Abstract
The thermal instability of the anticoagulant heparin is associated, in part, with the solvolytic loss of N-sulfo groups. This study describes a new method to assess the increased content of unsubstituted amino groups present in thermally-stressed and autoclave-sterilized heparin formulations. N-acetylation of heparin samples with acetic anhydride-d6 is followed by exhaustive heparinase treatment, and disaccharide analysis by hydrophilic interaction chromatography mass spectrometry. The introduction of stable isotopic label provides a sensitive probe for the detection and localization of the lost N-sulfo groups potentially providing valuable insights into degradation mechanism and the reasons for anticoagulant potency loss.
Keywords: heparin, sulfate, stability assay, amino group, mass spectrometry
Heparin is a sulfated polysaccharide that is widely used as an anticoagulant drug in extracorporeal therapy, surgical procedures and for treatment of clotting disorders [1]. The complex chemical structure of this heterogeneous (multiple disaccharide sequences), polydisperse (average molecular weight ~ 16,000 Da with an MW/MN ~ 1.6) polysaccharide makes its analysis quite difficult [2]. This is exemplified by the recent introduction of a detailed compendium by the United States Pharmacopeia (USP) to assess the identity and purity of heparin active pharmaceutical ingredient (API). Interest in heparin analysis peaked in 2008 during a contamination crisis, in which heparin was adulterated, leading to many severe reactions including patient deaths [3,4].
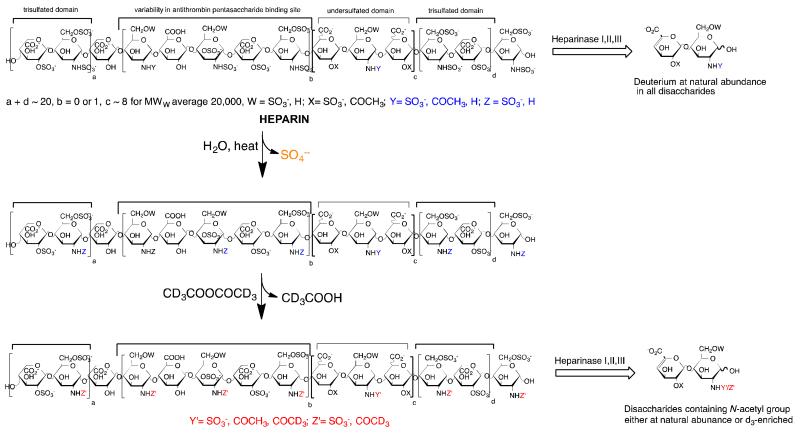
As part of a continued focus on developing improved methods for heparin analysis, our laboratory has been assessing stability determining assays for heparin that are critical in ensuring drug potency and establishing appropriate shelf-life for formulated heparin products. Surprisingly, there is very little published literature on stability determining assays for heparin and most commonly its anticoagulant potency is the sole method used to assess its stability on formulation and storage. Stability studies of thermally-stressed and pH-stressed heparin formulations, first published by our group nearly 20 years ago [5], suggested several major mechanisms of non-oxidative decomposition of heparin leading to loss of potency. The hydrolysis of glycosidic linkages and β-elimination of the glycosidic linkage to uronic acid residues result in decreased molecular weight, while the solvolytic loss of sulfate, particularly the more labile N-sulfo groups, can reduce heparin potency without significantly impacting its molecular weight. The loss of potency in autoclaving formulated heparin has be attributed to damage done to heparins antithrombin III (AT)-binding site, resulting in a decreased binding to AT, as determined by competitive binding studies using surface plasmon resonance (SPR) leading to loss in anticoagulant potency [6]. Heparin (and the structurally related polysaccharide heparan sulfate) contains a small number of unsubstituted amino groups [7] further complicating the analysis of such groups as they form when heparin decomposes. These unsubstituted amino groups can be determined using NMR or through fluorescent labeling [7] and the inorganic sulfate formed can be determined using ion chromatography [8]. The current study explores the use of hydrophilic interaction chromatography (HILIC)-mass spectrometry (MS) to measure unsubstituted amino groups formed in thermally-stressed heparin formulations.
USP heparin sodium (200 U/mg, Celsus, Cincinnati, OH) was prepared at 1000 U/mL in 0.9% aqueous sodium chloride at pH 7. Heparin sample (60 mL) was divided into six equal portions. One portion was retained (control), two portions were treated under 65 °C and 85 °C for 2 h, respectively (thermally stressed). The three remaining heparin samples were sterilized in an autoclave at 121°C for 0.5, 1, and 2 h, respectively (sterilized heparin) [6]. One mL of each sample, containing 5 mg of heparin, was lyophilized.
Samples (each consisting of 5 mg of polysaccharide), including control heparin, thermally-stressed heparins, sterilized heparins, and chemically N-desulfonated heparin (CNDS) prepared as previously described [9], were dissolved in the 1 mL of 50 mM aqueous sodium carbonate containing 10% methanol (v/v). Aliquots (8 μL) of acetic anhydride-d6 (or acetic anhydride in the case of one of the CNDS samples (negative control)) were added into the heparin solution every 20 min over a total reaction time of 2 h. After the chemical N-acetylation, all samples were desalted using 3-KDa molecular weight cut-off (MWCO) membranes and lyophilized. One-dimensional (1D) 1H- nuclear magnetic resonance (NMR; 600 MHz, Bruker Bio Spin, Billerica, MA) of CNDS confirmed the presence of unsubstituted amino groups and their conversion to N-acetyl groups following treatment with acetic anhydride (Fig. S1) and deuterium-labeling of CNDS was confirmed using 1D 2H-NMR (Fig. S2). Two-dimensional (2D) heteronuclear single-quantum coherence (HSQC) NMR allowed for spectral assignments and confirmed that all the amino groups had been N-acetylated (Fig. S3) [2,10].
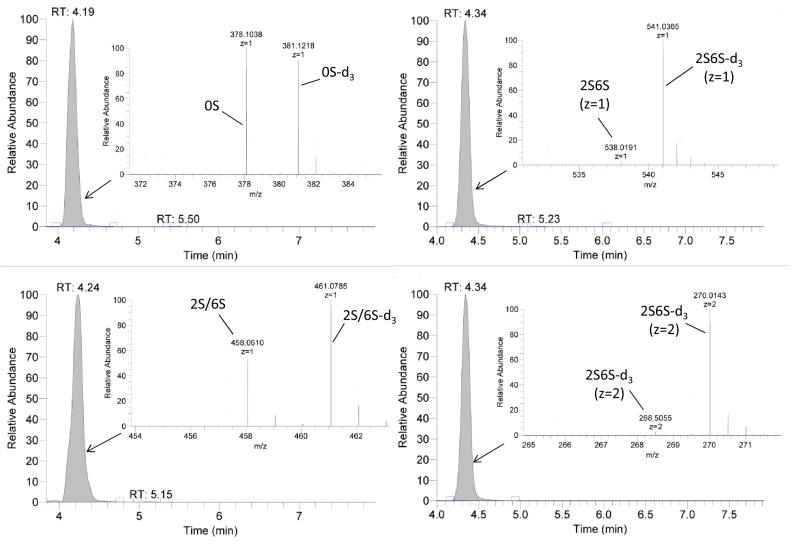
Treatment of each heparin sample (10 μL 10 μg/μL) with recombinant Flavobacterial heparin lyase I, II, and III [11] (10 mU each) in 90 μL of 40 mM ammonium acetate and 2 mM calcium acetate aqueous (pH 7.0) at 33°C for 10 h to completely degraded the heparin samples to their constituent disaccharides. Some residues with unsubstituted amino groups are resistant to heparin lyase degradation and heparin samples containing such residues [7]. Moreover, most of the unsubstituted disaccharides are isobaric with other disaccharides. Thus, we decided to N-acetylate with acetic anhydride-d6 to afford disaccharide products with unique masses with retention times matched to readily available disaccharide standards. After enzymatic treatment, samples were heated in 100 °C water bath for 10 min to thermally inactivate the enzymes and centrifuged (5,000 × g, 10 min) to remove precipitated protein [10] and the supernatants were collected for LC-MS analysis using a Luna HILIC column (2.0 × 50 mm, 200 Å, Phenomenex, Torrance, CA) connected online to the standard ESI source of LTQ-Orbitrap XL FT-MS (Thermo Fisher Scientific, San-Jose, CA) [2]. The short column selected to provides sufficient resolution for rapid analysis (base peak width of ~0.2-0.5 min) and removes salts and proteins from the analyte. Longer (2.0 × 150 mm) columns markedly improve resolution but result in lower sample throughput [12]. Gradient elution used 5-70% 5 mM aqueous ammonium acetate with 95-30% 5 mM aqueous ammonium acetate in 98% acetonitrile over 7 min at 250 μL/min. Unsaturated heparin disaccharide standards were analyzed at different concentrations to prepare a standard curve [2]. All the disaccharides and deuterated disaccharides detected (Fig. S4) and their mass are provided in Table 1. The mass percentages of these disaccharides are presented in Table 2. Little if any deuterated disaccharide (natural abundance of ~0.02%) was detected in the negative controls corresponding to CNDS (0.06%) and N-acetylated-d3 CNDS (0.02%) samples (Table 2). In contrast, the positive control, N-acetylated-d3 CNDS showed the expected high percentage (~90%) of deuterated disaccharide (Fig. 2). These data are consistent with the NMR results showing complete N-acetylation of unsubstituted amine groups and suggesting the low background and high sensitivity of this assay method. Heparin API, examined in triplicate, showed a relatively low level of unsubstituted amino groups 0.27 ± 0.02% establishing the excellent repeatability and stability of the current method. The heparin sample treated for 48 h at 85 °C generated twice as many unsubstituted amino groups than the sample treated at 65 °C and the autoclaved samples showed the greatest loss of N-sulfo groups. N-sulfo groups appeared to be lost in equal amounts from monosulfated, disulfated and trisulfated sequences suggesting that this solvolysis reaction is not selective (Table 2). Prior studies have demonstrated that autoclaving heparin reduces its anticoagulant potency [2].
Table 1.
Structure of disaccharides detected by HILIC-MS analysis.
| Disaccharide Units | M/Z | Deuterated Disaccharides | M/Z |
|---|---|---|---|
| ΔUA-GlcNAc (0S) | [378.10]1− | ΔUA-GlcNAc-d3 (0S) | [381.12]1− |
| ΔUA2S-GlcNAc (2S) | [458.06]1− | ΔUA2S-GlcNAc-d3 (2S) | [461.07]1− |
| ΔUA-GlcNAc6S (6S) | [458.06]1− | ΔUA-GlcNAc-d3 6S (6S) | [461.07]1− |
| ΔUA2S-GlcNAc6S (2S6S) | [268.50]2−, [538.01]1− | ΔUA2S-GlcNAc-d3 6S (2S6S) | [270.01]2−, [541.03]1− |
| ΔUA-GlcNS (NS) | [416.04]1− | ||
| ΔUA2S-GlcNS (NS2S) | [247.50]2−, [496.01]1− | ||
| ΔUA-GlcNS6S (NS6S) | [247.50]2−, [496.01]1− | ||
| ΔUA2S-GlcNS6S (Tri S) | [287.48]2−, [575.96]1− |
Table 2.
Disaccharide compositional analysis of heparin samples following N-acetylation.
| Samples | Unlabeled disaccharides (%) | D-labeled disaccharides (%) | Total D-labeled disaccharides (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 0S | 2S/6S | 2S6S | NS | NS2S/ NS6S |
Tri S | 0S | 2S/6S | 2S6S | ||
| CNDS | 9.91 | 13.9 | 5.90 | 68.50 | 0.36 | 1.38 | 0.04 | < 0.01 | 0.02 | 0.06 |
| Ac-CNDS | 3.85 | 14.0 | 81.7 | 0.18 | 0.01 | 0.23 | < 0.01 | < 0.01 | 0.02 | 0.02 |
| Ac-d3-CNDS | 2.00 | 5.14 | 2.30 | 0.17 | < 0.01 | 0.25 | 1.69 | 10.3 | 78.2 | 90.1 |
|
Heparin
(n=3) |
3.98 ±0.42 |
4.97 ±0.78 |
4.44 ±0.43 |
7.40 ±1.15 |
9.20 ±0.98 |
69.7 ±1.28 |
0.07 ±0.01 |
0.04 ±0.01 |
0.16 ±0.01 |
0.27 ±0.02 |
| 65°C-48hr | 4.65 | 6.23 | 4.79 | 11.19 | 0.28 | 72.56 | 0.08 | 0.04 | 0.17 | 0.30 |
| 85°C-48hr | 5.12 | 6.39 | 5.19 | 10.39 | 0.21 | 72.05 | 0.10 | 0.07 | 0.47 | 0.64 |
| 121°C-0.5hr | 4.18 | 4.61 | 4.18 | 8.36 | 8.08 | 70.09 | 0.08 | 0.06 | 0.35 | 0.49 |
| 121°C-1hr | 4.22 | 4.99 | 4.51 | 7.05 | 9.61 | 68.79 | 0.08 | 0.08 | 0.67 | 0.83 |
| 121°C-2hr | 4.01 | 4.40 | 4.71 | 9.55 | 8.80 | 64.94 | 0.15 | 0.25 | 3.19 | 3.58 |
The IC50 values determined in competitive AT binding measured by SPR (See Fig. S5) are heparin 1.38 U/mL, 65°C- 48 h 1.52 U/mL, 85°C- 48 h 1.76 U/mL, 121°C- 0.5 h 1.84 U/mL, 121°C- 1 h 1.96 U/mL, and 121°C- 2 h 3.81 U/mL.
Figure 2.
Extracted ion chromatography and mass spectra of the detected D-labeled disaccharides from N-acetylated-d3-CNDS using HILC-MS.
Solution competition studies between surface immobilized heparin and different concentrations (0-10 μg/mL) of soluble heparin sample and soluble AT (250 nM) to measure IC50 were performed using surface plasmon resonance (SPR) on a BIAcore 3000 (GE Healthcare) as previously described [2]. AT-binding affinity of heparin is dependent on the presence of an N-sulfo group-containing AT-binding site within a heparin chain [1] and this measured IC50 correlates to in vitro potency assays [2]. As expected, increased temperature and longer heat exposure times result in higher N-sulfo group loss (Table 1) and higher IC50 values (Table 2).
In conclusion, a facile assay for the presence and formation of unsubstituted amino groups within heparin is described. This assay shows that loss of N-sulfo groups in formulated heparin is proportional to the temperature and length of exposure in thermally-stressed samples. Moreover, this loss of N-sulfo groups, while nonselective, results in decreased heparin affinity for AT corresponding to reduced potency.
Supplementary Material
Figure 1.
Schematic of solvolytic loss of heparin N-sulfo groups leading to decreased AT-affinity and loss of potency in formulated heparin samples and method for determining N-sulfo group loss.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Linhardt RJ. Heparin: Structure and activity. J. Med. Chem. 2003;46:2521–2564. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 2.Fu L, Li G, Yang B, Onishi A, Li L, Sun P, Zhang F, Linhardt RJ. Structural characterization of pharmaceutical heparins prepared from different animal tissues. J. Pharm. Sci. 2013;102:1447–1457. doi: 10.1002/jps.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrini M, Beccati D, Shriver Z, Naggi AM, Bisio A, Capila I, Lansing J, Guglieri S, Fraser B, Al-Hakim A, Gunay S, Viswanathan K, Zhang Z, Robinson L, Venkataraman G, Buhse L, Nasr M, Woodcock J, Langer R, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Oversulfated chondroitin sulfate is a major contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008;26:669–775. doi: 10.1038/nbt1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26:313–321. doi: 10.1039/b819896a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jandik KA, Kruep D, Cartier M, Linhardt RJ. Accelerated stability studies of heparin. J. Pharm. Sci. 1996;85:45–51. doi: 10.1021/js9502736. [DOI] [PubMed] [Google Scholar]
- 6.Beaudet JM, Weyers A, Solakyildirim K, Yang B, Takieddin M, Mousa S, Zhang F, Linhardt RJ. Impact of autoclave sterilization on the activity and structure of formulated heparin. J. Pharm. Sci. 2011;100:3396–3404. doi: 10.1002/jps.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toida T, Yoshida H, Toyoda H, Koshiishi I, Imanari T, Hileman RE, Fromm JR, Linhardt RJ. Structural differences and the presence of unsubstituted amino groups in heparan sulphates from different tissues and species. Biochem. J. 1997;322:499–506. doi: 10.1042/bj3220499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Linhardt RJ, Zhang Z. Quantitative analysis of free and covalent anions in glycosaminoglycans and application in heparin stability studies. Carbohydr. Polym. 2014 doi: 10.1016/j.carbpol.2014.02.076. in press. [DOI] [PubMed] [Google Scholar]
- 9.Linhardt RJ. Chemical and enzymatic methods for the depolymerization and modification of heparin. In: Ogura H, Hasegawa A, Suami T, editors. Carbohydrates-Synthetic Methods and Applications in Medicinal Chemistry. Kodansha/VCH; Tokyo: 1992. pp. 385–401. 1992. [Google Scholar]
- 10.Fu L, Zhang F, Li G, Onishi A, Bhaskar U, Sun P, Linhardt RJ. Structure and activity of a new low-molecular-weight heparin produced by enzymatic ultrafiltration. J. Pharm. Sci. 2014 doi: 10.1002/jps.23939. in press. DOI 10.1002/jps.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Jones CL, Liu J. Using an enzymatic combinatorial approach to identify anticoagulant heparan sulfate structures. Chem. & Biol. 2007;14:986–993. doi: 10.1016/j.chembiol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li L, Zhang F, Zaia J, Linhardt RJ. Top-down approach for the direct characterization of low molecular weight heparins using LC-FT-MS. Anal. Chem. 2012;84:8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.