Rate
Pre-transplant GFR was estimated by the four variable MDRD formula (16).
Pre-transplant Albuminuria and Proteinuria
Random urine albumin (ACR) and total protein (PCR) where measured in the study urine samples using immunoturbidimetry and the Pyrogallol Red dye method respectively. Both were corrected for creatinine and expressed as mg/g of creatinine. Urinary creatinine was measured by the Jaffe reaction (17).
Renal Histology
Renal pathology reports from the native kidneys (excluding ADPKD cases) and post-transplant were reviewed. Electron microscopy was used to detect diffuse foot process effacement.
Statistical Analysis
Data are presented as mean ± standard deviation if normally distributed and median [25%, 75% percentile] if not. Differences in means were compared by the Student’s t test with equal variance not assumed, and if more than two groups, by one-way ANOVA. For highly skewed data the Mann Whitney U test and Kruskall Wallis tests were used for two groups or more respectively. Spearman’s ρ was used to test correlations. Differences in proportions were assessed by the Fisher’s exact test. Logistic regression was used to predict FSGS and recurrent FSGS.
STATA 12 (STATA Corp, College Station, TX) was used for the analyses.
The Mayo Clinic Institutional review board approved the collection of all samples and the conduct of this study.
Acknowledgements
Parts of this project were presented in abstract form at Kidney Week 2012 of the American Society of Nephrology and submitted to the 2013 congress of the European Society for Organ Transplantation. Funds to carry out the study were provided by the Division of Nephrology and Hypertension, the Department of Laboratory Medicine and Pathology, the William J von Liebig Transplant Center of Mayo Clinic, Rochester, MN, and Fernando G Cosio, MD. The authors wish to thank Walter K Kremers Ph.D. for statistical advice. This project was also supported by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Support
This project was supported by funds from the Division of Nephrology and Hypertension, Department of Laboratory Medicine and Pathology, the William J von Liebig Transplant Center of Mayo Clinic, Rochester, MN
CRFP received support from the CTSA of Mayo Clinic through Grant Number UL1 TR000135 from the National Center for Advancing Translational Science (NCATS)
Abbreviations
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- DN
Diabetic Nephropathy
- R-FSGS
Recurrent FSGS
- IgAN
IgA Nephropathy
- MN
Membranous Nephropathy
- N-R FSGS
Non Recurrent FSGS
- FSGS
Focal Segmental Glomerulosclerosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Participated in Research Design
Participated in writing the paper
Participated in the Performance of the research
Contributed new reagents of analytical tools
Participated in data analysis
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
References
- 1.Scandling JD. Kidney transplant candidate evaluation. Seminars in dialysis. 2005;18(6):487. doi: 10.1111/j.1525-139X.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Hickson LJ, Gera M, Amer H, et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87(8):1232. doi: 10.1097/TP.0b013e31819f12be. [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan S, Leung N, Lager DJ, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clinical journal of the American Society of Nephrology : CJASN. 2006;1(3):483. doi: 10.2215/CJN.00710805. [DOI] [PubMed] [Google Scholar]
- 4.Swaminathan S, Lager DJ, Qian X, Stegall MD, Larson TS, Griffin MD. Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants. Nephrol Dial Transplant. 2006;21(9):2607. doi: 10.1093/ndt/gfl225. [DOI] [PubMed] [Google Scholar]
- 5.Audard V, Kamar N, Sahali D, et al. Rituximab therapy prevents focal and segmental glomerulosclerosis recurrence after a second renal transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2012;25(5):e62. doi: 10.1111/j.1432-2277.2012.01462.x. [DOI] [PubMed] [Google Scholar]
- 6.Araya CE, Dharnidharka VR. The factors that may predict response to rituximab therapy in recurrent focal segmental glomerulosclerosis: a systematic review. Journal of transplantation. 2011;2011:374213. doi: 10.1155/2011/374213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kattah AG, Fervenza FC, Roccatello D. Rituximab-based novel strategies for the treatment of immune-mediated glomerular diseases. Autoimmunity reviews. 2012 doi: 10.1016/j.autrev.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nature medicine. 2011;17(8):952. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei C, Trachtman H, Li J, et al. Circulating suPAR in Two Cohorts of Primary FSGS. Journal of the American Society of Nephrology : JASN. 2012;23(12):2051. doi: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiser J, Wei C, Tumlin J. Soluble urokinase receptor and focal segmental glomerulosclerosis. Current opinion in nephrology and hypertension. 2012;21(4):428. doi: 10.1097/MNH.0b013e328354a681. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz G, Koksal I, Karahan SC, Mentese A. The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in systemic inflammatory response syndrome. Clinical biochemistry. 2011;44(14–15):1227. doi: 10.1016/j.clinbiochem.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Wittenhagen P, Andersen JB, Hansen A, et al. Plasma soluble urokinase plasminogen activator receptor in children with urinary tract infection. Biomarker insights. 2011;6:79. doi: 10.4137/BMI.S6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Disease markers. 2009;27(3):157. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehestedt T, Lyngbaek S, Eugen-Olsen J, et al. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis. 2011;216(1):237. doi: 10.1016/j.atherosclerosis.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 15.Pawlak K, Pawlak D, Mysliwiec M. Tissue factor and urokinase-type plasminogen activator system are related to the presence of cardiovascular disease in hemodialysis patients. Thrombosis research. 2007;120(6):871. doi: 10.1016/j.thromres.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hervey GR. Determination of creatinine by the Jaffe reaction. Nature. 1953;171(4364):1125. doi: 10.1038/1711125a0. [DOI] [PubMed] [Google Scholar]