Abstract
Langerhans cells are epidermal dendritic cells responsible for antigen presentation during an immune response. Langerhans cells associate intimately with epidermal sensory axons. While there is evidence that Langerhans cells may produce neurotrophic factors, a role in regulating cutaneous innervation has not been established. We used genetically engineered mice in which the diphtheria toxin (DT) receptor is targeted to Langerhans cells (Lang-DTR mice) to assess sensory axon-dendritic cell interactions. Diphtheria toxin administration to wild type mice did not affect epidermal structure, Langerhans cell content, or innervation density. A DT administration regimen supramaximal for completely ablating epidermal Langerhans cells in Lang-DTR mice reduced PGP 9.5–immunoreactive total innervation and calcitonin gene related peptide–immunoreactive peptidergic nociceptor innervation. Quantitative real-time polymerase chain reaction showed that epidermal gene expression of brain derived neurotrophic factor was unchanged, but nerve growth factor and glial cell line-derived neurotrophic factor mRNAs were reduced. Behavioral testing showed that, while thermal sensitivity was unaffected, mice depleted of Langerhans cells displayed mechanical hypersensitivity. These findings provide evidence that Langerhans cells play an important role in determining cutaneous sensory innervation density and mechanical sensitivity. This may involve alterations in neurotrophin production by Langerhans or other epidermal cells, which in turn may affect mechanical sensitivity directly or as a result of neuropathic changes.
Keywords: Dendritic cells, Neural-immune interactions, Sensory axons, Neurotrophic factors, Behavioral testing
1. Introduction
Intraepidermal nerve fibers (IENFs) represent terminations of dorsal root ganglion sensory axons [17]. They are critical for identifying tactile, thermal, and noxious stimuli [17, 19] and in mounting local inflammatory responses [31]. Accordingly, IENFs are critical components of skin and their loss in conditions such as diabetes [13] and Guillain-Barré syndrome [25] can contribute substantially to pathology phenotypes.
Mechanisms responsible for establishing and maintaining cutaneous innervation remain unclear. Keratinocytes, the predominant epidermal cell type, are known to produce neurotrophic factors (NTFs) [21, 23, 33]. However, melanocytes also synthesize neurotrophic proteins [20]. Similarly, Langerhans cells (LC) represent a substantial component of the skin [6] and have also been shown to produce NTFs [34]. Therefore, cells in addition to keratinocytes may be important in regulating epidermal innervation.
Langerhans cells have a well-established role in immunity. As the primary resident epidermal antigen-presenting cell, they capture and translocate foreign antigens to lymph node T cells, thus participating in innate and adaptive immune responses [2, 4]. LCs are distinguishable from other cutaneous and neural cells by their expression of langerin, a protein involved in ligand internalization and antigen presentation [36].
Despite their role in immunity, LCs have long been suspected to have neurally-related functions, a concept going back to their initial description [14]. Subsequent studies show that LCs make intimate contacts with IENFs [6–8], and LC density is apparently regulated by cutaneous innervation [9, 28, 30]. However, a role of LCs in regulating epidermal innervation has not been explored. The availability of genetically engineered mice where LCs can be selectively ablated provides an opportunity to explore the relationship between LCs and cutaneous innervation more thoroughly.
2. Materials and methods
All animal protocols were approved by the University of Kansas Medical Center's Animal Care and Use Committee, and comply with the National Institute of Health guidelines for the care and use of laboratory animals.
2.1 Langerhan cell depletion
Lang-DTR mice (Lang-DTREGFP, a kind gift from Dr. Bernard Malissen, Centre d'Immunologie de Marseille Luminy) are C57BL/6 mice genetically engineered to express the human diphtheria toxin receptor (DTR) fused to enhanced green fluorescent protein under control of the langerin gene [11]. Administration of diphtheria toxin (DT) at concentrations not toxic to wild type mice produces selective LC depletion [11].
LCs were depleted beginning at 1 month of age by intraperitoneal injections of DT (1μg in 100μl, Sigma Aldrich, St. Louis, MO, n=5) or vehicle (water, n=5) on d0 (first injection), 4, 8, and 12 to Lang-DTR mice (see Fig 4 for an experimental time line); this regimen is supramaximal for depleting epidermal LCs but is necessary to ensure complete and sustained elimination of all cutaneous langerin-ir cells [11]. To exclude effects that DT may have independent of LC depletion, we administered saline (n=6) or DT at the same dosage (n=6) to C57BL/6 mice (Jackson Laboratories).
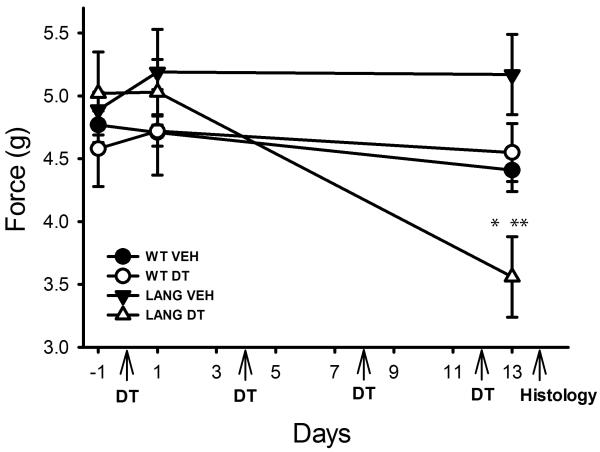
Figure 4. Langerhans cell depletion promotes mechanical hypersensitivity.
Wild type (WT) or Lang-DTR (Lang) mice received vehicle (VEH) or diphtheria toxin (DT) injections beginning on day 0 and repeated on days 4, 8 and 12 (arrows). Mechanical force required to elicit a response was determined 1 day prior to initiation of injections (−1), one day following the first injection, and one day following completion of the injection regimen (13). Lang-DTR mice at 13d of treatment showed increased sensitivity as compared to measurements prior to DT treatment (*p<0.001) and relative to Lang-DTR mice receiving vehicle injections (**p<0.001). N=5 mice per group.
Two days following the final DT injection, mice were anesthetized with isoflurane (Abbott) and right hind paws excised and fixed in Zamboni's solution for 24 hours, rinsed in PBS for 14d, immersed in 30% sucrose for 3–5d, frozen in tissue freezing medium (Electron Microscopy Sciences), and sectioned serially at 20 μm in sagittal orientation. Left hind paws were removed for real time quantitative RT-PCR.
2.2 Immunohistochemistry
Sections were treated with 100mM glycine in PBS for 30 minutes, rinsed and immersed for 1 hour in SuperBlock buffer (ThermoScientific), and stained overnight with primary antisera to protein gene product 9.5 (PGP9.5, 1:800, rabbit IgG, AbD Serotec), calcitonin gene-related peptide (CGRP, 1:600, sheep IgG, Enzo Life Sciences), and langerin (1:800, goat IgG, Santa Cruz Biotechnology), which have been characterized previously [6, 32]. After rinsing in PBST, sections were incubated for 1h with Cy2-conjugated donkey anti-goat (1:200), Cy3-conjugated donkey anti-rabbit (1:800), or Cy3-conjugated donkey anti-sheep (1:400) IgG secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.), rinsed in PBS and coverslipped with Fluoromount G. Three sections at 240μm intervals were analyzed per animal.
2.3 Langerhans cell analysis
Images of langerin immunostained cells were captured from distal, middle, and proximal regions in each section with a 40X oil immersion objective on a Nikon Eclipse 90i microscope. LC density was measured by dividing numbers of LCs by epidermal area (μm2) measured planimetrically, excluding the stratum corneum. Langerin immunostaining was used because GFP fluorescence can be attenuated by fixation and to allow direct comparison of LC features in Lang-DTR and wildtype mice. We evaluated LC size, an indicator of functional maturity [24], by dividing the langerin-ir area (μm2) measured by thresholding, by numbers of LCs. To determine the percentage of epidermal area occupied by LCs, langerin-ir area was divided by total epidermal area and multiplied by 100.
2.4 Epidermal nerve fiber quantification
PGP9.5, a pan-neuronal marker [37], was used to identify all IENFs and CGRP to identify `peptidergic' axons [35]. Three regions 0.42mm in length spaced equidistantly along each section were selected to provide indices of innervation at the distal, middle, and proximal regions of the footpad. Individual IENFs crossing the dermal-epidermal junction were counted, excluding secondary branching [15]. Counts were divided by epidermal length and expressed as IENF/mm. Epidermal thickness was measured at the center of each image.
2.5 Neurotrophin mRNA expression in epithelium after Langerhans cell depletion
Left hind footpads were excised and placed in 20mM EDTA for 1h at 37°C, allowing isolation of the epidermis as a single sheet. Epidermal sheets were rinsed in cold 0.1M PBS for 5 minutes, frozen on dry ice, and stored at −80°C. Total RNA was extracted in TRIzol (Invitrogen) and concentrations measured using a NanoDrop ND-1000 spectrophotometer (ThermoScientific). Complementary DNA was generated using 1μg of mRNA and SuperScript II Reverse Transcriptase (Invitrogen) in an MJ Mini Personal Thermal Cycler (BioRad). cDNA was amplified using iQ SYBR Green Supermix (BioRad) and an iCycler iQ Multicolor Real-Time PCR detection system (BioRad). Cycle number was recorded with the iCycler program (BioRad). Primer sets were: nerve growth factor (NGF); sense: TTTAAGAAACGGAGACTC and anti-sense: CTGTTGAAAGGGATTGTA; brain derived neurotrophic factor (BDNF; sense: AGAGTGATGACCATCCTT and anti-sense: TGGACGTTTACTTCTTTCA; glial cell line derived neurotrophic factor (GDNF); sense: TTAACTGCCATACACTTA and anti-sense: CTACTTTGTCACTTGTTAG. For normalization, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used: sense: CTCTACCCACGGCAAGTTC and anti-sense: CTCAGCACCAGCATCACC (Integrated DNA Technologies, Coralville, IA).
2.6 Behavioral testing
Thermal and mechanical sensitivity were measured 1d prior to and 1 and 13d after treatment was initiated. For thermal testing, mice were allowed to acclimate on a glass surface for 30 minutes and a radiant heat source (PAW Thermal Stimulator [UCSD], 4.3A) applied to the hind paw plantar surface and time required for the animal to withdraw its paw measured [3]. Each animal was tested 3 times on each hind paw with 5–10 minutes between tests.
After thermal testing, animals were acclimated for 30 minutes on a wire mesh and an electronic von Frey apparatus (IITC Life Science) was used to apply pressure to the hindpaw and force required to elicit withdrawal recorded [3]. Each animal was tested 3 times with 5–10 minutes between tests.
2.7 Statistical Analyses
Differences in epidermal thickness, LC density, size, area occupied by LCs, IENF density, and gene expression were assessed by one-way analysis of variance and Student-Newman-Keuls post-hoc test. Behavioral data were analyzed using two way repeated measures analysis of variance and Student-Newman-Keuls post-hoc analysis. Differences were considered significant at p ≤0.05. Data are expressed as Mean ± Standard Error Mean (SEM).
3. Results
3.1 DT depletes Langerhans cells in Lang-DTR mice
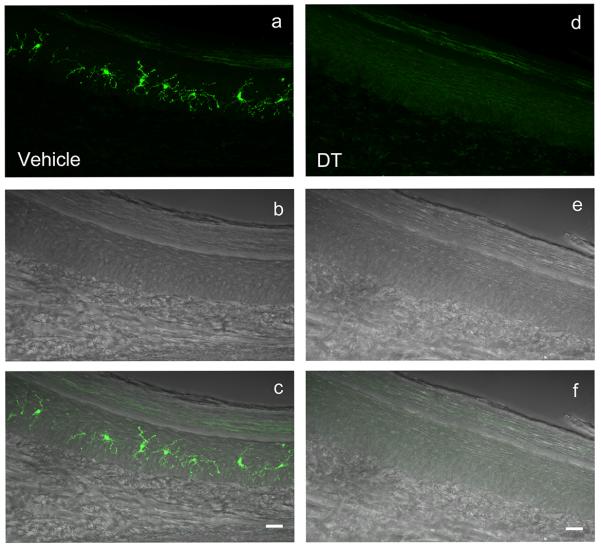
Langerin-immunoreactive (ir) LCs were present throughout the epidermis of vehicle-treated Lang-DTR mice (Fig 1a–c) in a pattern similar to that reported previously [6]. Following DT administration, langerin-ir cells were absent in footpad epidermis (Fig 1d–f), as described for the ear [11]; langerin mRNA was below limits detectable by quantitative PCR (data not shown).
Figure 1. LC depletion in Lang-DTR mice.
Images of footpad sections from vehicle- (a–c) and DT-treated (d–f) Lang-DTR mice immunostained for langerin. Langerin-ir cells (a, fluorescence microscopy) are confined to the epidermis (b, differential interference microscopy) as confirmed by superimposition of images (c). Following DT-administration fluorescent Langerhans cells are absent (d, f) while the epidermis is unaffected (e). Scale bars = 20μm.
3.2 Langerhans cell depletion reduces cutaneous innervation
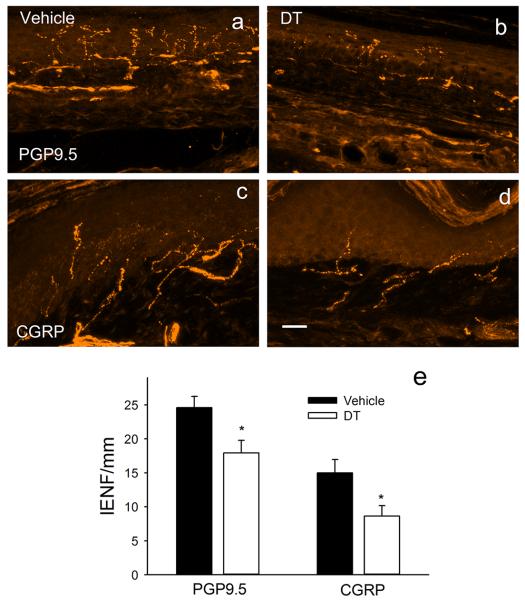
In vehicle-injected Lang-DTR mice, PGP9.5-ir axons were abundant within epidermis and dermis (Fig 2a). Many axons were also CGRP-ir, although these were largely restricted to the dermis (Fig 2b). Following DT, Lang-DTR mice showed reductions in both PGP9.5-ir (Fig 2c) and CGRP-ir fibers (Fig 2d). Quantitative analysis (Fig 2e) showed that LC depletion caused a 28% decrease in PGP9.5-ir fiber density (p= 0.036) and a 43% reduction in CGRP-ir fiber density (p=0.045).
Figure 2. Langerhans cell depletion reduces epidermal innervation.
In vehicle-injected Lang-DTR mice, epidermis contains abundant innervation immunoreactive for PGP9.5 (a). Following LC depletion by DT administration, PGP95-ir innervation appears to be reduced (b). Fibers immunoreactive for calcitonin gene-related peptide (CGRP) comprise a subpopulation in vehicle-injected Lang-DTR mice (c), and these are reduced following DT (d). Quantitative analysis (e) confirms reductions in both PGP9.5 and CGRP-ir populations (* p<0.05). Scale bar in d = 20μm in all micrographs.
3.3 Diphtheria toxin does not affect Langerhans cells or nerves in wild type mice
Because DT is a foreign antigen and potential toxin that may by itself alter LC status and innervation, we assessed epidermal features in wild type mice receiving DT. DT administration according to the protocol used to induce LC depletion in Lang-DTR mice did not alter epidermal thickness, LC density, LC size, or the proportion of epidermis occupied by these cells. Similarly, DT did not alter the numbers of PGP9.5- or CGRP-ir axons (see Supplemental Data).
3.4 Langerhans cell depletion reduces epidermal neurotrophin gene expression
To determine if LC depletion influences neurotrophin gene expression, we analyzed epidermal sheets from mice receiving vehicle or DT injections using quantitative RT-PCR. BDNF mRNA expression was not affected by DT-induced LC depletion (Fig 3a). However, NGF gene expression was reduced by approximately 75% (p=0.012, Fig 3b) and GDNF gene expression by approximately 90% (p=0.015, Fig 3c). DT injections did not affect NTF gene expression in wild type C57BL/6 mice (data not shown).
Figure 3. Langerhans cell depletion reduces epidermal neurotrophic factor mRNA.
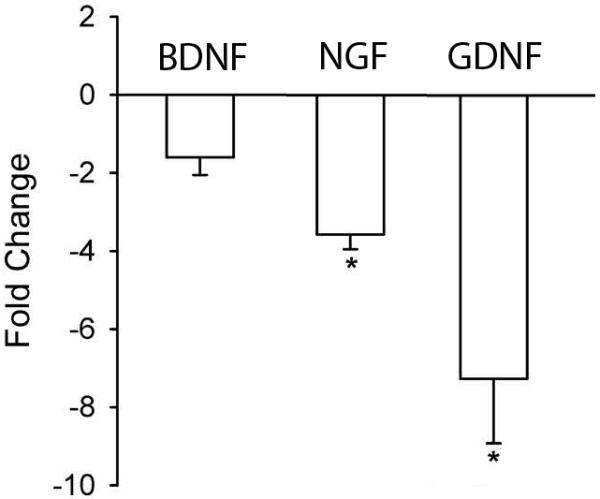
Real-time quantitative polymerase chain reaction was used to compare gene expression in epithelial sheets from vehicle injected and DT-injected Lang-DTR mice. Fold change is DT-injected relative to vehicle-injected Lang-DTR mice. Langerhans cell depletion did not affected expression of brain derived neurotrophic factor (BDNF) mRNA, but reduced mRNA for nerve growth factor (NGF, *p=0.012) and for glial cell line derived neurotrophic factor (GDNF, p=0.015).
3.5 Langerhans cell depletion increases mechanical sensitivity
Wild type C57BL6-J and Lang-DTR mice were evaluated immediately before and 1 or 13d after initiating injections. Thermal sensitivity did not change over the testing period and responses in all groups remained comparable (data not shown). Responses to mechanical stimulation of the hind paws were also comparable in all groups prior to and after the first injection of DT (Fig 4). However, following 13d of DT treatment Lang-DTR mice showed increased sensitivity to mechanical pressure relative to all other groups (p<0.001, Fig 4).
4. Discussion
Paul Langerhans first described LCs in 1868 [14] and ascribed to them a neural function, a view that persisted until their role as immune cells was confirmed nearly a century later [26]. The close relation between LCs and the nervous system is receiving renewed recognition. Sensory nerves show intimate physical associations with LCs. LCs express neuropeptide receptors, which when activated by sensory peptides suppress antigen presentation [5, 12]. Moreover, IENFs influence LC dynamics as denervation increases LC numbers [9, 16]. These observations suggest that LCs may be innervated and regulated by cutaneous sensory axons.
Findings presented here provide evidence that sensory nerves are, in turn, regulated by LCs. DT administration to wild type mice did not affect cutaneous innervation. However, when LCs were depleted in Lang-DTR mice, PGP9.5-ir axons were reduced suggesting that LCs are necessary for maintaining cutaneous innervation. CGRP-ir innervation, a major subset of cutaneous nerves, was also reduced; whether other subpopulations are affected remains to be assessed. It should be noted that we used an aggressive DT regimen that depletes all cutaneous langerin-ir cells, so effects of depletion in other compartments should also be considered. Nonetheless, the overall effect of LC depletion is a reduction in total number of cutaneous nerves, suggesting loss of trophic support.
Mechanisms by which LCs maintain cutaneous innervation are not clear but likely involve NTFs necessary for neuronal survival and axon growth. Quantitative PCR shows that, while BDNF expression was not altered, both NGF and GDNF mRNA levels were markedly reduced by LC depletion. Therefore, LCs are necessary for the normal neurotrophin gene expression profile of skin. LCs may contribute to epidermal NTF production directly, as LCs are reported to produce neurotrophins [34]. However, keratinocytes also synthesize NGF and GDNF [21, 23, 33], and an alternative explanation is that LCs modulate NTF synthesis in keratinocytes, possibly through release of secondary factors such as cytokines [22]. In any event, the large effect of LC depletion on NTF mRNA suggests LCs play a robust role in determining epidermal neurotrophin synthesis, thereby regulating cutaneous innervation density.
It is known that different sensory axon modalities respond selectively to different neurotrophins. Reduced NGF mRNA is consistent with decreases in thermally responsive CGRP axons which respond to NGF via trkA receptors [10]. While we did not analyze the effects of LC depletion on other subpopulations, non-peptidergic mechanoreceptive nociceptors are responsive to GDNF [1] and may also have been influenced by reduced GDNF following LC depletion. Consistent with an effect on mechanoreceptive axons, we found that LC depletion resulted in increased responsiveness to mechanical (but not thermal) stimuli. Accordingly, LCs not only regulate numbers of cutaneous axons, but also influence responsiveness to potentially painful mechanical stimuli.
The mechanism by which LCs affect sensitivity is unclear. Painful neuropathies frequently occur degenerative loss of sensory fibers [18, 29]. Accordingly, peripheral neuropathic pain resulting from reduced NTFs derived from LCs and/or keratinocytes is fully consistent with our findings. However, keratinocytes have been implicated as direct participants in sensory transduction [27] so changes in sensitivity owing directly to LC loss or changes in keratinocyte properties secondary to LC depletion cannot be excluded.
5. Conclusion
LCs exert an important role in maintaining normal numbers of cutaneous sensory nerves, probably as a result of their contributions to NTF levels in the skin. Moreover, their presence appears to be important in establishing mechanical but not thermal sensitivity. Alterations in LCs may contribute to nerve loss and hypersensitivity that occur in some forms of peripheral neuropathy.
Supplementary Material
Depletion of cutaneous Langerhans cell reduces numbers of sensory axons
Langerhans cell depletion reduces epidermal NGF and GDNF mRNA
Mechanical sensitivity is increased following Langerhans cell depletion
Langerhans cells therefore regulate cutaneous innervation density and sensitivity
ACKNOWLEDGEMENTS
This work was supported by NIH grants R01 HD049615, RO1 NS053796, P30 HD002528, and F31 DK083845. We thank Dr. Bernard Malissen for his generous gift of the Lang-DTR mice and Drs. Dora Agbas, Don Warn and Aritra Bhattacherjee and Phil Shafer for assistance.
ABBREVIATIONS
- BDNF
brain derived neurotrophic factor
- CGRP
calcitonin gene-related peptide
- DT
diphtheria toxin
- GDNF
glial cell line derived neurotrophic factor
- IENF
intraepithelial nerve fiber
- -ir
immunoreactivity
- LC
Langerhans cell
- NGF
nerve growth factor
- NTF
neurotrophic factor
- PGP9.5
protein gene product 9.5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26:2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ayala-Garcia I, Hernandez-Segura AM, Castell-Rodriguez A, Alvarez Perez SJ, Tellez BH, Ramirez-Gonzalez MD. Participation of epidermal langerhans cells in human pathology and their potential as targets for drug development: a review of literature. Proc West Pharmacol Soc. 2005;48:13–20. [PubMed] [Google Scholar]
- [3].Chakrabarty A, Liao Z, Smith PG. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J Pain. 2013;14:1053–1065. doi: 10.1016/j.jpain.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].de Jong MA, Geijtenbeek TB. Langerhans cells in innate defense against pathogens. Trends Immunol. 2010;31:452–459. doi: 10.1016/j.it.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [5].Ding W, Wagner JA, Granstein RD. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-kappaB activation. The Journal of investigative dermatology. 2007;127:2357–2367. doi: 10.1038/sj.jid.5700858. [DOI] [PubMed] [Google Scholar]
- [6].Doss AL, Smith PG. Nerve-Langerhans cell interactions in diabetes and aging. Histol Histopathol. 2012;27:1589–1598. doi: 10.14670/hh-27.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gaudillere A, Misery L, Souchier C, Claudy A, Schmitt D. Intimate associations between PGP9.5-positive nerve fibres and Langerhans cells. The British journal of dermatology. 1996;135:343–344. doi: 10.1111/j.1365-2133.1996.tb01191.x. [DOI] [PubMed] [Google Scholar]
- [8].Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- [9].Hsieh ST, Choi S, Lin WM, Chang YC, McArthur JC, Griffin JW. Epidermal denervation and its effects on keratinocytes and Langerhans cells. Journal of neurocytology. 1996;25:513–524. doi: 10.1007/BF02284819. [DOI] [PubMed] [Google Scholar]
- [10].Jankowski MP, Koerber HR. Neurotrophic Factors and Nociceptor Sensitization. 2010. [PubMed] [Google Scholar]
- [11].Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, Saeland S, Davoust J, Malissen B. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- [12].Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol. 2004;173:6082–6088. doi: 10.4049/jimmunol.173.10.6082. [DOI] [PubMed] [Google Scholar]
- [13].Lacomis D. Small-fiber neuropathy. Muscle & nerve. 2002;26:173–188. doi: 10.1002/mus.10181. [DOI] [PubMed] [Google Scholar]
- [14].Langerhans P. Uber die Nerven der menschlichen Haut. Virchows Archiv : an international journal of pathology. 1868;44:325–337. [Google Scholar]
- [15].Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–758. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- [16].Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. Intraepidermal nerve fiber density in rat foot pad: neuropathologic neurophysiologic correlation. J Peripher Nerv Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- [17].Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009;54:273–285. doi: 10.1111/j.1365-2559.2008.03096.x. [DOI] [PubMed] [Google Scholar]
- [18].Lin YW, Tseng TJ, Lin WM, Hsieh ST. Cutaneous nerve terminal degeneration in painful mononeuropathy. Experimental neurology. 2001;170:290–296. doi: 10.1006/exnr.2001.7704. [DOI] [PubMed] [Google Scholar]
- [19].Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- [20].Marconi A, Panza MC, Bonnet-Duquennoy M, Lazou K, Kurfurst R, Truzzi F, Lotti R, De Santis G, Dumas M, Bonte F, Pincelli C. Expression and function of neurotrophins and their receptors in human melanocytes. Int J Cosmet Sci. 2006;28:255–261. doi: 10.1111/j.1467-2494.2006.00321.x. [DOI] [PubMed] [Google Scholar]
- [21].Marconi A, Terracina M, Fila C, Franchi J, Bonte F, Romagnoli G, Maurelli R, Failla CM, Dumas M, Pincelli C. Expression and function of neurotrophins and their receptors in cultured human keratinocytes. The Journal of investigative dermatology. 2003;121:1515–1521. doi: 10.1111/j.1523-1747.2003.12624.x. [DOI] [PubMed] [Google Scholar]
- [22].Matsue H, Cruz PD, Jr., Bergstresser PR, Takashima A. Langerhans cells are the major source of mRNA for IL-1 beta and MIP-1 alpha among unstimulated mouse epidermal cells. The Journal of investigative dermatology. 1992;99:537–541. doi: 10.1111/1523-1747.ep12667296. [DOI] [PubMed] [Google Scholar]
- [23].Montano JA, Perez-Pinera P, Garcia-Suarez O, Cobo J, Vega JA. Development and neuronal dependence of cutaneous sensory nerve formations: Lessons from neurotrophins. Microsc Res Tech. 2010;73:513–529. doi: 10.1002/jemt.20790. [DOI] [PubMed] [Google Scholar]
- [24].Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. The Journal of investigative dermatology. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- [25].Pan CL, Tseng TJ, Lin YH, Chiang MC, Lin WM, Hsieh ST. Cutaneous innervation in Guillain-Barre syndrome: pathology and clinical correlations. Brain. 2003;126:386–397. doi: 10.1093/brain/awg039. [DOI] [PubMed] [Google Scholar]
- [26].Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. The Journal of investigative dermatology. 2012;132:872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- [27].Schmelz M. Neuronal sensitivity of the skin. Eur J Dermatol. 2011;21(Suppl 2):43–47. doi: 10.1684/ejd.2011.1265. [DOI] [PubMed] [Google Scholar]
- [28].Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Experimental neurology. 2006;201:507–514. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- [30].Stankovic N, Johansson O, Hildebrand C. Increased occurrence of PGP 9.5-immunoreactive epidermal Langerhans cells in rat plantar skin after sciatic nerve injury. Cell and tissue research. 1999;298:255–260. doi: 10.1007/s004419900083. [DOI] [PubMed] [Google Scholar]
- [31].Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Archives of dermatology. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- [32].Tague SE, Clarke GL, Winter MK, McCarson KE, Wright DE, Smith PG. Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation. J Neurosci. 2011;31:13728–13738. doi: 10.1523/JNEUROSCI.3637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tokime K, Katoh-Semba R, Yamanaka K, Mizoguchi A, Mizutani H. Enhanced production and secretion of glial cell line-derived neurotrophic factor and nerve growth factor from the skin in atopic dermatitis mouse model. Arch Dermatol Res. 2008;300:343–352. doi: 10.1007/s00403-008-0856-z. [DOI] [PubMed] [Google Scholar]
- [34].Torii H, Yan Z, Hosoi J, Granstein RD. Expression of neurotrophic factors and neuropeptide receptors by Langerhans cells and the Langerhans cell-like cell line XS52: further support for a functional relationship between Langerhans cells and epidermal nerves. The Journal of investigative dermatology. 1997;109:586–591. doi: 10.1111/1523-1747.ep12337516. [DOI] [PubMed] [Google Scholar]
- [35].Vaalasti A, Tainio H, Johansson O, Rechardt L. Light and electron microscopic immunocytochemical demonstration of intraepidermal CGRP-containing nerves in human skin. Skin Pharmacol. 1988;1:225–229. doi: 10.1159/000210779. [DOI] [PubMed] [Google Scholar]
- [36].Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, Caux C, Lebecque S, Saeland S. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- [37].Wang L, Hilliges M, Jernberg T, Wiegleb-Edstrom D, Johansson O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell and tissue research. 1990;261:25–33. doi: 10.1007/BF00329435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.