Abstract
Previous research suggests that elevated pulse pressure (PP) is a risk factor for atrial fibrillation (AF) independently of mean arterial pressure (MAP). PP may serve as an indirect measure of aortic stiffness (reduced distensibility), but whether directly-measured aortic distensibility is related to risk of AF has not yet been studied. This analysis included 6,630 participants aged 45-84 years from the Multi-Ethnic Study of Atherosclerosis (MESA). At baseline, blood pressure (BP) and other relevant covariates were measured using standardized protocols. Magnetic resonance imaging-based aortic distensibility was measured in 3,441 participants. Incident AF was identified from hospitalization discharge codes and Medicare claims. Multivariable Cox models were used to estimate the association of BP components and aortic distensibility with AF risk. During a mean follow-up of 7.8 years, 307 AF events (137 among those with aortic distensibility measurements) were identified. In multivariable adjusted models simultaneously including MAP and PP, each 1-standard deviation increase in PP was associated with a 29% increased risk of AF (95% CI: 5%, 59%, p=0.02), with MAP not being associated with increased AF risk. Overall, aortic distensibility was not consistently associated with AF risk: after removing outliers, each 1-standard deviation increase in aortic distensibility was associated with a 9% increased risk of AF (95% CI: - 22%, 51%, p=0.63). In conclusion, in this large community-based cohort, we found that PP, but not MAP or aortic distensibility, was a significant risk factor for AF, emphasizing the importance of PP when assessing the risk of developing AF. Our results cast doubt upon the clinical utility of aortic distensibility as a predictor for the development of AF.
Keywords: blood pressure, atrial fibrillation, epidemiology
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in clinical practice, causing a large burden of morbidity and mortality in an increasingly aging population.1 Studies published over the last 2 decades have consistently shown that both elevated blood pressure (BP)2,3 and a diagnosis of hypertension4,5 are important risk factors for AF. More recently, an analysis of the Framingham Heart Study identified pulse pressure (PP) as a better predictor for the development of AF than mean arterial pressure (MAP),6 though these results were not confirmed in the Women’s Health Study.3 PP has also been associated with left atrial enlargement, a risk factor for AF.7,8 Increased PP can be a consequence of aortic stiffness (reduced aortic distensibility). However, no information exists on the association between directly-measured aortic stiffness and AF incidence in the general population. In the present study we used BP and magnetic resonance imaging (MRI)-based aortic distensibility data available from the Multi-Ethnic Study of Atherosclerosis (MESA), a community-based, multi-ethnic cohort of middle to older aged adults. First, we assessed whether PP is more strongly associated with AF than MAP in the MESA cohort. Second, we examined the role of aortic distensibility as a risk factor for AF in comparison to established BP measures.
METHODS
MESA is a prospective cohort study of risk factors for subclinical atherosclerosis conducted at 6 field centers in the US (Baltimore, MD; Chicago, IL; Saint Paul, MN; Los Angeles, CA; New York, NY; and Forsyth County, NC).9 At entry participants were aged 45 to 84 and self-reported no history of clinical cardiovascular disease. Recruitment and baseline examination of the original 6,814 MESA participants occurred between July 2000 and August 2002. A subsample of consenting participants with no contraindications underwent a cardiac MRI, with 3,541 of the MRIs including an assessment of the ascending aorta. Four additional examinations have been completed over the follow-up (most recently in 2010-2012). The study was approved by the institutional review boards of all participating institutions, and all participants provided written informed consent.
AF was ascertained through study electrocardiography, hospital discharge codes, and for participants age 65 years and older enrolled in fee-for-service Medicare (55% of the cohort), from Medicare claims data obtained from the Centers for Medicare & Medicaid Services (CMS). Annual follow-up phone calls to study participants through February 2012 were used to identify hospitalizations, and Medicare claims data were used to ascertain inpatient AF events through December 31, 2009. Discharges showing the International Classification of Diseases, ninth revision (ICD-9) codes 427.31 or 427.32 were classified as AF events. The date of AF incidence was defined as the date of the first record showing a diagnosis of AF. A review of 16 validation studies determined that the use of the ICD-9 codes to identify AF events has relatively good performance.10 At baseline, persons who self-reported AF or who had AF in the baseline electrocardiograph or in a Medicare claim prior to study enrollment were excluded.
Measures from physical examination and questionnaires were made at MESA baseline. Seated systolic and diastolic BP were defined as the average of the last 2 of 3 BP measurements taken after a 5 minute seated rest using an automated oscillometric sphygmomanometer (Dinamap Pro 100; Critikon, Tampa, FL). MAP is defined as the sum of diastolic BP and [(1/3) × systolic BP]. PP is defined as the difference between systolic and diastolic BP. Aortic distensibility was evaluated using 1.5-T whole-body MRI systems, Signa CV/I or Signa LX (General Electric Medical Systems, Waukesha, WI), as previously described.11 Covariate variables were measured using standard protocols, as described in Supplementary Methods.
Among the original 6,814 MESA participants, for our primary analysis we made the following exclusions: those who were ineligible (n=5); those with prevalent AF (n=58); those with no follow-up information (n=20); and those missing information on covariates (n=101). The subgroup analysis additionally excluded MESA participants who did not receive an aortic MRI at baseline or who had invalid MRI parameters (n=3,189).
Using restricted cubic splines, we determined that the shape of associations of blood pressure measures (systolic BP, diastolic BP, MAP, and PP) and aortic distensibility with incident AF were approximately linear. Thus, we decided to model each measure as a continuous variable divided by its respective standard deviation. Using Cox proportional hazards regression, we calculated adjusted hazard ratios (HR) and 95% confidence intervals (CI) for the associations of the BP measures and aortic distensibility with incident AF. Follow-up time was defined as time in days between baseline visit until incidence of AF, death, or last follow-up contact, whichever occurred first. Initial models were adjusted for age, sex, race/ethnicity, and MESA field center site, and fully-adjusted models were additionally adjusted for education level, height, body mass index, smoking status, antihypertensive medication use, diabetes, electrocardiography-based left ventricular hypertrophy, P-R interval (<120, 120–199, or >199 ms), and resting heart rate. Other than sex, site, education level, and heart rate, the other variables used for model adjustment were chosen in accordance with the CHARGE-AF risk score augmented model.12 In addition, we also ran analyses adjusting for MRI-based left ventricular mass in place of electrocardiography-based left ventricular hypertrophy (MRI-based left ventricular mass was used instead of electrocardiography-based left ventricular hypertrophy in all subgroup analyses). In further analyses we also adjusted for incident heart failure and myocardial infarction as time-dependent covariates, where events diagnosed on the same date as AF diagnosis were considered interim events. Lastly, we ran a sensitivity analysis adjusting for different types of antihypertensive medications (ACE inhibitors/angiotensin receptor blockers, beta blockers, diuretics, and other types). We evaluated effect modification by age, sex, and race/ethnicity by adding multiplicative terms into regression models. The proportional hazards assumption was tested by including time-covariate interactions for the BP measures and aortic distensibility, and no violation of the proportional hazards assumption was detected. All statistical analysis was performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
At baseline, there were 6,630 participants (3,130 men and 3,500 women) free of AF who met the inclusion criteria for the primary analysis (n=4,885 available for left ventricular mass-adjusted models). PP was strongly correlated with systolic BP (r=0.88, P<0.001), weakly correlated with diastolic BP (r=0.17, P<0.001), and moderately correlated with MAP (r=0.59, P<0.001). Table 1 presents the baseline characteristics of those MESA participants included in the primary analysis by incident AF status. Those who experienced incident AF during follow-up were older, taller, and more likely to be male, ever-smokers, and antihypertensive medication users. They also had higher levels of BP and left ventricular mass; lower levels of aortic distensibility; and longer P-R intervals. There were 3,441 participants (1,571 men and 1,870 women) meeting the inclusion criteria for the aortic distensibility-subgroup analysis, and their overall characteristics were similar to those included in the primary analysis (see Supplementary Table 1).
Table 1.
Baseline Characteristics by AF Status: Multi-Ethnic Study of Atherosclerosis, 2000-2002
| Characteristics and risk factors | No AF n=6,323 |
AF n=307 | P-Value for AF difference |
|---|---|---|---|
| Age (years), mean (SD) | 62(10) | 70 (8) | <0.0001 |
| Male | 2942 (47%) | 188 (61%) | <0.0001 |
| Race/Ethnicity | <0.0001 | ||
| White | 2366 (37%) | 166 (54%) | |
| Chinese American | 771 (12%) | 21 (7%) | |
| Black | 1766 (28%) | 67 (22%) | |
| Hispanic | 1420 (22%) | 53 (17%) | |
| College degree or higher | 2227 (35%) | 111 (36%) | 0.74 |
| Height (cm), mean (SD) | 166 (10) | 169 (10) | 0.0001 |
| Body mass index (kg/m2), mean (SD) | 28 (5) | 29 (6) | 0.25 |
| Systolic blood pressure (mmHg), mean (SD) | 126 (21) | 135 (22) | <0.0001 |
| Diastolic blood pressure (mmHg), mean (SD) | 72 (10) | 72 (10) | 0.91 |
| Mean arterial pressure (mmHg), mean (SD) | 90 (13) | 93 (13) | <0.0001 |
| Pulse pressure (mmHg), mean (SD) | 54 (17) | 63 (18) | <0.0001 |
| Aortic distensibility (mmHg−1)†, mean (SD) | 1.9 (1.2) | 1.6 (1.7) | 0.04 |
| Ever-smoker | 3114 (49%) | 180 (59%) | 0.001 |
| Any antihypertensives | 2271 (36%) | 172 (56%) | <0.0001 |
| ACE inhibitors/angiotensin receptor blockers | 1107(18%) | 90 (29%) | <0.0001 |
| Beta-blocker | 566 (9%) | 58 (19%) | <0.0001 |
| Diuretics | 808 (13%) | 70 (23%) | <0.0001 |
| Diabetes‡ | 789 (12%) | 46 (15%) | 0.20 |
| Left ventricular hypertrophy¥ | 144 (2%) | 12 (4%) | 0.07 |
| Left ventricular mass (g)€, mean (SD) | 144 (39) | 164 (46) | <0.0001 |
| P-R interval (msec), mean (SD) | 165 (24) | 174 (33) | <0.0001 |
| Resting heart rate, mean (SD) | 63 (10) | 63 (11) | 0.38 |
AF: atrial fibrillation
Available in 3,441 participants (137 with AF)
Diabetes is defined as fasting glucose > 126 mg/dL or use of glucose-lowering medication.
Defined using electrocardiograph by Cornell voltage criteria.
Measured by MRI; available in 4,885 participants (204 with AF).
There were 307 (4.6%) newly-diagnosed cases of AF over a mean follow-up period of 7.8±1.7 years (incidence rate 5.9 cases per 1,000 person-years). Table 2 presents the association between BP measures, modeled continuously per 1-standard deviation increase, and incident AF. After adjusting for age, sex, race/ethnicity, and site (model 1), increases in systolic BP, MAP, and PP, but not diastolic BP, were each significantly associated with higher risk of AF. Additional adjustment for education, resting heart rate, and the remaining CHARGE-AF risk factors (model 2) resulted in slightly attenuated hazard ratios, with only systolic BP and PP remaining significant. Models further adjusted for MRI-based left ventricular mass in place of electrocardiography-based left ventricular hypertrophy (model 3) and interim heart failure and myocardial infarction events (model 4) further attenuated the association, with none of the individual BP measures remaining significantly associated with AF risk. Sensitivity analyses further adjusting model 3 for specific antihypertensive medication types also slightly attenuated the association (see Supplementary Table 2). Diastolic BP showed an inverse association with incident AF in all models when modeled simultaneously with systolic BP. When modeled simultaneously with MAP, PP remained significantly positively associated with AF in all models (Table 2 and Figure 1). In model 3, systolic BP and MAP were each marginally negatively associated with incident AF when modeled simultaneously with PP. In all models, PP was more strongly associated with AF risk after adjustment for MAP or systolic BP when compared to models including PP alone. We found no significant interactions between BP measures and age, sex, or race/ethnicity (results not shown).
Table 2.
Cox proportional hazards models for blood pressure measures as risk factors of incident atrial fibrillation (Sample size: 6,630; AF events: 307): Multi-Ethnic Study of Atherosclerosis, 2000-2011.
| Model 1† | Model 2‡ | Model 3¥ | Model 4€ | |||||
|---|---|---|---|---|---|---|---|---|
| Predictor | HR (95% CI)§ | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Modeled separately | ||||||||
| Systolic blood pressure | 1.21 (1.09, 1.36) | 0.0007 | 1.16 (1.03, 1.31) | 0.01 | 1.04 (0.90, 1.20) | 0.59 | 1.07 (0.92, 1.25) | 0.36 |
|
| ||||||||
| Diastolic blood pressure | 1.03 (0.91, 1.16) | 0.65 | 1.00 (0.88, 1.13) | 0.95 | 0.91 (0.78, 1.05) | 0.20 | 0.95 (0.81, 1.01) | 0.48 |
|
| ||||||||
| Mean arterial pressure | 1.13 (1.01, 1.27) | 0.03 | 1.09 (0.97, 1.22) | 0.16 | 0.97 (0.84, 1.12) | 0.70 | 1.01 (0.87, 1.17) | 0.89 |
|
| ||||||||
| Pulse pressure | 1.28 (1.14, 1.44) | <0.0001 | 1.23 (1.09, 1.39) | 0.0006 | 1.13 (0.97, 1.32) | 0.12 | 1.15 (0.98, 1.34) | 0.10 |
|
| ||||||||
| Modeled jointly | ||||||||
| Systolic blood pressure | 1.39 (1.19, 1.61) | <0.0001 | 1.34 (1.14, 1.57) | 0.0003 | 1.24 (1.01, 1.52) | 0.04 | 1.24 (1.00, 1.52) | 0.05 |
| Diastolic blood pressure | 0.81 (0.69, 0.96) | 0.01 | 0.80 (0.68, 0.95) | 0.01 | 0.78 (0.63, 0.96) | 0.02 | 0.82 (0.66, 1.01) | 0.06 |
|
| ||||||||
| Pulse pressure | 1.42 (1.08, 1.86) | 0.01 | 1.44 (1.08, 1.91) | 0.01 | 1.53 (1.08, 2.16) | 0.02 | 1.41 (0.99, 2.02) | 0.06 |
| Systolic blood pressure | 0.90 (0.69, 1.17) | 0.42 | 0.85 (0.65, 1.12) | 0.25 | 0.73 (0.53, 1.01) | 0.06 | 0.81 (0.57, 1.13) | 0.21 |
|
| ||||||||
| Pulse pressure | 1.34 (1.14, 1.57) | 0.0003 | 1.32 (1.12, 1.56) | 0.001 | 1.29 (1.05, 1.59) | 0.02 | 1.26 (1.02, 1.55) | 0.04 |
| Mean arterial pressure | 0.94 (0.80, 1.10) | 0.42 | 0.91 (0.78, 1.07) | 0.25 | 0.83 (0.69, 1.01) | 0.06 | 0.88 (0.72, 1.07) | 0.21 |
Model 1: Adjusted for age, sex, race/ethnicity, and site.
Model 2: Model 1, additionally adjusted for education, height, body mass index, smoking status, antihypertensive medication use (yes/no), diabetes, electrocardiography-based left ventricular hypertrophy, P-R interval, and resting heart rate.
Model 3: Model 2, but adjusted for MRI-based left ventricular mass instead of electrocardiography-based left ventricular hypertrophy. This limits the sample size to 4,885 (N events: 204).
Model 4: Model 3, additionally adjusted for interim myocardial infarction and heart failure events.
Hazard ratio (confidence interval) per 1 standard deviation increase in predictor. Standard deviations for systolic, diastolic, mean, and pulse pressure are 21.5, 10.3, 12.6, and 17.2 mmHg, respectively.
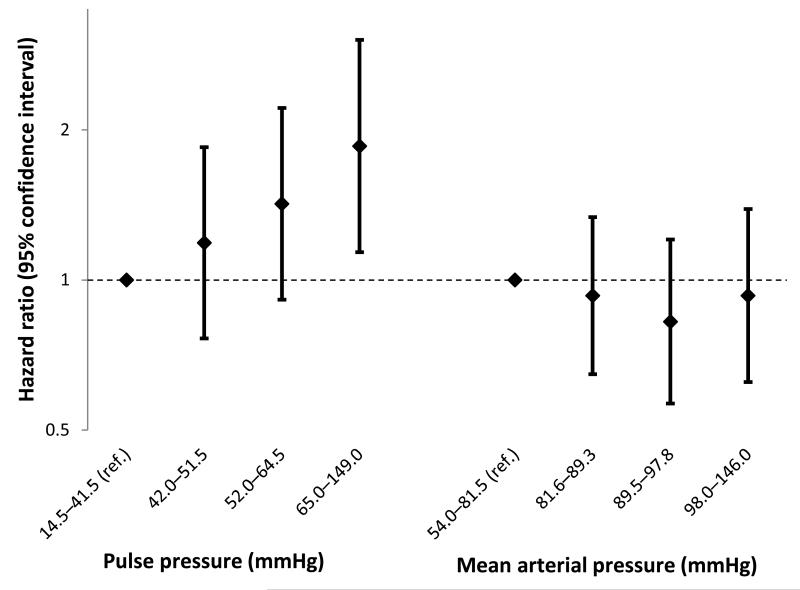
Figure 1.
Hazard ratios for blood pressure measures modeled jointly in quartiles (lowest quartile is reference) as risk factors of incident atrial fibrillation (Total N: 6,630; N Events: 307): Multi-Ethnic Study of Atherosclerosis, 2000-2011. Adjustment is made for age, sex, race/ethnicity, site, education, height, body mass index, smoking status, antihypertensive medication use (yes/no), diabetes, electrocardiography-based left ventricular hypertrophy, P-R interval, and resting heart rate.
There were 137 AF cases among participants in the aortic distensibility-subgroup analysis. The correlation between aortic distensibility and PP was negative and weak (r=−0.34). Table 3 presents the association of BP and aortic distensibility measures, modeled continuously per 1-standard deviation increase (using the standard deviations from the primary analysis), with incident AF. After model 1 adjustments, increases in systolic BP and PP, but not MAP or diastolic BP, were each significantly associated with higher risk of AF. Similar to results in the primary analysis, model 2 and 3 adjustments resulted in attenuated hazard ratios, and PP remained the only significant independent BP risk factor for AF when modeled separately. When PP and MAP were modeled together, PP was positively associated and MAP was negatively associated with AF risk. When modeled simultaneously with PP and MAP, there was a significant association between aortic distensibility and AF, whereby a 1-standard deviation increase in aortic distensibility was associated with a 16% increased risk of incident AF. In a sensitivity analysis excluding outliers that were more than 3 standard deviations away from the mean aortic distensibility value (N=30 observations removed, ranging in value from 5.7–24.2 mmHg×10−1), aortic distensibility was not associated with AF with simultaneous adjustment for PP and MAP.
Table 3.
Cox proportional hazards models for blood pressure and aortic distensibility measures as risk factors of incident atrial fibrillation among the magnetic resonance imaging subgroup (Sample size: 3,441; AF events: 137): Multi-Ethnic Study of Atherosclerosis, 2000-2011.
| Model 1† | Model 2‡ | Model 3€ | ||||
|---|---|---|---|---|---|---|
| Predictor | HR (95% CI)¥ | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Modeled separately | ||||||
| Systolic blood pressure | 1.25 (1.05, 1.48) | 0.01 | 1.11 (0.93, 1.33) | 0.26 | 1.11 (0.92, 1.33) | 0.30 |
|
| ||||||
| Diastolic blood pressure | 1.00 (0.84, 1.20) | 0.99 | 0.89 (0.74, 1.07) | 0.21 | 0.89 (0.73, 1.08) | 0.23 |
|
| ||||||
| Mean arterial pressure | 1.13 (0.95, 1.34) | 0.16 | 1.00 (0.83, 1.19) | 0.96 | 0.99 (0.82, 1.20) | 0.94 |
|
| ||||||
| Pulse pressure | 1.36 (1.14, 1.62) | 0.001 | 1.25 (1.04, 1.51) | 0.02 | 1.23 (1.02, 1.50) | 0.03 |
|
| ||||||
| Aortic distensibility | 1.09 (0.94, 1.27) | 0.23 | 1.13 (0.99, 1.29) | 0.07 | 1.14 (1.00, 1.30) | 0.04 |
| Modeled jointly | ||||||
| Pulse pressure | 1.48 (1.17, 1.88) | 0.001 | 1.47 (1.15, 1.89) | 0.002 | 1.43 (1.11, 1.84) | 0.005 |
| Mean arterial pressure | 0.88 (0.70, 1.11) | 0.29 | 0.79 (0.63, 1.00) | 0.05 | 0.80 (0.63, 1.02) | 0.07 |
|
| ||||||
| Aortic distensibility | 1.13 (0.98, 1.30) | 0.08 | 1.16 (1.01, 1.32) | 0.03 | 1.16 (1.02, 1.32) | 0.02 |
| Aortic distensibility§ | 0.98 (0.71, 1.36) | 0.92 | 1.09 (0.78, 1.51) | 0.63 | 1.10 (0.79, 1.55) | 0.57 |
| Pulse pressure | 1.50 (1.18, 1.91) | 0.001 | 1.50 (1.17, 1.92) | 0.001 | 1.45 (1.13, 1.87) | 0.004 |
| Mean arterial pressure | 0.89 (0.71, 1.12) | 0.32 | 0.79 (0.63, 1.00) | 0.05 | 0.82 (0.64, 1.03) | 0.08 |
Adjusted for age, sex, race/ethnicity, and site.
Model 1, additionally adjusted for education, height, body mass index, smoking status, antihypertensive medication use (yes/no), diabetes, MRI-based left ventricular mass, P-R interval, and resting heart rate.
Model 2, additionally adjusted for interim myocardial infarction and heart failure events.
Hazard ratio (confidence interval) per 1 standard deviation increase in predictor, using standard deviation values from the primary analysis. Standard deviations for systolic, diastolic, mean, and pulse pressure are 21.5, 10.3, 12.6, and 17.2 mmHg, respectively. Standard deviation for aortic distensibility is 1.3 mmHg × 10−1
HR (95% CI) for separate analysis with aortic distensibility outliers > 3 SD from the mean removed.
DISCUSSION
In this study of a large, multi-ethnic, community-based cohort, we found that higher levels of systolic BP and PP, but not MAP or diastolic BP, were each individually associated with increased risk of AF after adjustment for all CHARGE-AF risk factors, sex, site, education, and resting heart rate. In analysis considering PP and MAP simultaneously, higher PP was an independent risk factor of AF, while MAP showed no association. After adjustment for MRI-based left ventricular mass and interim cardiovascular events, associations between individual BP measures and AF were attenuated, but PP remained significantly associated when modeled simultaneously with MAP. The analysis in the subset of participants with aortic distensibility measurements demonstrated a similar pattern of results regarding the association of blood pressure measures and risk of AF. However, aortic distensibility was not consistently associated with the risk of AF.
Elevated BP is considered one of the most important risk factors for AF.13 However, our findings show that high PP, as opposed to high systolic BP or MAP, is most predictive of the development of AF. The importance of PP with regards to AF has been reported previously. An analysis of the Framingham Heart Study6 modeling PP and MAP simultaneously found that PP, but not MAP, was a significant risk factor for AF. Our results show a similar pattern for PP, though we found a marginally significant negative association of MAP with AF risk; we are uncertain as to the significance of this finding. Our results are also consistent with the recently developed CHARGE-AF risk score for the prediction of AF, which showed high systolic BP and low diastolic BP (and therefore increased PP) to be predictive of AF risk.12 In contrast, an analysis of middle-aged women from the Women’s Health Study3 found that when modeling systolic BP and diastolic BP together, diastolic BP was not significantly associated with AF risk. However, further analyses using directly reported continuous BP found that that women with lowest diastolic BP (<65 mmHg) had the highest risk of incident AF after adjustment for systolic BP, supporting the idea of PP being a risk factor for AF.3 Similarly, reports from 2 clinical trials14,15 and 2 observational studies16,17 in samples with other cardiovascular risk factors have also noted that PP is an independent risk factor for new-onset AF. The clinical implication of our primary results is that for risk assessment for the development of AF among middle-aged and older adults, focus should be placed on levels of PP rather than on systolic BP or MAP alone.
Our secondary hypothesis, based on the idea that PP serves as an indirect measure of aortic distensibility, was that aortic distensibility would explain the association between PP and incident AF. The correlation between aortic distensibility and PP, while fairly weak, was negative as expected (i.e., higher levels of PP being associated with lower aortic distensibility). Nevertheless, we found inconsistent evidence that aortic distensibility was associated with AF. Our secondary results seem to cast doubt upon the clinical utility of the measurement of MRI-defined aortic distensibility as a predictor for the development of AF. Alternative mechanisms explaining the association between PP and AF could involve left ventricular structural change and myocardial fibrosis. However, in this study we observed that PP remained an independent risk factor for AF after adjustment for P-R interval, electrocardiography-derived left ventricular hypertrophy, and MRI-derived left ventricular mass.
Strengths of this analysis include the longitudinal design, the diverse racial/ethnic make-up of the study population, and the availability of many important confounders related to atherosclerotic disease and the risk of AF. Limitations include the relatively small number of participants who underwent MRI measurement of the ascending aorta, the possibility of measurement error of AF and other covariates, and the relatively low rate of AF incidence. Given that approximately 50% of the total MESA cohort received an aortic MRI, the results from this subgroup analysis may not be generalizable to the entire MESA cohort. A single measurement of aortic distensibility may not be optimal; measuring change in aortic distensibility over time may correlate better with development of AF. Additionally, measuring aortic distensibility via MRI may not be the most adequate method. We depended on ICD-9 codes from hospital and Medicare claims for ascertainment of AF. While medical records perform relatively well at identifying AF cases,10 asymptomatic cases are probably missed. Similarly, Medicare claims were only available in a subset of the cohort. Also, MESA participants were free of cardiovascular disease at baseline and thus, incidence of AF is lower than in other cohorts that did not have this exclusion criterion.
Supplementary Material
ACKNOWLEDGMENTS
Mr. Roetker and Dr. Alonso had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
SOURCES OF FUNDING This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES Author Saman Nazarian is a consultant to Biosense Webster and receives research grants from the NIH and Biosense Webster.
REFERENCES
- 1.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 2.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 3.Conen D, Tedrow UB, Koplan BA, Glynn RJ, Buring JE, Albert CM. Influence of Systolic and Diastolic Blood Pressure on the Risk of Incident Atrial Fibrillation in Women. Circulation. 2009;119:2146–2152. doi: 10.1161/CIRCULATIONAHA.108.830042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 5.Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell GFVR. Pulse pressure and risk of new-onset atrial fibrillation. JAMA. 2007;297:709–715. doi: 10.1001/jama.297.7.709. [DOI] [PubMed] [Google Scholar]
- 7.Abhayaratna WP, Barnes ME, O’Rourke MF, Gersh BJ, Seward JB, Miyasaka Y, Bailey KR, Tsang TSM. Relation of arterial stiffness to left ventricular diastolic function and cardiovascular risk prediction in patients > or =65 years of age. Am J Cardiol. 2006;98:1387–1392. doi: 10.1016/j.amjcard.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size. The Framingham Heart Study. Hypertension. 1995;25:1155–1160. doi: 10.1161/01.hyp.25.6.1155. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Jensen PN, Johnson K, Floyd J, Heckbert SR, Carnahan R, Dublin S. A systematic review of validated methods for identifying atrial fibrillation using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):141–147. doi: 10.1002/pds.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso A, Soliman EZ, Chen LY, Bluemke DA, Heckbert SR. Association of blood pressure and aortic distensibility with P wave indices and PR interval: the multi-ethnic study of atherosclerosis (MESA) J Electrocardiol. 2013;46:359.e1–6. doi: 10.1016/j.jelectrocard.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508. doi: 10.1161/CIRCULATIONAHA.110.009035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdecchia P, Dagenais G, Healey J, Gao P, Dans AL, Chazova I, Binbrek AS, Iacobellis G, Ferreira R, Holwerda N, Karatzas N, Keltai M, Mancia G, Sleight P, Teo K, Yusuf S. Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint TrialTelmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease Investigators. Blood pressure and other determinants of new-onset atrial fibrillation in patients at high cardiovascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease studies. J Hypertens. 2012;30:1004–1014. doi: 10.1097/HJH.0b013e3283522a51. [DOI] [PubMed] [Google Scholar]
- 15.Larstorp ACK, Ariansen I, Gjesdal K, Olsen MH, Ibsen H, Devereux RB, Okin PM, Dahlöf B, Kjeldsen SE, Wachtell K. Association of pulse pressure with new-onset atrial fibrillation in patients with hypertension and left ventricular hypertrophy: the Losartan Intervention For Endpoint (LIFE) reduction in hypertension study. Hypertension. 2012;60:347–353. doi: 10.1161/HYPERTENSIONAHA.112.195032. [DOI] [PubMed] [Google Scholar]
- 16.Ciaroni S, Bloch A, Lemaire M-C, Fournet D, Bettoni M. Prognostic value of 24-hour ambulatory blood pressure measurement for the onset of atrial fibrillation in treated patients with essential hypertension. Am J Cardiol. 2004;94:1566–1569. doi: 10.1016/j.amjcard.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 17.Valbusa F, Bonapace S, Bertolini L, Zenari L, Arcaro G, Targher G. Increased pulse pressure independently predicts incident atrial fibrillation in patients with type 2 diabetes. Diabetes Care. 2012;35:2337–2339. doi: 10.2337/dc12-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.