Abstract
Objective
To summarize the published effects of systemic glucocorticoid therapy on bone mineral density (BMD) and fractures in children.
Methods
We performed a systematic review and meta-analysis of existing literature, using Medline, CINAHL, and Cochrane databases to identify studies of BMD or fractures in children ≤ 18 years taking systemic glucocorticoid therapy. We excluded studies of inhaled glucocorticoids, chemotherapy, and organ transplantation. Two authors reviewed abstracts for inclusion, read full-text articles to extract data, and rated each study using the Downs–Black scale.
Results
A total of 16 studies met eligibility criteria, including 10 BMD (287 children) and six fracture (37,819 children) studies. Spine BMD was significantly lower (−0.18; 95% CI = −0.25; −0.10 g/cm2) in children taking glucocorticoid therapy, compared to age- and gender-matched healthy controls. Spine BMD was also lower (−0.14; 95% CI = −0.27; 0.00 g/cm2) in children taking glucocorticoids, compared to children with the same disease not taking glucocorticoids. Incident clinical fracture rates varied from 2% to 33%. Morphometric vertebral fracture incidence ranged from 6% to 10%, and prevalence was 29–45%.
Conclusion
Published data suggest that children treated with glucocorticoid therapy have lower spine BMD compared to healthy children. Whether children receiving glucocorticoid therapy have lower spine BMD compared to children with milder disease not requiring such therapy is not certain. Clinical and morphometric vertebral fractures are common, although only one study assessed fracture rates in healthy controls. Additional well-designed, prospective studies are needed to evaluate the skeletal effects of glucocorticoid therapy in children.
Keywords: Bone mineral density, Children, Fracture, Glucocorticoid therapy, Systematic review, Meta-analysis
Introduction
Glucocorticoid-induced osteoporosis (GIO) is the most common form of secondary osteoporosis [1]. Systemic glucocorticoid therapy is associated with an initial increase in bone resorption [2,3] and more importantly, subsequent reduced bone formation [4], leading to microarchitectural deterioration and increased fracture risk. Epidemiologic studies have unequivocally established a higher risk of fracture among adults taking systemic glucocorticoid therapy [1,5–7]. The risk of fracture in adults is dose-dependent, with higher daily doses of oral glucocorticoids conferring a greater risk of fracture. Several medications are FDA-approved for the prevention and treatment of GIO in adults, and guidelines from the American College of Rheumatology can inform clinical decisions about whom to treat and which medication to select [8]. However, no evidence-based guidelines exist to assist clinicians caring for children who require long-term oral glucocorticoid therapy.
The absence of a synthesized evidence base regarding glucocorticoid use in children is a barrier to guidelines to monitor bone health in this patient population [4]. Glucocorticoid use in growing children might decrease peak bone mass and increase life-long risk of fracture. However, in the disease for which glucocorticoid therapy is given might also adversely affect skeletal health, glucocorticoid therapy might improve appetite, increase physical activity, control the underlying disease, and/or reduce inflammatory cytokines, thereby improving skeletal health [9].
The aim of this study was to examine the effect of systemic glucocorticoid therapy on skeletal health in children by systematic review and meta-analysis. We hypothesized that children taking systemic glucocorticoid therapy would experience higher rates of symptomatic (clinical) and morphometric vertebral fractures and lower bone mineral density (BMD) and/or content (BMC), compared to baseline measures, age-matched healthy peers, and children with the same disease who were not taking glucocorticoids.
Methods
With assistance from an academic librarian, we searched PubMed, CINAHL, and Cochrane databases from January 1, 1966 to February 5, 2013 to identify relevant articles published in any language on children 0–18 years. We used the MeSH terms glucocorticoids, corticosteroids, methylprednisolone, prednisone, prednisolone, hydrocortisone, triamcinolone, and dexamethasone crossed with the following terms: fracture(s), bone density, bone mineral density, and bone mineral content. To identify additional articles we added the following search terms: juvenile rheumatoid arthritis, juvenile idiopathic arthritis (JIA), juvenile systemic lupus erythematosus (SLE), inflammatory bowel disease, and juvenile dermatomyositis. We also reviewed the reference lists of the full-length articles undergoing level 2 review to identify additional articles meeting predefined inclusion criteria. Literature was reviewed again in October 2013 to identify interim publications.
Our primary outcomes were spine and/or total body less head BMD or BMC measurements [10] and clinical and/or morphometric fractures associated with systemic glucocorticoid therapy. We included studies in which children age ≤ 18 years taking systemic glucocorticoid therapy underwent clinical and/or radiographic evaluation for fracture and/or measurements of lumbar spine and/or total body BMD (less head), with comparison to their baseline measurements or controls. Inclusion required that authors report the dose and duration of glucocorticoid therapy. We excluded studies related to inhaled or topical glucocorticoid therapy, concurrent glucocorticoid and chemotherapy, and organ or bone marrow transplant. We excluded the treatment arm of prospective studies involving bone active medications such as bisphosphonates or vitamin D, which could potentially obscure the true effects of glucocorticoids on skeletal health. We excluded case series and case reports due to potential for selection and detection bias. We included cross-sectional, cohort, and interventional studies.
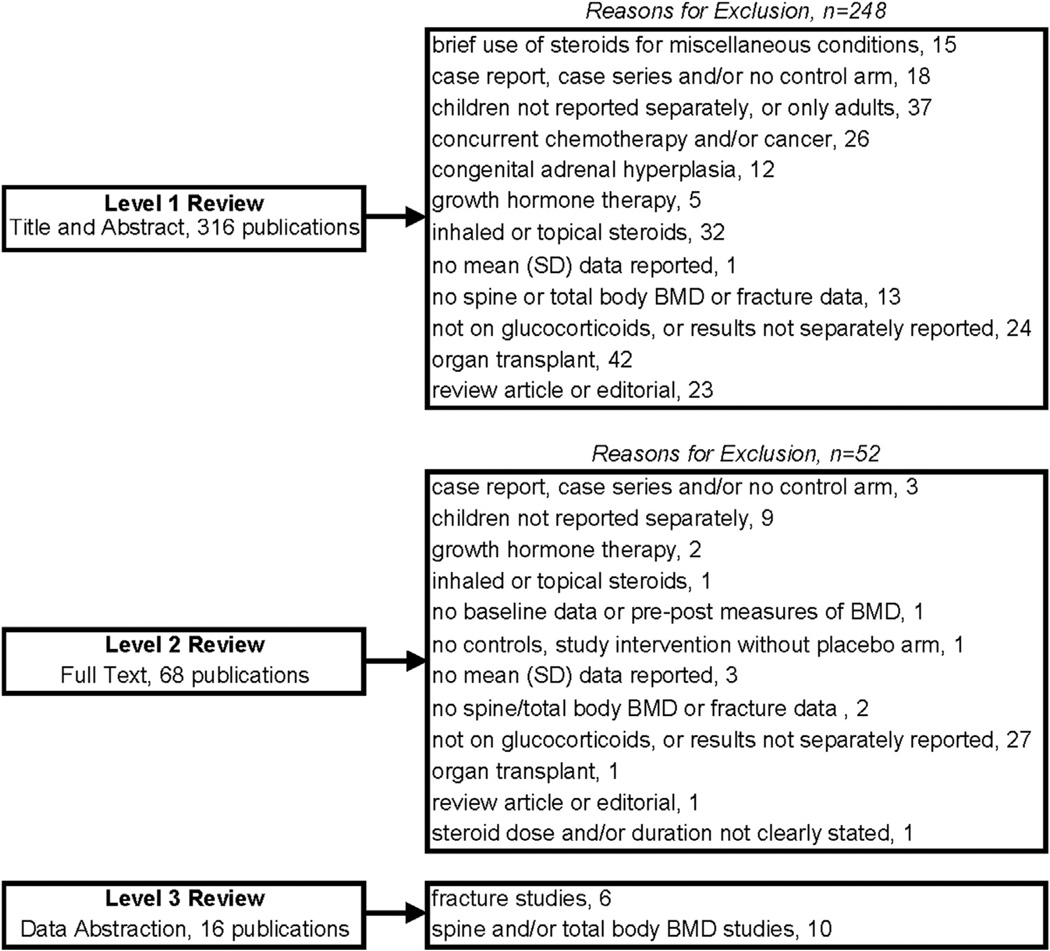
Two authors reviewed all the abstracts to determine potential eligibility for inclusion (level 1 review, Fig. 1). Next, two authors read the full text of all articles reaching level 2 review. During level 3 review, two authors independently extracted data from each study using a standardized form to record the populations, interventions (form, dose, and duration of glucocorticoid therapy), main outcome measures (bone mineral density and fractures), and study design. The first author of each study publication was contacted twice by email for missing data (e.g., standard deviation for mean values) if the overall study design was otherwise acceptable.
Fig. 1.
Summary of articles reviewed.
Two reviewers rated the quality of each study using the Downs and Black [11] checklist which assesses five domains: reporting, external validity, bias, confounding, and power. We resolved discrepancies in data extraction and scoring by consensus. The final score for each study was determined using the average of the two reviewers' scores, divided by the number of possible points, with results rounded to the nearest whole number. We used the PRISMA 2009 checklist [12] to generate the current report.
Statistical analysis
We compared BMD measures in ill children taking and not taking glucocorticoids. Likewise, we compared BMD measures in ill children taking glucocorticoids to BMD measures in healthy age and gender-matched controls. We also analyzed paired changes in children's BMD in prospective studies of ongoing glucocorticoid therapy [13,14]. Data were analyzed using the R software (version 3.0.1) with the packages “meta” for unpaired comparisons and “metafor” for paired comparisons [15].
Forest plots were generated to summarize composite data. For forest plot comparisons of independent groups of subjects, we reported the pooled difference between the means for cases and controls. For forest plot comparisons of paired data, we reported the standardized effect size (i.e., the difference between means for cases and controls, divided by the standard deviation of the cases). In one study [16], the mean BMD was reported with an associated range rather than standard deviation. For that study, we used the range as the standard deviation. In another study [17], the standard error of the mean was converted to the standard deviation to permit forest plot analysis.
Heterogeneity between studies was assessed using the Cochrane Q test and I2 statistic, with 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively [18]. Funnel plots were reviewed to detect publication bias. Agreement between two reviewers for scoring the quality of studies was assessed using the intraclass correlation coefficient.
Only one of six fracture studies assessed fracture rates in healthy controls. Additionally, some studies reported clinical, and others morphometric, fracture rates. Therefore, fracture studies were summarized descriptively. Although we hoped to explore whether glucocorticoid therapy demonstrated a dose–response relationship with fracture risk, the heterogeneity of the studies (including the glucocorticoid agent, dose, and duration) was too great to allow such analysis.
Results
From 316 citations undergoing level 1 review (title and abstract), 68 articles underwent level 2 review (full text), leading to identification of 16 studies for level 3 review. Figure 1 summarizes the number of studies identified and reasons for exclusion.
Effects of systemic glucocorticoid therapy on BMD
A total of 10 studies reported spine BMD and/or Z-scores in a total of 287 children taking systemic glucocorticoid therapy.
Cross-sectional studies
Four cross-sectional studies [16,19–21] compared spine BMD and/or Z-scores in 63 children requiring glucocorticoid therapy to measures in 42 children not requiring glucocorticoid therapy for the same condition or to 123 healthy controls (Table 1). In the first study [16], spine BMD was compared in 40 healthy controls and 40 pre-pubertal children with JIA, 16 receiving oral daily deflazacort and 24 treated with non-steroidal anti-inflammatory drugs (NSAIDs). The three groups were similar except for fewer boys in the group requiring glucocorticoid therapy, compared to the NSAID and control groups (25%, 42%, and 35%, respectively). Mean spine BMD was numerically lower (0.623 g/cm2; range: 0.475–0.759 g/cm2) in steroid-treated children compared to children taking NSAIDs (0.710 g/cm2; range: 0.572–0.821 g/cm2; p value not reported) and significantly lower than healthy controls (0.722 g/cm2; range: 0.584–0.887 g/cm2; p < 0.001). The second study [20] compared spine BMD in 45 age-matched healthy controls and 28 children with JIA, including 17 treated with prednisone for ≥ 5 months at a dose of ≥ 7.5 mg/day. Mean spine BMD was 0.492 ± 0.150 g/cm2 in children treated with prednisone, 0.595 ± 0.850 g/cm2 in children treated with NSAIDs, and 0.636 ± 0.120 g/cm2 in healthy controls (p < 0.0005 versus prednisone group).
Table 1.
Cross-sectional studies of spine bone mineral density in children on glucocorticoid therapy
| References | Condition, sample size | Glucocorticoid dose and duration |
Quality score |
|---|---|---|---|
| Falcini et al. [16] | JIA cases, n = 16 | Deflazacort median 0.8 mg/kg/day | 15/21 |
| JIA controls, n = 24 | 0.3–2.0 mg/kg/day | ||
| Healthy controls, n = 40 | Range: 6 months to 3 years | ||
| Celiker et al. [20] | JIA cases, n = 17 | Prednisolone | 14/20 |
| JIA controls, n = 11 | ≥ 7.5 mg/day | ||
| Healthy controls, n = 45 | ≥ 5 months | ||
| Brik et al. [19] | JIA cases, n = 10 | Prednisone | 16/21 |
| JIA controls, n = 7 | 0.4 mg ± 0.2 mg/kg/day | ||
| Healthy controls, n = 18 | 2.5 ± 1.3 years | ||
| Kashef et al. [21] | JIA or SLE cases, n = 20 | GC not specified | 17/21 |
| Healthy controls, n = 20 | 6.6 ± 3.2 mg/day | ||
| ≥ 6 months |
JIA = juvenile inflammatory arthritis; SLE = systemic lupus erythematosus; GC = glucocorticoid therapy.
Another study [19] compared spine BMD in 18 age- and gender-matched healthy controls and 17 children with JIA, including 10 taking prednisone 0.4 ± 0.2 mg/kg/day for 2.5 ± 1.3 years. Mean spine BMD was lower, 0.690 ± 0.230 g/cm2 in glucocorticoid, 0.930 ± 0.250 g/cm2 in NSAID, and 0.860 ± 0.150 g/cm2 in the control group (p < 0.05, prednisone versus control). The fourth study [21] compared spine BMD in 20 healthy controls and 20 children with SLE or JIA taking oral daily glucocorticoids for ≥ 6 months. The form of glucocorticoid therapy was not reported. Mean spine BMD was 0.700 ± 0.190 g/cm2 in the children taking glucocorticoids and 0.980 ± 0.200 g/cm2 in healthy controls (p < 0.001).
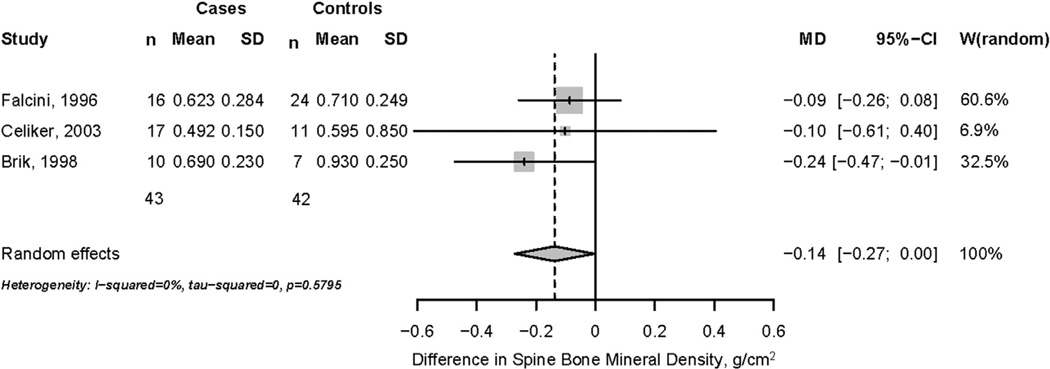
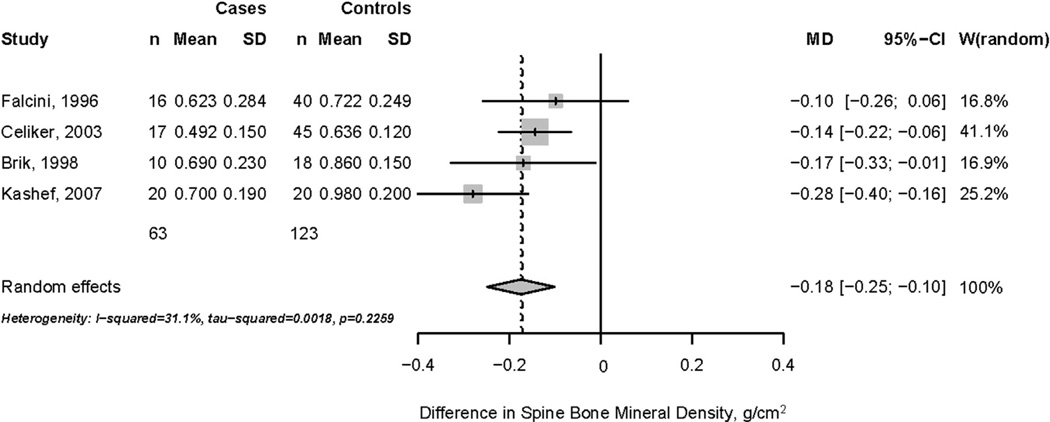
Based on a pooled random-effects model, spine BMD was lower but of borderline statistical significance (−0.14 g/cm2; 95% CI = −0.270; 0.000 g/cm2) in 43 children taking systemic glucocorticoid therapy, compared to 42 children with the same condition but not taking systemic glucocorticoids (Fig. 2). No significant heterogeneity in BMD results (I2 = 0%) was noted across publications, but heterogeneity is likely underestimated due to variations in disease severity, glucocorticoid agent, dose, and duration. By contrast, a random-effects model indicated that spine BMD was significantly lower (−0.180 g/cm2; 95% CI = −0.250; −0.100 g/cm2) in 63 children taking systemic glucocorticoid therapy, compared to 123 healthy age- and gender-matched controls (Fig. 3). Analysis revealed moderate heterogeneity in BMD results (I2 = 31%) between studies.
Fig. 2.
Difference in spine bone mineral density, cases and controls with same disease.
Fig. 3.
Difference in spine bone mineral density, cases and healthy controls.
Prospective studies
Six prospective cohort studies [17,22–26] measured changes in spine BMD (n = 3) and/or Z-score (n = 3) related to ongoing use of glucocorticoid therapy in 224 children (Table 2). In studies of bone active medications, only the placebo subjects were included in the meta-analysis.
Table 2.
Prospective cohort studies of spine bone mineral density and/or Z-scores in children taking glucocorticoid therapy
| References | Condition, n | Glucocorticoid dose and duration | Quality score |
|---|---|---|---|
| Rudge et al. [17] | Rheumatic diseases, n = 11 | Prednisone median 0.4 mg/kg/day | 22/27 |
| Median = 0.7 years | |||
| Bak et al. [24] | Nephrotic syndrome, n = 20 | Prednisolone 2 mg/kg/day × 4 weeks, then 2 mg/kg every other day × 4 weeks | 18/27 |
| Kim and Cho [23] | Nephropathy, n = 22 | Methylprednisolone IV daily × 3 days, then prednisone 1 mg/kg/day × 14 days × 5 cycles | 20/27 |
| Ferrara et al. [22] | ITP, n = 29 | Prednisone, methylprednisolone, dexamethasone, variable dose, and duration | 14/22 |
| Escolar et al. [25] | Duchenne muscular dystrophy, n = 25 | Prednisone 0.75 mg/kg/day 12 months | 26/27 |
| Rodd et al. [26] | Rheumatic diseases, n = 117 | Prednisone 16 ± 13 mg/m2/day | 15/18 |
| Median = 342 days |
ITP = immune-mediated thrombocytopenia.
Changes in spine bone mineral density
Researchers measured 1-year changes in spine BMD related to alendronate or placebo in 22 children with rheumatic diseases requiring prednisone therapy [17]. At randomization, placebo-treated subjects (n = 11) reported a median prednisone dose and duration of 0.4 mg/kg/day (range: 0.1–2.4 mg/kg/day) over 0.7 years (range: 0.3–5 years). Among 8 of 11 subjects completing the study, spine BMD increased (0.691 ± 0.235 g/cm2 to 0.741 ± 0.209 g/cm2, p = 0.041). At week 52, the prednisone dose had decreased to 0.3 mg/kg/day, but it was not separately reported for each treatment arm. The second study [24] evaluated the effects of vitamin D and calcium supplements on BMD in children receiving daily prednisolone for nephrotic syndrome. At week 8, children randomized to placebo (n = 20) exhibited a significant decline in spine BMD (0.520 ± 0.180 g/cm2 to 0.450 ± 0.160 g/cm2, p < 0.001). The third study [23] evaluated the effect of pamidronate or placebo (n = 22) on spine BMD in children with nephropathy treated with daily intravenous methylprednisolone followed by oral prednisone 1 mg/kg/day for 2 weeks. Over 3 months in the placebo arm, spine BMD decreased (0.654 ± 0.069 g/cm2 to 0.631 ± 0.070 g/cm2, p = 0.002).
In summary, two studies [23,24] of high-dose short-duration (≤ 3 months) glucocorticoid therapy demonstrated declines in spine BMD, whereas a 52-week study [17] reported a significant increase in spine BMD despite low-dose glucocorticoid therapy. A forest plot summarizing these studies demonstrates a non-significant reduction in spine BMD (standardized effect size = −0.240; 95% CI: −0.500 to 0.020; Fig. 4). Heterogeneity across studies was low (I2 = 12%).
Fig. 4.
Prospective changes in spine BMD in children taking glucocorticoid therapy.
Changes in spine Z-score
Four prospective studies reported changes in spine Z-scores among children taking glucocorticoid therapy (Table 2). The first study [17], described above, measured changes in spine Z-score in 22 children randomized to alendronate or placebo, with three drop-outs in the placebo arm. In eight remaining placebo-treated subjects, spine Z-score increased (−0.35 ± 3.55 to 0.02 ± 3.05, p = 0.157). Another study [22] reported 1-year changes in spine Z-score associated with three glucocorticoid regimens to treat immune-mediated thrombocytopenia. Ten children treated with oral prednisone 2 mg/kg/day over 2–4 weeks for three cycles experienced a decline in spine Z-score (0.50 ± 0.05 to −1.30 ± 0.20). Eight children treated with oral dexamethasone 24 mg/m2 4 days monthly for 6 months appeared to have stable spine Z-scores (0.60 ± 0.30 to 0.50 ± 0.01). Eleven children treated with intravenous methylprednisolone 9 mg/kg/day for 5 days with 3–4 cycles of therapy likewise appeared to have stable Z-scores (0.60 ± 0.20 to 0.40 ± 0.30). Authors reported that at 1 year, spine Z-scores were significantly lower (p < 0.05) in children treated with daily oral prednisone, compared to children treated with dexamethasone or methylprednisolone.
A randomized double-blind trial [25] measured 1-year changes in spine BMD in boys with Duchenne muscular dystrophy randomized to weekend (5 mg/kg on Saturday and Sunday) or daily prednisone (0.75 mg/kg/day). Daily prednisone therapy was associated with a decline in spine Z-score from −1.08 ± 0.85 to −1.38 ± 0.91. The final prospective study evaluated rates of incident vertebral fracture in children taking systemic glucocorticoid therapy for rheumatic diseases. Among 117 children, the spine Z-score was −0.60 ± 1.20 at baseline and −0.80 ± 1.20 at 12 months (p < 0.001) following daily prednisone 16 ± 13 mg/m2/day.
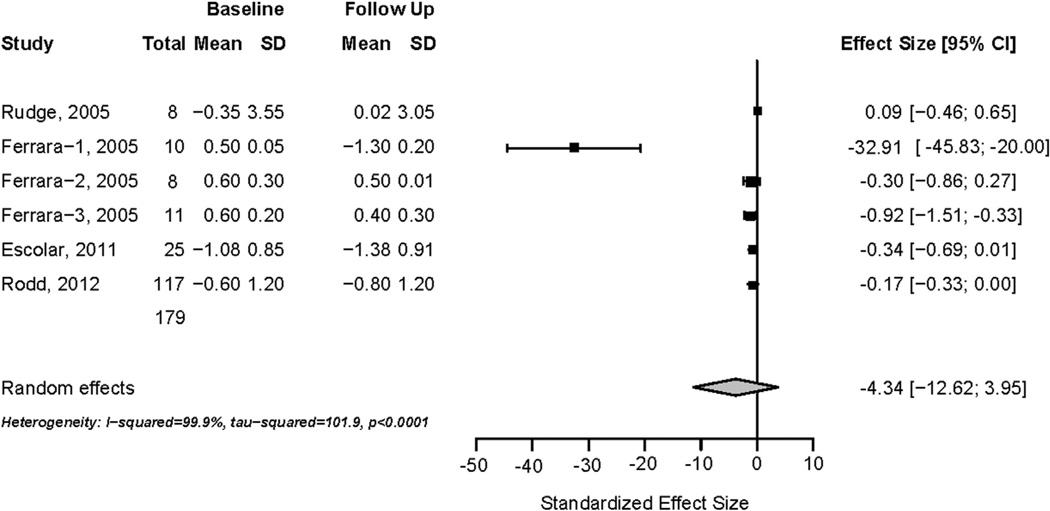
In summary, 179 children treated with varying glucocorticoid regimens exhibited variable changes in spine Z-score over a 1-year observation period. A forest plot summarizing these data indicates a non-significant reduction in spine Z-score associated with glucocorticoid therapy (standardized effect size = −4.34; 95% CI: −12.62 to 3.95; Fig. 5). Heterogeneity was high (I2 = 99%) across these studies and a funnel plot suggested significant publication bias (data not shown).
Fig. 5.
Prospective changes in spine Z-score in children taking glucocorticoid therapy.
Fractures
Six studies reported fracture rates related to glucocorticoid therapy. Two studies reported symptomatic (clinical) fractures in 37,577 children, and four studies reported morphometric fractures in 242 children. Only one study reported fracture rates in subjects and controls, precluding meta-analyses of these data.
Clinical fractures
The two studies of symptomatic clinical fractures reported disparate fracture rates (Table 3). One study [27] of 15 children with juvenile dermatomyositis reported 12 symptomatic vertebral fractures in five children (33%) taking glucocorticoid therapy for median of 1.2 years (range: 0–4.5 years). In contrast, a medical records study [28] using the General Practice Research Database reported clinical fractures in only 2% (746 of 37,562) of children prescribed an average of 2.4 glucocorticoid prescriptions for a mean of 6.4 days each.
Table 3.
Studies of fracture associated with glucocorticoid therapy
| References | Condition, n | GC inclusion requirement | Spine imaging | Results | Quality score |
|---|---|---|---|---|---|
| Stewart et al. [27] | JDM, n = 15 | GC not required, but all had prior or current use | None | Clinical incidence 5/15 (33%) | 19/23 |
| van Staa et al. [28] | Any illness, n = 37,562 | Any GC use | None | Clinical incidence 746/37,562 (2%) | 18/22 |
| Rodd et al. [26] | Rheumatic diseases, n = 118 | GC onset within 30 days of study entry | Baseline and 1 year | Radiographic incidence 7/118 (6%) | 15/18 |
| Loftus et al. [30] | JIA, n = 29 | ≥ 1 year of GC therapy at a dose of ≥ 5 mg/day | Once | Radiographic prevalence 13/29 (45%) | 17/27 |
| Incidence 3/31 (10%) | |||||
| Nakhla et al. [31] | Rheumatic diseases, n = 49 | Prior or current exposure to GC or methotrexate | Once | Radiographic prevalence 14/49 (29%) | 19/23 |
| Varonos et al. [32] | JIA, n = 46, ½ with fracture | ≥ 1 year of GC therapy, mean dose ≥ 5 mg/day | Yearly | Higher mean GC dose in those with fracture | 13/22 |
JDM = juvenile dermatomyositis; GC = glucocorticoid therapy; JIA = juvenile inflammatory arthritis.
Morphometric vertebral fractures
Only one study was designed to prospectively detect incident morphometric vertebral fractures related to new-onset glucocorticoid therapy. In that study, 134 children beginning systemic glucocorticoid therapy for rheumatic diseases underwent thoracic and lumbar spine radiographs at baseline [29]; 117 had x-rays 1 year later [26]. At baseline (defined as ≤ 30 days of glucocorticoids), nine children (7%) had prevalent morphometric vertebral fractures [29]. Over 1 year, seven children (6%) experienced 12 incident vertebral fractures [26]. Children with incident fracture received a higher average and cumulative dose of glucocorticoid therapy compared to those without fracture.
A randomized prospective study [30] compared the effects of prednisone and deflazacort on BMD and fracture rates in children with JIA. Thirty-four children with JIA were randomized to ≥ 1 year of prednisone or deflazacort ≥ 5mg/day. Of 29 children who underwent baseline spine films, 13 had vertebral fractures, suggesting a prevalence of 45%. Overall, 31 children completed spine x-rays 1 year later and three had incident fractures, with a 1-year fracture incidence of 10%.
In another report [31], the prevalence of vertebral fracture was determined by lateral spine radiographs in 90 children with rheumatic diseases, including 49 exposed to systemic glucocorticoid therapy. Of 49 children exposed to glucocorticoid therapy (29%), 14 had vertebral fractures, versus 3 of 21 (14%) without exposure. In multivariate linear models, cumulative glucocorticoid dose was a significant predictor of vertebral fracture, along with male gender and body mass index Z-score.
A final case–control study [32] did not report the total number of children undergoing radiographs, precluding estimates of fracture rates, but compared characteristics of 23 children with JIA and incident fracture to 23 JIA children without fractures. Children with fracture received a higher mean daily prednisolone dose (0.62 mg/kg versus 0.27 mg/kg, p < 0.01) but not a higher cumulative dose, compared to children without fracture. Authors noted a correlation between the mean daily prednisolone dose and the time to first vertebral fracture (r = −0.67, p < 0.001).
Skeletal effects of glucocorticoid therapy by gender and Tanner stage
All studies recorded participant gender, but only two studies analyzed study results by gender. Rodd et al. [26] found no difference in gender between children with incident vertebral fracture and children without incident vertebral fracture. By contrast, Nakhla et al. [31] reported that boys were more likely to have incidents of fracture than girls were (odds ratio = 6.04; 95% CI: 2.85–12.81; p < 0.001). However, in multivariate analysis controlling for multiple fractures, gender was no longer an independent risk factor for fracture.
Surprisingly, only four studies [16,24,26,27,31] determined pubertal status and/or Tanner stage. However only two studies [26,31] analyzed study results as a function of Tanner stage. Neither study found Tanner stage to be different between children with and without fracture.
Study quality assessment and publication bias
Across the 15 studies, the average ± SD Downs and Black quality score was 17 ± 3 for both reviewers. The two reviewer's scores highly correlated across the 16 included studies (ICC r = 0.94; 95% CI = 0.84; 0.98). Based on the study design, the total possible score for the studies was 23 ± 2 points. Funnel plots suggested low bias, but this was likely underestimated as we did not include symposia, abstracts, or poster presentations in the meta-analysis.
Discussion
A surprisingly small number of studies reported bone health outcomes in children taking systemic glucocorticoid therapy with comparisons to controls. Although limited in total sample size, published data suggest that ongoing glucocorticoid therapy associates with lower spine BMD in children, compared to measures in age- and gender-matched healthy controls. Whether children receiving glucocorticoid therapy for systemic diseases have lower spine BMD compared to children with milder disease not requiring such therapy is not certain. Among six prospective studies measuring changes in spine BMD and/or spine Z-score related to glucocorticoid therapy, there was inconsistent reduction in spine BMD or Z-score across studies.
As observed in adults taking glucocorticoids, children taking glucocorticoids had higher rates of morphometric, as opposed to clinical, vertebral fracture. Clinical fracture rates varied from 2% to 33% per year. Incident morphometric fracture rates ranged 6–10% per year, and prevalent fractures varied from 7% (within 30 days of beginning glucocorticoid therapy) to 45%. Only one study was specifically designed to determine rates of incident morphometric vertebral fractures related to recently initiated systemic glucocorticoid therapy in children, reporting a rate of 6% per year.
To our knowledge, this is the first systematic review and meta-analysis on the skeletal effects of glucocorticoid therapy in children, yet the results must be interpreted with regard to strengths and weaknesses. We limited our analysis to measures of spine and/or total body less head BMD, as the International Society for Clinical Densitometry [10] states these two measures are the most reliable indicators of skeletal status in children. We contacted authors to obtain additional data necessary for meta-analysis, permitting inclusion of studies that would otherwise be excluded. Two authors reviewed each abstract, publication, and performed data extraction, providing consistent data for interpretation. Though most studies recruited age- and gender-matched controls, our meta-analysis was not able to control for covariates such as disease activity, bone age, pubertal status, physical activity, or dietary habits. Only two studies recruited >100 children taking glucocorticoid therapy. The small sample size of most studies was likely due to rarity of pediatric diseases requiring glucocorticoid therapy. No studies determined sample size a priori. BMD outcomes differed across studies, with some reporting data in g/cm2 and others reporting Z-scores; this fact limited our ability to pool data across all studies. Moreover, some studies reported fracture incidence, and others reported prevalence, and only one study reported fracture rates in healthy controls.
Additional well-designed, long-term prospective research studies are needed to evaluate the skeletal effects of glucocorticoid therapy in children. We suggest several future research priorities such as the following:
Evaluate whether glucocorticoid therapy in children exhibits a dose–response relationship, with higher doses and longer duration conferring greater skeletal harm.
Evaluate to what extent the underlying disease, and/or its severity, contributes to low BMD and fractures in children with and without exposure to glucocorticoids.
Evaluate whether other patient characteristics, such as race, gender, and Tanner stage, alter the effects of glucocorticoid therapy on BMD and fracture.
Evaluate the sensitivity and specificity of vertebral fracture assessment (VFA) as a tool to detect morphometric vertebral fractures in children, compared to the gold-standard thoracolumbar spine films with greater radiation exposure.
Evaluate whether cessation of glucocorticoid therapy is associated with recovery of BMD and/or reduced fracture risk, as it seems to be in adults [1].
Examine the public health burden of childhood glucocorticoid exposure, including life-long fracture risk.
Conclusion
In summary, limited data suggest that children treated with glucocorticoid therapy have lower spine BMD and higher rates of morphometric fractures compared to healthy children. It remains uncertain whether children receiving glucocorticoid therapy have lower spine BMD compared to children with milder disease not requiring glucocorticoid therapy. Surprisingly, only a few studies have prospectively evaluated changes in BMD and fracture related to glucocorticoid therapy. Existing data suggest that morphometric vertebral fractures are highly prevalent in children treated with glucocorticoid therapy, although only one study reported fracture rates in healthy controls. Future prospective, well-designed studies are needed to systematically investigate the skeletal effects of glucocorticoid therapy in children.
Acknowledgments
We thank Paulina Czapiga, MD, Eva Procek, MA, and Michael G. Johnson, MS, for translation of articles in non-English languages. We thank Heidi Marleau, Associate Director, Ebling Library for assistance with searching the literature. We thank Drs. Leanne Ward and Diana Escolar for providing additional unpublished data to facilitate these analyses. We acknowledge University of Wisconsin Professor William T. Hoyt and Nick S. Keuler for guidance with statistical analysis.
Sources of Support: K.E.H. received support from NIH (R01 AG028739) during the conduct of this study. C.M.B. received support from NIH-NIAMS K23AR062381. N.S. received support from NIH-NIA R03AG040669 and a VA MERIT award. The views expressed in this manuscript do not necessarily represent those of the Department of Veterans Affairs.
Footnotes
Competing Interests: K.E.H. provides consulting to Deltanoid Pharmaceuticals; other authors have no competing interests.
References
- 1.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 2.Chappard D, Legrand E, Basle MF, Fromont P, Racineux JL, Rebel A, et al. Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Miner Res. 1996;11:676–685. doi: 10.1002/jbmr.5650110516. [DOI] [PubMed] [Google Scholar]
- 3.Dalle Carbonare L, Arlot ME, Chavassieux PM, Roux JP, Portero NR, Meunier PJ. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res. 2001;16:97–103. doi: 10.1359/jbmr.2001.16.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13:777–787. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 6.Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 7.van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–198. doi: 10.1093/qjmed/hci029. [DOI] [PubMed] [Google Scholar]
- 8.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62:1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 9.Leonard MB. Glucocorticoid-induced osteoporosis in children: impact of the underlying disease. Pediatrics. 2007;119(Suppl 2):S166–S174. doi: 10.1542/peds.2006-2023J. [DOI] [PubMed] [Google Scholar]
- 10.Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. (W64) [DOI] [PubMed] [Google Scholar]
- 13.Del Re AC, Hoyt WT. MAd: Meta-analysis with mean differences. R package version 0.9 ed. 2010 [Google Scholar]
- 14.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36:1–48. [Google Scholar]
- 15.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 16.Falcini F, Trapani S, Civinini R, Capone A, Ermini M, Bartolozzi G. The primary role of steroids on the osteoporosis in juvenile rheumatoid patients evaluated by dual energy X-ray absorptiometry. J Endocrinol Invest. 1996;19:165–169. doi: 10.1007/BF03349860. [DOI] [PubMed] [Google Scholar]
- 17.Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology. 2005;44:813–818. doi: 10.1093/rheumatology/keh538. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brik R, Keidar Z, Schapira D, Israel O. Bone mineral density and turnover in children with systemic juvenile chronic arthritis. J Rheumatol. 1998;25:990–992. [PubMed] [Google Scholar]
- 20.Celiker R, Bal S, Bakkaloglu A, Ozaydin E, Coskun T, Cetin A, et al. Factors playing a role in the development of decreased bone mineral density in juvenile chronic arthritis. Rheumatol Int. 2003;23:127–129. doi: 10.1007/s00296-002-0265-0. [DOI] [PubMed] [Google Scholar]
- 21.Kashef S, Saki F, Karamizadeh Z, Kashef MA. Bone mineral density in children wth systemic lupus erythematosus and juvenile rheumatoid arthritis. Ann Saudi Med. 2007;27:427–431. doi: 10.5144/0256-4947.2007.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara M, Borrelli B, Greco N, Coppola L, Coppola A, Simeone G, et al. Side effects of corticosteroid therapy in children with chronic idiopathic thrombocytopenic purpura. Hematology. 2005;10:401–403. doi: 10.1080/10245330500168740. [DOI] [PubMed] [Google Scholar]
- 23.Kim SD, Cho BS. Pamidronate therapy for preventing steroid-induced osteoporosis in children with nephropathy. Nephron Clin Pract. 2006;102:c81–c87. doi: 10.1159/000089664. [DOI] [PubMed] [Google Scholar]
- 24.Bak M, Serdaroglu E, Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr Nephrol. 2006;21:350–354. doi: 10.1007/s00467-005-2118-z. [DOI] [PubMed] [Google Scholar]
- 25.Escolar DM, Hache LP, Clemens PR, Cnaan A, McDonald CM, Viswanathan V, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodd C, Lang B, Ramsay T, Alos N, Huber AM, Cabral DA, et al. Incident vertebral fractures among children with rheumatic disorders 12 months after glucocorticoid initiation: a national observational study. Arthritis Care Res. 2012;64:122–131. doi: 10.1002/acr.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart WA, Acott PD, Salisbury SR, Lang BA. Bone mineral density in juvenile dermatomyositis: assessment using dual x-ray absorptiometry. Arthritis Rheum. 2003;48:2294–2298. doi: 10.1002/art.11211. [DOI] [PubMed] [Google Scholar]
- 28.van Staa TP, Cooper C, Leufkens HG, Bishop N. Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res. 2003;18:913–918. doi: 10.1359/jbmr.2003.18.5.913. [DOI] [PubMed] [Google Scholar]
- 29.Huber AM, Gaboury I, Cabral DA, Lang B, Ni A, Stephure D, et al. Prevalent vertebral fractures among children initiating glucocorticoid therapy for the treatment of rheumatic disorders. Arthritis Care Res. 2010;62:516–526. doi: 10.1002/acr.20171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loftus J, Allen R, Hesp R, David J, Reid DM, Wright DJ, et al. Randomized, double-blind trial of deflazacort versus prednisone in juvenile chronic (or rheumatoid) arthritis: a relatively bone-sparing effect of deflazacort. Pediatrics. 1991;88:428–436. [PubMed] [Google Scholar]
- 31.Nakhla M, Scuccimarri R, Duffy KN, Chedeville G, Campillo S, Duffy CM, et al. Prevalence of vertebral fractures in children with chronic rheumatic diseases at risk for osteopenia. J Pediatr. 2009;154:438–443. doi: 10.1016/j.jpeds.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Varonos S, Ansell BM, Reeve J. Vertebral collapse in juvenile chronic arthritis: its relationship with glucocorticoid therapy. Calcif Tissue Int. 1987;41:75–78. doi: 10.1007/BF02555248. [DOI] [PubMed] [Google Scholar]