Abstract
Combinatorial use of iron oxide nanoparticles (IONPs) and an alternating magnetic filed (AMF) can induce local hyperthermia in tumors in a controlled and uniform manner. Heating B16 primary tumors at 43°C for 30 minutes activated dendritic cells (DCs) and subsequently CD8+ T cells in the draining lymph node (dLN) and conferred resistance against rechallenge with B16 (but not unrelated Lewis Lung carcinoma) given 7 days post hyperthermia on both the primary tumor side and the contralateral side in a CD8+ T cell-dependent manner. Mice with heated primary tumors also resisted rechallenge given 30 days post hyperthermia. Mice with larger heated primary tumors had greater resistance to secondary tumors. No rechallenge resistance occurred when tumors were heated at 45°C. Our results demonstrate the promising potential of local hyperthermia treatment applied to identified tumors in inducing anti-tumor immune responses that reduce the risk of recurrence and metastasis.
Keywords: ironoxide, nanoparticle, local hyperthermia, heat, anti-tumor immune
Background
Nanomaterials, with the ability to function at cellular and molecular levels, have opened up novel treatment approaches in cancer therapy. Recent progress in nanotechnology not only improves current therapies used routinely, such as chemotherapy(1), but also provide opportunities to reevaluate potential therapies that were thought to be ineffective by themselves without the help of nanotechnology, such as local hyperthermia therapy.
There is considerable interest and active research into how to use the immune system to treat cancer, and this effort has led to numerous novel immunotherapies. Some drugs, such as Ipilimumab (anti-CTLA4), directly interact with and activate immune cells(2, 3), while some therapies, such as GVAX (GM-CSF transduced tumor vaccine), make the tumor more immunostimulatory(4). Even many traditional chemotherapies are now found to work partially through immune activation(5-7). Hyperthermia therapy has historically been used to treat various types of cancer, but is often employed in combination with and to enhance the efficacy of chemotherapy and radiation therapy(8) and its potential immunostimulatory effect is generally ignored. In association with expanded appreciation of the roll of the immune system in cancer therapy, the potential of hyperthermia by itself to stimulate anti-tumor responses and to treat cancer is now being investigated using nanotechnology.
Multiple studies show that local hyperthermia therapy can improve anti-tumor immunity. Heat-stressed cancer cells release heat-shock proteins (HSPs) and many HSPs bind to and activate antigen-presenting cells (APCs)(9-11). Since HSPs are often bound to cancer antigens, once the released HSP-antigen complex is phagocytosed by APCs, APCs can now present the antigen to T cells to initiate adaptive immune responses(12, 13). Heat-stressed cancer cells also release exosomes containing antigens and chemokines(14, 15) and therefore not only provide an antigen source for APCs but also help recruit APCs and T cells to tumors. In addition, heat alters the visibility of tumors to immune cells. Tumor cells heated at 39.5 or 43 °C express higher levels of MICA, an NKG2D ligand(16), or MHC class I(17), respectively, making tumors more sensitive to lysis by NK or CD8+ T cells, respectively. Lastly, local hyperthermia at 42°C increases the permeability of tumor vasculature(18, 19), which in turn improves perfusion in the tumor and may facilitate better immune cell trafficking between tumors and lymphoid organs.
Compared to traditional methods of inducing local hyperthermia, such as inserting a metal antenna into tumors and applying a radiofrequency electromagnetic field(20), combinatorial use of metallic nanoparticles and appropriate external energy sources has better potential to heat tumors uniformly at a given temperature for a desired time(21) as we also confirm here, which makes the method attractive for inducing temperature-dependent effects. Melanoma, despite the feasibility of surgery, is still a life-threatening disease due to its highly metastatic tendency. There have been studies implying that heating melanomas induces resistance against rechallenge, which correlates with immunological changes(17, 22-26). However, these studies the comparison of mice whose primary tumors were heated with naïve mice rather than with mice that experienced primary tumors that were never heated but removed surgically limited the conclusions that could be proved. While these previous studies made a number of important observations, they did not reveal many details of immune mechanisms involved. Therefore, our studies were undertaken to refine and extend the understanding of how hyperthermia treatment of a tumor may induce systemic anti-tumor immunity. Here, using an excisable dermal tumor model, we demonstrate that local hyperthermia therapy consisting of intratumoral injection of IONPs and AMF application prior to surgical excision generates resistance against rechallenge in a CD8+ T cell-dependent manner. Our studies underscore the potential of nanoparticle-mediated hyperthermia prior to surgery to stimulate immune responses against metastatic disease.
Methods
Animals and cell line
Female BALB/c and C57BL/6 mice were purchased from the NCI. Animal experiments were approved by the IACUC of Geisel Medical School at Dartmouth. CT26, a mouse colon carcinoma cell line, B16F10, a mouse melanoma cell line, and LLC, a mouse Lewis lung carcinoma cell line, were maintained in RPMI medium containing 10% fetal bovine serum.
Dermal tumor models
BALB/c or C57BL/6 mice (6-8 week old) were injected intradermally (ID) on day 0 with 1×105 CT26 or 1.25×105 B16F10 cells, to establish dermal tumors of about 5 mm × 6 mm on day 7 (the day of hyperthermia, unless otherwise stated). Alternatively, 0.625×105, 1.25×105, 2.5×105, or 5×105 B16F10 cells were injected (Figure 2E-F). For rechallenge experiments, primary tumors both in “unheated group” and “heated group” were surgically removed 3 days post hyperthermia, and at indicated timings mice were reinjected ID with 1.25×105 B16F10 or LLC cells right next to where the primary tumor was (primary tumor side) and on the other side (contralateral side). Tumor sizes, described as (larger diameter) × (smaller diameter) mm2, were measured every 2-3 days.
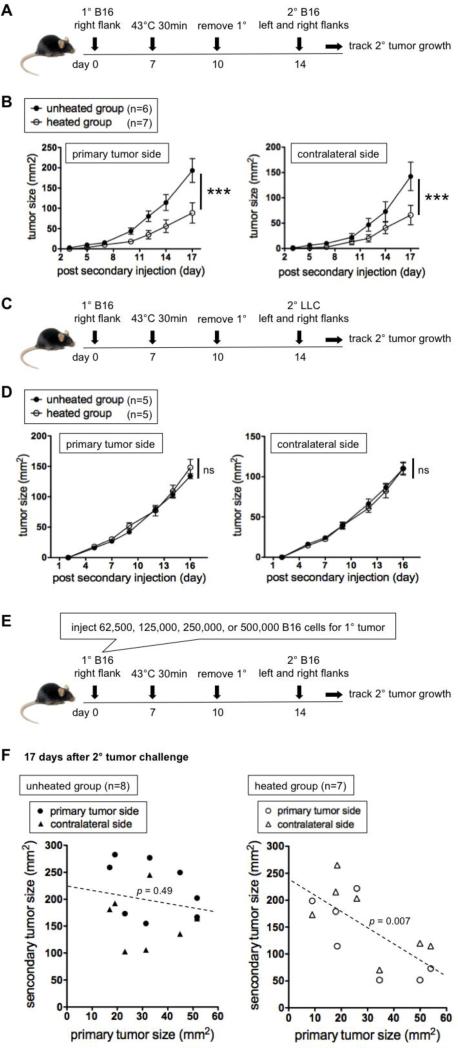
Figure 2.
Local hyperthermia (43°C 30 min) of B16 primary tumors slows secondary B16, but not LLC, tumors in a primary tumor size dependent manner. (A) Experimental design to test how heating primary tumors affects secondary B16 tumors given 7 day post hyperthermia. (B) Growth kinetics of secondary tumors. Representative of six experiments. (C) Experiment design to test the effect on irrelevant secondary tumors. (D) Growth kinetics of irrelevant secondary LLC tumors. Representative of two experiments. (E) Experimental design to test the effect of the primary tumor size at the time of hyperthermia treatment on the secondary tumor growth. (F) Relation between the primary tumor size on the day of hyperthermia treatment (x axis) and the secondary tumor size 17 days after rechallenge (y axis). Representative of two experiments. P values in F represent the probability that there is not a negative correlation between the primary and secondary tumor sizes.
Local hyperthermia treatment
Mice in “heated group” were anesthetized with isoflurane. 10 l, equivalent to 140 g of iron, of BNF-Starch coated IONPs of 100 nm diameter (Micromod, Germany) were injected ID immediately before AMF application. A fiber optic temperature probe (Luxton/LumaSense and FISO, Canada) was placed in the tumor and rectum. Mice were placed in a solenoid coil attached to 10 kW generator (Huttinger Elektronik, Germany). During AMF application, temperatures were monitored using the module and software Evolution (FISO, ± 0.4°C accuracy). AMF field strength was manually adjusted between 450-550 Oe (167.5 kHz) so that the tumor temperature was maintained at 42.5-43°C for 30 min (except Figure 3A-C) or 44.5-45°C for 20 min (Figure 3A-C). When possible, uniform tumor temperature was confirmed with multiple assessment points (Supplemental Figure 1). The cumulative thermal dose was calculated as Cumulative Equivalent Minutes at 43°C (CEM) by Evolution. CEM is defined as ΣtR(43-Tavg) , where t is time interval, R is a constant (R = 0.5 when > 43°C and R = 0.25 when < 43°C) and Tavg is the average temperature during a desired time interval(27).
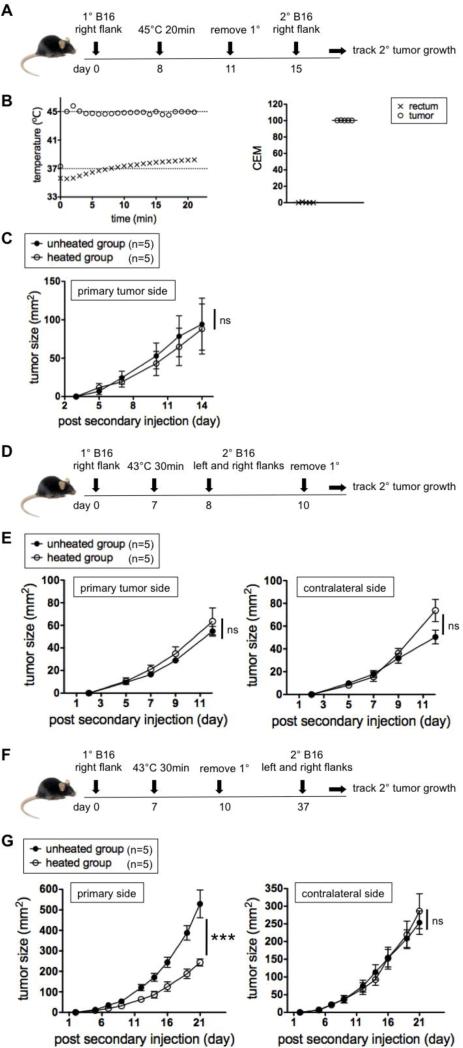
Figure 3.
Heating temperature and secondary challenge timing affect outcomes. (A) Experimental design to test if local hyperthermia at 45°C also gives resistance against secondary tumors given 7 day post hyperthermia. (B) Representative heating curve (left) and cumulative thermal doses (right) in the tumor and rectum. (C) Growth kinetics of secondary tumors. Representative of two experiments. (D) Experimental design to test how local hyperthermia affects the growth of secondary tumors given 1 day post hyperthermia. (E) Growth kinetics of secondary tumors. Representative of two experiments. (F) Experimental design to test how local hyperthermia affects secondary tumors given 30 day post hyperthermia. (G) Growth kinetics of secondary tumors. Representative of two experiments.
Intratumoral cytokine and chemokine concentrations
5 days post hyperthermia, tumors were homogenized in T-PER Tissue Protein Extraction Reagent (Thermo Scientific, MA) containing Protease Inhibitor Cocktail (Roche, IN). The digest was spun at 9,000g for 10 min at 4°C twice. Cytokine and chemokine concentrations in the supernatant (pg per ml) were measured using MILLIPLEX Mouse Premixed 32-plex (Millipore, MA). Total protein concentrations in the supernatant (mg per ml) was measured using Epoch Micro-Volume Spectrophotometer System and Gen5 software (BioTek, VT). Cytokine and chemokine concentrations (pg per mg total protein) were then calculated.
Flow cytometry
3 or 7 days post hyperthermia, draining lymph nodes (dLN) were digested in HBSS containing 1 mg/ml collagenase D and 0.12 mg/ml DNAse (Roche, IN) at 37°C for 40 min and were filtered through 40 mm strainers. 0.5-1×106 cells were blocked with anti-CD16/32 (eBioscience, CA), stained with appropriate antibodies and analyzed by 7-Color MACSQuant (Miltenyi Biotec, CA). Anti-mouse CD45 (clone 30-F11), CD11c (N418), CD80 (16-10A1), CD86 (GL-1), CD3e (145-2C11), CD8a (53-6.7), CD44 (IM7), and CD69 (H1.2F3) antibodies (BioLegend, CA) were used for staining. Isotype control antibodies for CD11c, CD80, CD86, CD8a, CD44 and CD69 were negative controls.
CD8 depletion
Depleting anti-CD8 (clone 2.43) antibodies were produced as bioreactor supernatant. 200 ml, equivalent to 250 mg of the antibody, was administered intraperitoneally 1 day prior to hyperthermia and every 4 days thereafter. Greater than 95% depletion of CD8+ T cells was confirmed by flow cytometry.
Statistical analysis
Statistical differences between groups in tumor growth kinetics were analyzed by 2-way ANOVA. Differences in cytokine/chemokine levels and cell populations were analyzed by Student's t-test. For tumor-size dependency test (Figure 2F), regression analysis was used. Figures denote statistical significance of *p < 0.05, **p < 0.01, and ***p < 0.001. P > 0.05 was considered non-significant (ns). “n” represents the number of mice used per group. Error bars, standard error of the mean (SEM).
Results
Local hyperthermia (43°C 30 min) of a tumor retards the growth of distal tumors
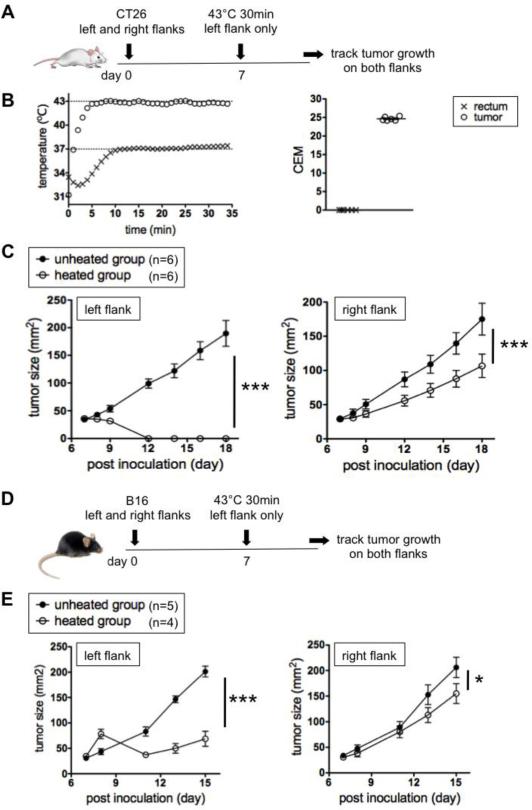
First we tested the impact of heating one tumor on another distal tumor. As a metastatic model, BALB/c mice were ID challenged with syngeneic CT26 colon cancer cells to form dermal tumors of about 30 mm2 on both the left and right flanks. In the heated group, IONPs were directly injected only into left-flank tumors and mice were treated with an AMF (Figure 1A), so that the left tumors, but not right tumors, were heated at 42.5-43°C for 30 min (Figure 1B left). The average cumulative thermal dose (CEM) in the tumor was 24.6 (Fig 1B right). The ending rectal temperature was typically 35.5-37.5°C, so the rest of the body was not heated above their normal body temperature. Heated tumors on the left flank disappeared completely in 5 days (Figure 1C left). In support of the hypothesis that heating one tumor would immunologically impact growth of the other tumor, right-flank tumors (not heated) in the heated group grew slower than in the unheated group (Figure 1C right).
Figure 1.
Local hyperthermia (43°C 30 min) of tumors on one flank slows tumors on the other flank. (A) Experimental design to test the effect of local hyperthermia in CT26 model. (B) Representative heating curve (left) and cumulative thermal doses (right) in the tumor and rectum. (C) Growth kinetics of CT26 tumors. Representative of three experiments. (D) Experimental design to test the effect of local hyperthermia in B16 model. (E) Growth kinetics of B16 tumors. Representative of two experiments.
CT26 is an immunogenic tumor against which immune responses are mounted by a syngeneic mouse(28). In order to further investigate this effect in an orthotopic but poorly immunogenic(29) tumor model, the same experiment was done using C57BL/6 mice bearing B16F10 melanoma dermal tumors (Figure 1D). Unheated tumors (right flank) in the heated group did grow slower than in the unheated group, but the difference was less pronounced (Figure 1E right) compared to the CT26 model. The right-flank tumors in mice that received either IONPs only or AMF only had similar growth kinetics to tumors in the unheated group in both CT26 and B16 models (Supplemental Figure 2).
In general, anti-tumor immune responses against immunogenic tumors are easier to boost and hence immunotherapies work better than against poorly immunogenic tumors(30, 31). It is possible that the difference in the treatment efficacy between CT26 (immunogenic) and B16 (poorly immunogenic) means that the immune system is involved in the treatment efficacy. Alternatively, since left-flank tumors are smaller in the heated than unheated group in both CT26 and B16 models (Figure 1C left and E left), it is also possible that the slower growth of right-flank tumors in the heated group (Figure 1C right and E right) is due other potential systemic effects of having smaller tumors on the left flank.
Local hyperthermia (43°C 30 min) on B16 primary tumors slows the growth of secondary B16, but not irrelevant LLC, tumors
To exclude the effect of size differences of heated tumors, thereby better visualizing the immune-mediated effects, we utilized a different experimental approach (Figure 2A). Primary tumors in both unheated and heated groups were surgically removed 3 days after hyperthermia, and then mice were rechallenged with B16F10 on both the primary tumor side and contralateral side 7 days after hyperthermia. Secondary tumors in the heated group on both sides grew slower than in the unheated group (Figure 2B), meaning that this one-time hyperthermia treatment is sufficient in inducing resistance at anatomically distant sites from the primary tumor. The same experiment was performed using the immunogenic CT26 model to see if there is better efficacy than in the B16 model, but all mice completely rejected secondary tumors regardless of whether the tumors were heated or not (data not shown).
Secondary tumors in mice that received either IONPs only or AMF only had growth kinetics similar to tumors in the unheated group (Supplemental Figure 3). Importantly, when mice were rechallenged with irrelevant LLC tumors instead of B16F10, mice had no resistance against secondary tumors (Figure 2C-D), showing that the resistance is tumor specific.
Larger primary when treated gives better resistance to rechallenge
Upon careful analysis of the data from experiments in Figure 2A-B, it was noted that there is a trend in the heated group that mice with larger primary tumors resisted better against secondary tumors. To specifically test this hypothesis, we injected four different quantities of B16F10 cells (62,000, 125,000, 250,000, or 500,000) for primary tumor inoculation in order to get mice with primary tumors of different sizes on the day of hyperthermia (Figure 2E), and a similar experiment as in Figure 2A was performed.
In Figure 2F, secondary tumor sizes 17 days after rechallenge (y axis) are plotted against primary tumor sizes at the time of hyperthermia (x axis). Indeed, there is a negative correlation between the primary and secondary tumor sizes in the heated group (Figure 2F right). Since there was no correlation between the primary and secondary tumor sizes in the unheated group (Figure 2F left), the stronger rechallenge resistance when the primary treated tumor was larger cannot be simply attributed to the possibility that stronger anti-tumor immune responses were induced by larger primary tumors.
No treatment efficacy when primary tumor is heated at 45°C
Another interesting finding is that the treatment efficacy is dependent on the heating temperature. When primary tumors were heated at 45°C instead of 43°C (Figure 3A and B left), which gave tumors the average CEM of 100.3 (Figure 3B right), the resistance against secondary tumors was not established (Figure 3C). However, this 45°C treatment was enough to destroy the entire primary tumor even without surgery (data not shown). This demonstrates that a hyperthermia treatment that completely eliminates the primary treated tumor is not the best approach to developing resistance to potential metastatic disease.
Secondary challenge timing affects outcomes
We next evaluated the impact of different rechallenge timings (Figure 3D-G). When the secondary tumors are inoculated 1 day instead of 7 days after hyperthermia, there was no resistance against rechallenge on either the primary tumor side or contralateral side (Figure 3E). In order to investigate the longevity of the resistance, mice were rechallenged 30 days after hyperthermia. Secondary tumors on the primary tumor side grew more slowly but there was no reduction in growth on the contralateral side (Figure 3G). The fact that it takes more than 1 day for the resistance to develop and that the resistance lasts for 30 days at least on the primary tumor side suggests that the adaptive immune system may be involved.
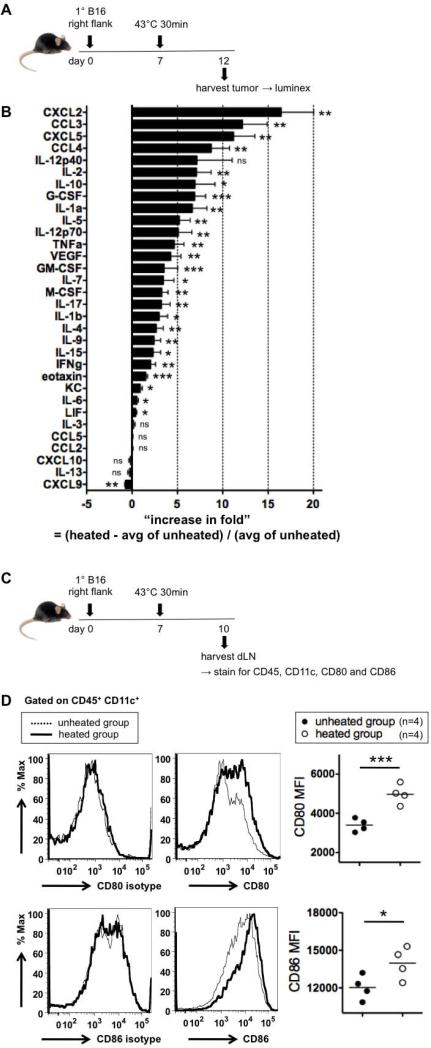
Local hyperthermia (43°C 30 min) induces broad increases in cytokines and chemokines in the tumor
To investigate immune responses induced by the hyperthermia treatment, we measured intratumoral cytokine and chemokine concentrations (pg / mg total protein) 5 days after hyperthermia (Figure 4A). 25 out of 32 cytokines and chemokines analyzed were increased in the heated tumors (Figure 4B, see Supplemental Table I for absolute concentrations), indicating profound immunostimulation. This broad set of cytokine/chemokine changes suggests that multiple cell types are likely involved in the local response. The greatest increases were seen in chemokines, CXCL2, CCL3, CXCL5 and CCL4, whose function is to recruit innate immune cells such as granulocytes, neutrophils, NK cells, monocytes and DCs, cell types typically seen in many inflammatory settings(32).
Figure 4.
43°C 30 min induces broad increases in intratumoral cytokines and chemokines and activates DCs in dLN. (A) Experimental design to test the changes in intratumoral cytokine and chemokine concentrations by hyperthermia. (B) Increase in fold of the intratumoral concentration of each cytokine or chemokine (pg per mg total protein), compared to the unheated group. 4 mice per group. (C) Experimental design to test if local hyperthermia activates DCs in dLN. (D) Representative histograms for CD80, CD86 and isotype stainings and MFI. Representative of three experiments.
In particular, CCL3 and CCL4 are known to recruit NK cells, one of the important cell types in anti-tumor immunity(33, 34). Therefore, we tested whether NK cells are required for treatment efficacy. The resistance agaist rechallenge was still generated in mice depleted of NK cells (Supplemental Figure 4), showing that NK cells are not necessary.
Local hyperthermia (43°C 30 min) activates DCs in draining lymph nodes (dLN)
CCL3 and CCL4 are also known to attract DCs(35-37). DCs are critical in anti-tumor immunity, because DCs, the professional antigen presenting cells (APCs), ingest antigens and if sufficiently stimulated become activated and traffic to dLN to present antigens to T cells to initiate adaptive anti-tumor immune responses(38). We therefore determined whether DCs in dLN are activated by hyperthermia. Tumors were heated at 43°C, and 3 days later dLN was harvested (Figure 4C). DCs in dLN from the heated group were more activated than the unheated group, as shown by higher expression of APC costimulatory molecules CD80 and CD86 (Figure 4D).
Local hyperthermia (43°C 30 min) increases and activates CD8+ but not CD4 T+ cells in dLN
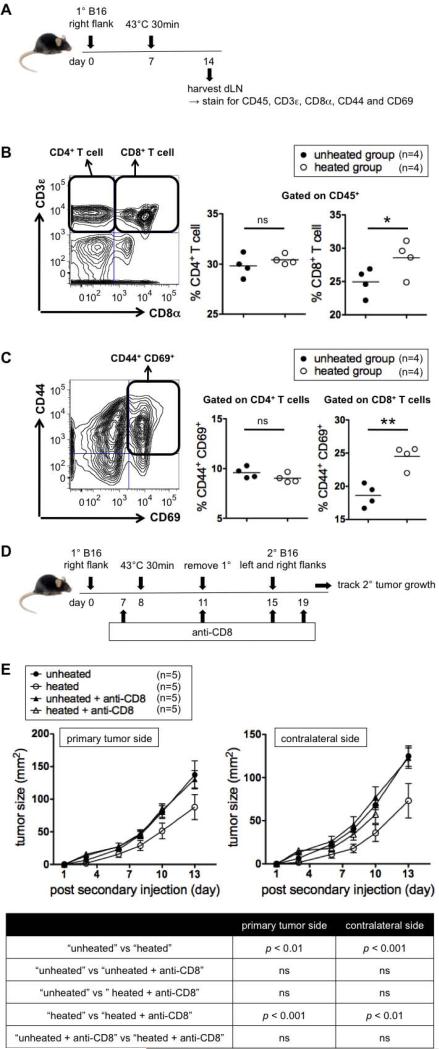
APCs primed with tumor cells are able to present antigens to CD8+ T cells through MHC class I (cross presentation). Since the hyperthermia treatment activates DCs in dLN (Figure 4C-D), we determined how hyperthermia affects CD8+ T cells in dLN. Tumors were heated at 43°C, and 7 days later dLN was harvested (Figure 5A). The percentage of CD8+ T cells within total leukocytes in dLN was higher in the heated group (Figure 5B right) and the percentage of Ag-experienced, recently-activated (CD44+ CD69+) CD8+ T cells within total CD8+ T cells in dLN was also higher in the heated group (Figure 5C right). Interestingly, heating tumors at 45°C instead of 43°C still increased frequencies of CD8+ T cells but did not activate them. (Supplemental Figure 6).
Figure 5.
43°C 30 min increases and activates CD8+ T cells in dLN and CD8+ T cells are required for treatment efficacy. (A) Experimental design to test if local hyperthermia increases and activates CD4+ and CD8+ T cells. (B) Gating strategy for CD4+ and CD8+ T cells (left) and % CD4+ T cells and % CD8+ T cells among leukocytes (CD45+ cells) in dLN (right). Representative of two experiments. (C) Gating strategy for CD44+ CD69+ cells (left) and % CD44+ CD69+ cells among CD4+ and CD8+ T cells in dLN (right). Representative of two experiments. (D) Experimental design to test if CD8+ T cells are required for treatment efficacy. (E) Growth kinetics of secondary tumors (top) and statistics (bottom). Representative of two experiments.
Primed APCs are also able to present antigens to CD4+ T cells through MHC class II and induce differentiation of naïve CD4+ T cells into type-1 helper T (Th1) cells, a subset of T cells that further help activation of CD8+ T cells(39). Since Th1 differentiation requires IL-12(40-42), a cytokine upregulated by hyperthermia treatment (Figure 4B), we tested whether IL-12 is required for treatment efficacy. The resistance against rechallenge was successfully generated in IL-12p35 knockout mice (Supplemental Figure 5), showing that IL-12 is not required and suggesting that Th1 cells play a minimal role in developing the resistance. In line with this result, the hyperthermia treatment did not influence either the percentage of CD4+ T cells (Figure 5B) or the percentage of Ag-experienced, recently-activated CD4+ T cells (Figure 5C) in dLN. No increase or activation of either CD8+ or CD4+ T cells in the non-draining lymph node or spleen was observed (Supplemental Table II).
CD8+ T cells are required for the treatment efficacy
In order to determine the role of CD8+ T cells in resistance to rechallenge, mice were treated with CD8 depleting antibodies and used to test the treatment efficacy (Figure 5D). CD8 depletion did not alter the secondary tumor growth kinetics either on the primary tumor side or contralateral side in mice whose primary tumor was not heated (Figure 5E). However, resistance to rechallenge was completely abrogated in the heated group by CD8+ T cell depletion. Since CD8 depleting antibody treatment did not affect major immune populations other than CD8+ T cells (Supplemental Figure 7), this result shows that CD8+ T cells are required for inducing resistance against secondary tumors. The roll of T cells in our treatment is further supported by upregulation of IL-2 and IL-7 (Figure 4B), cytokines required for effector T cell growth, proliferation, differentiation and maintenance(43).
Discussion
Dr. Mayo's observation and progress in nanotechnology
In 1913 Dr. William Mayo reported less dissemination of tumors post surgery and increased cures when he heated cervical tumors with a cautery before vaginal hysterectomy(44). Interestingly, he observed “little if any difference in the ultimate results” when tumors had been heated immediately before surgery. This suggests that his improved outcome was not simply because tumor cells were killed in a more thorough manner by adding heat, but rather there is a protective mechanism that requires some time to develop. Despite Dr. Mayo's significant finding, the potential of heating tumors before surgery in stimulating anti-tumor immune responses was not further explored for many years. It is at least in part due to lack of a well-controlled technique that heats tumors uniformly at a particular temperature, which made it difficult to obtain consistent treatment efficacy. With the striking progress within the last 20 years in the field of nanotechnology in medicine, more precise heating methods, such as the combinatorial use of metallic nanoparticles and external energy sources, have become available.
Proposal of the mechanism
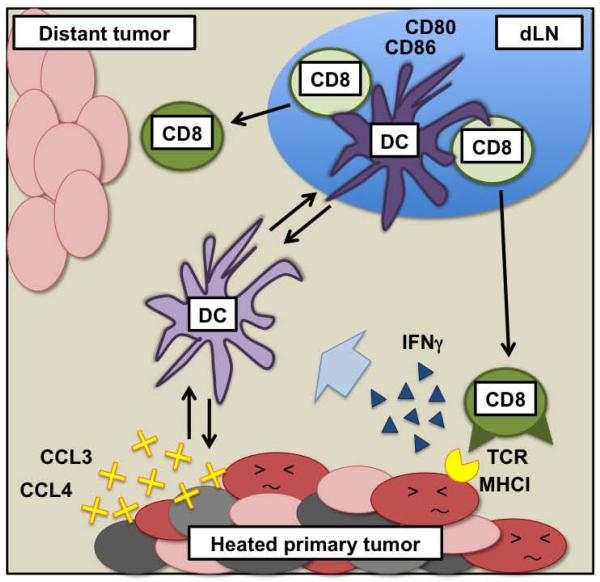
Here we demonstrate that local hyperthermia (43°C 30 min) treatment on a primary tumor before surgery induces various cytokines and chemokines in the tumor, activates DCs and then CD8+ T cells in dLN, and generates resistance against secondary tumors both at the primary tumor site and at a distant site in a CD8+ T cell-dependent manner. Anti-tumor CD8+ T cell responses can be amplified by Th1 CD4 cells(39), but based on our observations that there is no CD4+ T cell increase or activation in dLN and that the treatment works in mice lacking IL-12 (Supplemental Figure 5), there is no evidence that the treatment efficacy requires Th1 cell help. We propose the major mechanism for our therapeutic efficacy is the induction of cytokines and chemokines by hyperthermia in the tumor that recruits DCs to the tumor, DCs take up tumor cells and become activated, activated DCs go to dLN and crosspresent tumor antigens to and activate CD8+ T cells, and CD8+ T cells traffic to and attack unheated tumors. This proposed anti-tumor immune mechanism induced by local hyperthermia treatment is summarized in Figure 6.
Figure 6.
Mechanism of anti-tumor immune resistance induced by local hyperthermia treatment.
As Kobayashi's group has reported(17), we also observed that heating B16 cells at 43°C 30 min in vitro increases surface expression of MHC class I (Supplemental Figure 8). This may help enhance the immune effect by hyperthermia; tumor-specific CD8+ T cells that already existed before hyperthermia may enter the heated tumor, recognize antigens on upregulated MHC class I on tumor cells, and produce cytokines to amplify subsequent immune responses.
Size dependency, temperature dependency, importance of in situ heating
One of the interesting findings is that the treatment works better in mice that had larger primary tumors (Figure 2E-F). While inclusion of all treated tumors gives statistically significant resistance to rechallenge, if only tumors below the size of 30 mm2 are included there is no effect of the treatment. This suggests that the tumor has to be a minimum size of 30 mm2 to get the immune stimulation required for the effect. This could be because larger heated primary tumors make more tumor antigens available to DCs recruited to the tumor, leading to more robust downstream responses. It is also possible that mice with larger primary tumors have already developed more tumor-specific CD8+ T cells in dLN that are not necessarily fully activated prior to the treatment possibly due to the immunosuppressive pressure by the tumor, but hyperthermia activates those CD8+ T cells, resulting in stronger anti-tumor efficacy. Alternatively, heat could directly activate tumor-infiltrating leukocytes and therefore heating larger tumors containing more leukocytes could lead to better immune activation. However, we found directly heating tumor-infiltrating leukocytes at 43°C in vitro does not activate T cells (Supplemental Figure 9). It is generally believed that almost all cancer treatments work better when tumors are treated when they are smaller. However, this result provides evidence that while larger treated tumors may be more resistant to the treatment, the impact on systemic anti-tumor immune responses may be improved.
The fact that there is no efficacy when tumors are heated at a modesly higher temperature (45°C) (Figure 3A-C) was surprising, because those tumors should equally be able to function as antigen sources scale-wise regardless of heating temperatures. This has significant implications for the treatment of tumors by nanoparticle-mediated hyperthermia since it is easy to assume that more heat induces stronger effects. High heat with associated rapid and complete necrotic death of primary tumors is possible with current technology, but there seems to be a narrow range of optimal temperature for heating primary tumors in order to induce systemic immune responses. This temperature dependency could be because different temperatures may provide different levels of impacts on cytokine/chemokine milieus, tumor vasculature, and stress on tumor and stromal cells. More specifically, for example, 43°C may have increased the vasculature permeability(18, 19), better facilitating trafficking of DCs between tumors and dLN, but 45°C may have destroyed the vasculature. In addition, as shown in an in vitro study(45), tumor cells heated at 45°C may have produced much less HSPs, resulting in minimal downstream effect. The range of desirable temperature being narrow, it is particularly important to be able to heat tumors uniformly and to monitor temperatures precisely. In this sense, compared to traditional heating strategies such as radiofrequency ablation, the use of metallic nanoparticles and external energy sources that enables uniform heating, as we confirmed in Supplemental Figure 1, is more suitable for producing immunogenic temperature environments.
A simple method to look for the most immunogenic temperature range would be using tumor cells heated in vitro at different temperatures for prophylactic cell vaccinations. Surprisingly, studies by our group (Supplemental Figure 10) and others(46) using irradiated and heated CT26 tumor cells as vaccine demonstrate clearly that the in vitro heating of CT26 cells at 43°C dampens anti-tumor responses that could have been stimulated by unheated CT26 cells. This highlights the importance of “in situ” heating and the difficulty extrapolating from in vitro heated tumor cell vaccines. Heating tumors in situ induces numerous changes not only in tumor cells but also in the stroma, endothelial cells and the local immune system(47). When the balance among heat effects on all these factors is optimized, the best anti-tumor immune responses and treatment efficacy may be achieved. Despite considerable previous research(17, 22-26), there is much that is not understood about how local hyperthermia influences systemic anti-tumor immunity.
Strengths of local hyperthermia therapy, towards clinical application
Standard care for most types of cancer involves surgery, which is imperfect when there are undetected metastases. More sensitive detection methods are being developed to locate metastases, but it is inconceivable that all metastases will be located and surgically resectable in the foreseeable future. On the other hand, even a single cancer cell could be detected by the immune system when it is properly trained, and the local hyperthermia approach is one tool to do this.
In clinical situations tumor antigens are not always identified. However heating the whole tumor in situ allows the immune system to be exposed to all the expressed antigens and choose which antigens to attack, including unknown antigens. Also, by doing so immune responses are generated against multiple antigens. One challenge of cancer immunotherapies targeting particular tumor antigens such as antibody therapies is the resistance mounted by tumors for example by reducing the expression of targeted antigens(48). On the other hand, therapies like local hyperthermia that use the whole tumor as an antigen source are less subject to this kind of resistance and therefore the treatment likely stays effective for a longer period.
Immunological memory is a valuable aspect of immunotherapy for cancer if it can be adequately developed. The longer the immune response lasts, the more likely it will be that all tumor cells are found and eliminated. Indeed, we showed that the resistance against secondary tumors lasted for 30 days on the primary tumor side in a model in which immunological memory is very difficult to develop (Figure 3F-G). Upregulation of IL-7 and IL-15 (Figure 4B), cytokines important in maintaining CD8+ memory T cells(49), may be helping establish the memory response. Further optimizing the treatment (temperature, length, timing, number of treatments, etc) and/or combining with other immunotherapies will make this local hyperthermia a more promising approach for clinical application.
Supplementary Material
Acknowledgement
We would like to acknowledge Jennifer L. Fields and the Dartmouth Transgenic and Genetic Construct Shared Resource for maintaining IL-12p35 knockout mice and assistance with surgical excision of dermal tumors, DartLab for Luminex and flow cytometry, and Ele Clancy-Thomson for technical help on cytokine/chemokine analysis.
Funding
Dartmouth Center of Nanotechnology Excellence NIH 1 U54 CA151662
Center for Molecular, Cellular, and Translational Immunological Research NIGMS P20 RR15639
Norris Cotton Cancer Support Grant P30 CA023108
Dartmouth Immunobiology of Myeloid and Lymphoid Cells 5T32AI007363-22
List of abbreviations
- IONP
iron oxide nanoparticle
- AMF
alternating magnetic field
- DC
dendritic cell
- dLN
draining lymph node
- HSP
heat-shock protein
- APC
antigen presenting cell
- ID
intradermally
- CEM
cumulative equivalent minutes at 43°C
- SEM
standard error of the mean
- Th1
type-1 helper T
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No Conflicts of interest
No commercial association
References
- 1.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–98. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 2.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459–65. doi: 10.1084/jem.182.2.459. PMCID: 2192127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90(8):3539–43. doi: 10.1073/pnas.90.8.3539. PMCID: 46336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 7.Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71(14):4821–33. doi: 10.1158/0008-5472.CAN-11-0950. [DOI] [PubMed] [Google Scholar]
- 8.Palazzi M, Maluta S, Dall'Oglio S, Romano M. The role of hyperthermia in the battle against cancer. Tumori. 2010;96(6):902–10. [PubMed] [Google Scholar]
- 9.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1(2):151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 10.Bethke K, Staib F, Distler M, Schmitt U, Jonuleit H, Enk AH, et al. Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J Immunol. 2002;169(11):6141–8. doi: 10.4049/jimmunol.169.11.6141. [DOI] [PubMed] [Google Scholar]
- 11.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, et al. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163(3):1398–408. [PubMed] [Google Scholar]
- 12.Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, et al. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169(10):5424–32. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 13.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci U S A. 1997;94(24):13146–51. doi: 10.1073/pnas.94.24.13146. PMCID: 24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, et al. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11(20):7554–63. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 15.Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J Immunol. 2011;186(4):2219–28. doi: 10.4049/jimmunol.1002991. [DOI] [PubMed] [Google Scholar]
- 16.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82(5):1322–31. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 17.Ito A, Tanaka K, Kondo K, Shinkai M, Honda H, Matsumoto K, et al. Tumor regression by combined immunotherapy and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Sci. 2003;94(3):308–13. doi: 10.1111/j.1349-7006.2003.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer RE, Braun RD, Rosner GL, Dewhirst MW. Local 42 degrees C hyperthermia improves vascular conductance of the R3230Ac rat mammary adenocarcinoma during sodium nitroprusside infusion. Radiat Res. 2000;154(2):196–201. doi: 10.1667/0033-7587(2000)154[0196:lchivc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumor vasculature. Cancer Res. 2001;61(7):3027–32. [PubMed] [Google Scholar]
- 20.Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6(1):14–23. doi: 10.1634/theoncologist.6-1-14. [DOI] [PubMed] [Google Scholar]
- 21.Petryk AA, Giustini AJ, Gottesman RE, Trembly BS, Hoopes PJ. Comparison of magnetic nanoparticle and microwave hyperthermia cancer treatment methodology and treatment effect in a rodent breast cancer model. Int J Hyperthermia. 2013;29(8):819–27. doi: 10.3109/02656736.2013.845801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato A, Tamura Y, Sato N, Yamashita T, Takada T, Sato M, et al. Melanoma-targeted chemo-thermo-immuno (CTI)-therapy using N-propionyl-4-S-cysteaminylphenol-magnetite nanoparticles elicits CTL response via heat shock protein-peptide complex release. Cancer Sci. 2010;101(9):1939–46. doi: 10.1111/j.1349-7006.2010.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Antitumor immunity induction by intracellular hyperthermia using magnetite cationic liposomes. Jpn J Cancer Res. 1998;89(7):775–82. doi: 10.1111/j.1349-7006.1998.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito A, Yamaguchi M, Okamoto N, Sanematsu Y, Kawabe Y, Wakamatsu K, et al. T-cell receptor repertoires of tumor-infiltrating lymphocytes after hyperthermia using functionalized magnetite nanoparticles. Nanomedicine (Lond) 2013;8(6):891–902. doi: 10.2217/nnm.12.142. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Shinkai M, Honda H, Kobayashi T. Anticancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res. 2003;13(2):129–35. doi: 10.1097/00008390-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother. 2004;53(1):26–32. doi: 10.1007/s00262-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 28.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci U S A. 1996;93(18):9730–5. doi: 10.1073/pnas.93.18.9730. PMCID: 38497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon TJ, Kim JY, Kim H, Hong C, Lee H, Lee CK, et al. Anti-tumor immunostimulatory effect of heat-killed tumor cells. Exp Mol Med. 2008;40(1):130–44. doi: 10.3858/emm.2008.40.1.130. PMCID: 2679326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le HN, Lee NC, Tsung K, Norton JA. Pre-existing tumor-sensitized T cells are essential for eradication of established tumors by IL-12 and cyclophosphamide plus IL-12. J Immunol. 2001;167(12):6765–72. doi: 10.4049/jimmunol.167.12.6765. [DOI] [PubMed] [Google Scholar]
- 31.Norton JA, Li M, Lee NC, Tsung K. Inhibition of host signal transducer and activator of transcription factor 6 results in cure with cyclophosphamide and interleukin 12 immunotherapy. Ann Surg Oncol. 2006;13(1):118–24. doi: 10.1245/ASO.2006.03.514. [DOI] [PubMed] [Google Scholar]
- 32.Bendall L. Chemokines and their receptors in disease. Histol Histopathol. 2005;20(3):907–26. doi: 10.14670/HH-20.907. [DOI] [PubMed] [Google Scholar]
- 33.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155(8):3877–88. [PubMed] [Google Scholar]
- 34.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Activation of NK cells by CC chemokines. Chemotaxis, Ca2+ mobilization, and enzyme release. J Immunol. 1996;156(1):322–7. [PubMed] [Google Scholar]
- 35.Zhang Y, Yoneyama H, Wang Y, Ishikawa S, Hashimoto S, Gao JL, et al. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1alpha in mice. J Natl Cancer Inst. 2004;96(3):201–9. doi: 10.1093/jnci/djh024. [DOI] [PubMed] [Google Scholar]
- 36.Chiba K, Zhao W, Chen J, Wang J, Cui HY, Kawakami H, et al. Neutrophils secrete MIP-1 beta after adhesion to laminin contained in basement membrane of blood vessels. Br J Haematol. 2004;127(5):592–7. doi: 10.1111/j.1365-2141.2004.05242.x. [DOI] [PubMed] [Google Scholar]
- 37.Charmoy M, Brunner-Agten S, Aebischer D, Auderset F, Launois P, Milon G, et al. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 2010;6(2):e1000755. doi: 10.1371/journal.ppat.1000755. PMCID: 2816696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 39.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–8. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, et al. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177(4):1199–204. doi: 10.1084/jem.177.4.1199. PMCID: 2190961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 42.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14(7):335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 43.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 44.Mayo WJ. Grafting and traumatic dissemination of carcinoma in the course of operations for malignant disease. J Amer Med Assoc. 1913;60:512–3. [Google Scholar]
- 45.Ito A, Fujioka M, Tanaka K, Kobayashi T, Honda H. Screening of cytokines to enhance vaccine effects of heat shock protein 70-rich tumor cell lysate. J Biosci Bioeng. 2005;100(1):36–42. doi: 10.1263/jbb.100.36. [DOI] [PubMed] [Google Scholar]
- 46.Huang C, Zhao J, Li Z, Li D, Xia D, Wang Q, et al. Multi-chaperone-peptide-rich mixture from colo-carcinoma cells elicits potent anticancer immunity. Cancer Epidemiol. 2010;34(4):494–500. doi: 10.1016/j.canep.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28(6):528–42. doi: 10.3109/02656736.2012.677933. [DOI] [PubMed] [Google Scholar]
- 48.Davis TA, Czerwinski DK, Levy R. Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res. 1999;5(3):611–5. [PubMed] [Google Scholar]
- 49.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.