Abstract
Glucagon-like peptide-1 (GLP-1) is an incretin hormone that has been shown to have hemodynamic and cardioprotective capacity in addition to its better characterized glucoregulatory actions. Because of this, emerging research has focused on the ability of GLP-1 based therapies to drive myocardial substrate selection, enhance cardiac performance and regulate heart rate, blood pressure and vascular tone. These studies have produced consistent and reproducible results amongst numerous laboratories. However, there are obvious disparities in findings obtained in small animal models versus those of higher mammals. This species dependent discrepancy calls to question, the translational value of individual findings. Moreover, few studies of GLP-1 mediated cardiovascular action have been performed in the presence of a pre-existing comorbidities (e.g. obesity/diabetes) which limits interpretation of the effectiveness of incretin-based therapies in the setting of disease. This review addresses cardiovascular and hemodynamic potential of GLP-1 based therapies with attention to species specific effects as well as the interaction between therapies and disease.
INTRODUCTION
Glucagon-like peptide-1 (GLP-1), a natural product of the intestinal L-cells, has been shown to be physiologically important in maintenance of glucose homeostasis via insulin stimulation and inhibition of glucagon secretion [1–3]. These glucoregulatory effects are reportedly mediated predominantly by actions of full-length GLP-1 (i.e. GLP-1 (7-36)) on its cognate receptor GLP-1R [4]. Based on these reported actions, GLP-1 (7-36) was identified to have potential therapeutic benefits in patients with impaired glucose tolerance [5, 6]. A broadly expressed protease, dipeptidyl-peptidase IV (DPP-4), rapidly truncates GLP-1 (7-36) in the circulation via cleavage at the penultimate amino acid to GLP-1 (9-36) [7]. This truncated form of GLP-1 does not activate the GLP-1R and is inactive as an insulinotropic agent (although there is some debate on this point) [8]. DPP-4 action is sufficiently rapid to degrade exogenously administered GLP-1 (7-36) to GLP-1 (9-36) in minutes. Because of this, administration of GLP-1 (7-36) results in proportionate increases in GLP-1 (9-36) levels. Accordingly, DPP-4 resistant analogues (e.g. exendin-4, liraglutide) have been developed to allow for clinical exploitation of the GLP-1 pathway [6].
Early studies performed soon after the discovery of GLP-1 suggested that this gut-derived peptide has cardiovascular effects [9–11]. Subsequent work has clearly demonstrated that GLP-1 modulates myocardial substrate selection and that GLP-1-based agents (GLP-1 fragments, GLP-1 mimetics, DPP-4 inhibitors) mitigate myocardial ischemia/reperfusion injury [8, 12–18]. Interestingly, these actions appear to occur independent of the glucoregulatory actions of GLP-1 and its analogues, and some evidence suggests they may be mediated in part by contributions from GLP-1 (9-36) [8]. Here we review the effects of GLP-1 and related agents on hemodynamic regulation, including blood pressure, heart rate, and cardiac function. Although studies evaluating cardiovascular effects of GLP-1 have been conducted in a variety of animal models and in humans, findings to date have yielded model-dependent discrepancies and, in some cases, contradictory findings between species. Nevertheless, evidence supports that GLP-1 and related agents elicit important cardiovascular effects, especially on cardiac contractile function in the setting of ischemic injury and heart failure.
GLP-1 EFFECTS ON BLOOD PRESSURE
Significant pressor effects of exogenous GLP-1 were first reported in rats by Barragan et al. in 1994, following systemic infusion of GLP-1 [10]. Subsequent studies have established that there is a demonstrable and significant dose-dependent relationship between experimentally-increased GLP-1 concentration and elevations in blood pressure in otherwise healthy rodent models [10, 11, 19–23] with significant effects (+20mmHg) demonstrated at concentrations as low as the picomolar range [20]. Not all GLP-1 related peptides exert these effects; rather, these hypertensive responses to GLP-1 in rodent models appear to be induced only by those agents capable of activating the GLP-1R (i.e. GLP-1 (7-36), exenatide, liraglutide) [19, 20, 24]. In particular, there is strong evidence to support that exendin-4 induces significant and sustained elevations in systemic pressure in rodents [21–23].
Interestingly, hypertensive responses have been produced with a wide variety of dosing and exposure timing approaches. Bolus infusions lasting only seconds in duration [10, 22] and longer infusions on the order of hours [20] both result in similar increases in systemic pressure. This observation has informed studies examining mechanisms of GLP-1R mediated elevations in pressure. Work by Barragan, Bojanowska, Isbil-Buyunkcoskun and Yamamoto has established that central and peripheral factors both contribute to GLP-1R mediated pressor responses [22–25]. In these studies, intra-cerebroventricular (ICV) administration of GLP-1 (7-36) or exendin-4 results in elevations in systemic pressure in anesthetized [24, 26] and in conscious [22] Sprague Dawley rats. Identical findings have been demonstrated in Wistar rats, indicating that this phenomenon is not strain specific [19, 23]. However, there appear to be differences in the response to ICV versus intravenous infusions. Notably, intravenous administration produces more rapid increases in pressure of greater magnitude, whereas ICV administration results in longer-lasting hypertensive responses [24]. Additionally, ICV but not intravenous administration of the GLP-1R competitive antagonist GLP-1 (9-39) was able to abolish the effects of ICV GLP-1 (7-36) and blunt the effects of systemically administered GLP-1 (7-36). However, systemically administered GLP-1R blockade with GLP-1 (9-39) had no effect on ICV administered GLP-1 (7-36) effects [11, 24]. These observations point to an important central locus of the pressor effect of GLP-1 in rats.
Pharmacological studies also support a role for central GLP-1R mediated autonomic effects mediating hypertensive responses in rodents. Systemic administration of the β-blocker propranolol enhanced pressor responses to systemically administered exendin-4 whereas treatment with the α–blocker phentolamine resulted in dose dependent decreases in mean arterial pressure in response to increasing levels of exendin-4 [26]. However, GLP-1 effects to augment pressor responses were not seen in either adrenal demedullated rats or animals receiving treatment with phentolamine [21]. Further evidence for central autonomic effects include observations of GLP-1R mediated activation of “catecholamine neurons” and stimulation of central nicotinic and muscarinic receptors respectively [22, 23]. In other studies, the physiologic state of the animal has proven to be a modifier of the effects of GLP-1 on blood pressure, in that increases in blood pressure are observed in rodent models during hypovolemic challenge [19] whereas decreases in pressure are found following GLP-1R activation in the setting of insulin resistant obesity and salt sensitive hypertension [27–29].
Despite the near universal observation of increases in blood pressure following GLP-1 administration in rodent models, studies in higher mammals have failed to show this “hypertensive” effect. In the earliest such report, systemic pressure effects of GLP-1 (7-36) administration in conscious calves were evaluated. There was no observable pressor response to GLP-1 (7-36) administered intravenously at 35pmol/kg/min for 10 minutes [9]. In normal mongrel dogs, direct intracoronary administration of GLP-1 (7-36) elicited no change in systemic pressures across a range from 10pM to 1nM [30]. These studies used relatively acute exposures to GLP-1 (~30 min); three studies by the Shannon laboratory which used more prolonged exposures (~24–72 hours) similarly demonstrate essentially no effect of intravenous GLP-1 (7-36) on mean arterial pressures in normal healthy dogs or in dogs following the development of pacing induced cardiomyopathy [8, 31, 32]. In swine GLP-1 [33, 34], exendin-4 [35] or the long acting analogue liraglutide [36] have all failed to exert pressor effects. These swine studies employed treatment durations ranging from minutes [34] to days [36], minimizing the likelihood of effects being missed due to insufficient duration of exposure.
Many GLP-1 related agents are now approved for clinical use in the treatment of diabetes, and hemodynamic effects were monitored during the registration trials. Similar to observations in dogs and swine but in contrast to observations in rodents, these clinical studies in humans have not revealed a definitive effect of GLP-1 related agents to increase systemic blood pressure. To the contrary, a meta-analysis of multiple clinical studies comparing GLP-1 analogues with placebo suggested that exenatide and liraglutide elicit modest decreases in systolic blood pressure (~2.39 & 1.79 mmHg respectively) [37]. These modest decreases may be attributable to findings reported by the Drucker group where cardiac atrial GLP-1 receptor activation was found to promote secretion of atrial natriuretic peptide; a finding with potential implications regarding mechanisms by which GLP-1 based therapies may elicit decreases in systemic pressure [38].
In summary, the preponderance of evidence from human studies indicates that GLP-1R activation does not result in elevations in blood pressure. Similarly, studies in large animal models demonstrate no hypertensive response to GLP-1. These observations are in contrast to pronounced hypertensive responses seen in rodents. From this we conclude that the rodent is not a strong model of this aspect of human physiology. Is this difference itself informative, however? The data explored above suggest that the pressor effect in rodents is predominantly a phenomenon of regulation of central autonomic function; it is possible that these observations highlight differences between rodents and humans in transfer of GLP-1 into the central compartments, or in the sensitivity of central regulatory pathways to effects of GLP-1. There is a dearth of information regarding potential central effects of GLP-1R on hemodynamics in larger animal models. Recent findings of GLP-1R expression in monkey and human brain [39, 40] highlight the potential relevance of such central effects that should be further explored.
GLP-1 EFFECTS ON HEART RATE
An early observation suggesting an effect of GLP-1R on heart came from the GLP-1R knockout mice. The development of the heart is not normal in these animals, and at 2 months of age these animals maintain a resting heart rate that is ~100 beats/min faster than their parent strain. This tachycardia is lost by 5 months of age via an undefined compensatory mechanism [41]. This observation is confounded by the alteration in heart size, but highlights the potential relevance of GLP-1 signaling in heart rate regulation. More direct assessment of chronotropic effects has been evaluated in rodents and in higher mammals, and again these effects differ across species. Perhaps not surprisingly, GLP-1R mediated effects on heart rate are most pronounced in species exhibiting robust pressor responses to GLP-1 administration. A GLP-1 dose-dependent tachycardia is consistently reported in rodent models [10, 11, 19–23]. This phenomenon is particularly notable as it is contrary to the otherwise expected baroreflexive response to elevated systemic pressure, further supporting the notion of centrally mediated GLP-1 dependent cardiac actions. As outlined above, these effects depend on activation of the classical receptor since infusions of the degradation product GLP-1 (9-36) fail to elevate resting heart rates [11, 24]. Co-administration of the nicotinic receptor antagonist mecamylamine is sufficient to abolish GLP-1 mediated increases in heart rate in male Wistar rats [23], suggesting a role for autonomic modulation in the tachycardia response. Further, exendin-4 administered ICV produced dose-dependent increases in heart rate in conscious Sprague Dawley rats [22].
As was seen with the blood pressure effects of GLP-1, tachycardic effects are generally absent in higher mammals [8, 14, 30–35]. Human studies have however reported an extremely modest tachycardic response: A meta-analysis of 32 clinical trials concluded that GLP-1 agonists increased heart rate an average of 1.86 beats/min versus placebo [37]. The relevance of these observations to long-term cardiovascular effects of GLP-1 related treatments is uncertain, particular in context of the modest reductions in blood pressure.
GLP-1 EFFECTS ON CARDIAC CONTRACTILE FUNCTION
Effects of GLP-1 and related agents on cardiac performance have been evaluated in the context of normal physiology, in response to ischemia/reperfusion injury, and in the setting of heart failure. The effects to protect the myocardium against ischemic insults are reviewed elsewhere in this collection. Here we focus on the effects specifically on cardiac contractile function (i.e. ejection fraction, stroke volume, developed pressures, cardiac output, etc.), including evaluations of the functional effects of therapy in the setting of ischemia and heart failure.
GLP-1 is important in supporting normal force development in the heart. Mice genetically lacking a functional GLP-1R demonstrate significantly elevated left ventricular (LV) end diastolic pressure at 2 months of age, although this is lost by 5 months (note that this time course is concurrent with a similar self-limited interval of tachycardia observed in these animals; see above). Interestingly, mice lacking GLP-1R demonstrate no defects on the rate of LV pressure development or relaxation (+dP/dt or −dP/dt), but do develop significant LV hypertrophy by 5 months of age. This likely reflects a functional contractile deficit that is compensated by development of a LV hypertrophy [41]. GLP-1 (7-36) therapy in these animals is sufficient to increase LV developed pressure along with cardiac myocyte glucose uptake, indicating that these cardiac effects are independent of the classical GLP-1R [12]. These studies have implicated full-length GLP-1 (7-36) as well as the degradation product GLP-1 (9-36) in producing these actions [12]. Nevertheless, activation of the classical GLP-1R does appear to mediate at least some of the effects of GLP-1 and related factors, as suggested by observations of similar functional effects using the degradation-resistant GLP-1 analog liraglutide. In these studies, wild type mice subjected to 30 minutes of global myocardial ischemia and treated with liraglutide showed enhanced rates of cardiac fractional shortening and significantly increased LV developed pressure during recovery from ischemia [42].
The cardiac effects of exogenous GLP-1 (7-36) administration in rat models have been decidedly less consistent. Isolated buffer-perfused hearts from male Wistar rats exhibited GLP-1 dependent decreases in LV developed pressure and +dP/dt. In contrast, when identically handled and treated hearts were exposed to low-flow ischemia, GLP-1 administration was found to improve LV end diastolic pressure and LV developed pressure [16]. Similar studies interrogating mechanisms of GLP-1 mediated effects on cardiac performance have produced data demonstrating that exendin-4 and the GLP-1 degradation product GLP-1 (9-36) are both capable of augmenting LV performance in isolated rat hearts during recovery from ischemia, indicating receptor-dependent and receptor-independent pathways for GLP-1 mediated enhancements to cardiac performance [43].
These observations have largely been interpreted as demonstrating effects of GLP-1 based compounds on contractility (inotropy), i.e. increased myocardial force generation that occurs independent of ventricular preload. However, studies exposing isolated rat cardiomyocytes directly to GLP-1 (7-36) result in elevations to cAMP that occur independent of any positive effect on contractility [44]. Further, studies in isolated rat and mouse hearts where infusion of GLP-1 (7-36) demonstrated modest increases in LV mechanical performance during reperfusion subsequent to global ischemia could be accounted for by the beneficial effects of GLP-1 treatment to limit infarct size [45]. In obese Zucker rats in vivo GLP-1 therapies enhance cardiac performance via increases in LV diastolic function (as assessed by Tau, time constant of LV relaxation, and end diastolic pressure volume relationships) [27], i.e. an effect on cardiac output that is not mediated by changes in contractility. Taken together, these data suggest that GLP-1 mediated enhancements in cardiac performance could be related to increases in diastolic filling (i.e. Frank-Starling effect) as opposed to increases in inotropy/contractility per se. To date there exist no satisfactory dataset to evaluate load-independent contractile function in response to GLP-1 therapies. This avenue of investigation will be crucial for identifying mechanisms through which GLP-1 may act to enhance cardiac function. These analyses need be conducted over a variety of infusion ranges and conditions to address disparate results in the current literature that could be simply due to differences in in duration of therapy or experimental condition. In terms of molecular mechanisms that mediate these responses, an emerging body of evidence supports effects of GLP-1 in the setting of ischemia to modulate effects on function via 5′-AMP-activated protein kinase (AMPK) [46] without a dependence on Akt [16], with accompanying enhancements to cardiomyocyte SERCA activity [27].
GLP-1 effects to augment cardiac performance that are seen in rodents have also been observed in higher mammals. In 2004, Nikolaidis and colleagues first demonstrated GLP-1 dependent increases in LV +dP/dt, stroke volume and cardiac output with concurrent decreases in LV end diastolic pressure [14]. This same group reported that systemic GLP-1 administration (1.5 pmol/kg/min) was sufficient to protect against myocardial stunning resulting from a 10-minute occlusion of the left circumflex coronary artery followed by 24 hours of reperfusion, by improving regional wall motion and thereby enhancing isovolumic relaxation [32]. These reports were followed by two seminal studies in canines investigating differential effects of full length (7-36) vs truncated (9-36) GLP-1 on cardiac function [8, 14]. In the context of pacing induced cardiomyopathies, GLP-1 (7-36) and (9-36) both significantly reduced LV end-diastolic pressure and increased LV +dP/dt, indicating that both forms of GLP-1 are capable of augmenting cardiac performance in the setting of myocardial dysfunction [8]. Notably, in healthy canines the direct intracoronary delivery of GLP-1(7-36) exerted no effect on heart rate or degree of regional shortening [30]. These findings support the hypothesis that the cardiac effects of GLP-1 are predominantly evident in the setting of underlying disease such as myocardial ischemia and heart failure, and suggest that these effects are centrally mediated rather than depending on direct activation of myocardial GLP-1R.
Studies evaluating effects of GLP-1 and related agents on cardiac function in humans have relied on measures that are load-dependent, requiring careful interpretation in light of the above discussion. Nevertheless, the above observations in animal models aid in the interpretations of these measures of cardiac function. In one such study, GLP-1 (intravenous infusion at 1.5 pmol/kg/min for 72 hours) was added to background therapy in patients with acute myocardial infarction and ejection fractions < 40%. Under these conditions, GLP-1 administration significantly improved ejection fraction and wall motion [15]. Similar improvements in ejection fraction were reported with longer duration of therapy (2.5 pmol/kg/min subcutaneously for 5 weeks) in patients with class III/IV heart failure, but effects on ejection fraction were not observed in otherwise healthy control subjects [47]. Read and colleagues examined effects of GLP-1 therapies in a more acute setting to determine whether GLP-1 would convey protection against ischemic dysfunction resulting from a 1 minute coronary occlusion [48]. This study was the first in humans to provide real time pressure volume analyses of hearts receiving GLP-1 therapy. The study did not directly assess end systolic pressure volume relationships, but using pressure-volume loops they were able to demonstrate that GLP-1 therapy was sufficient to improve LV systolic and diastolic function during a 30 minute reperfusion period. GLP-1 infusion produced significant increases in load-dependent measures of contractility (+dP/dt) and improved relaxation (tau) and cardiac function (ejection fraction and stroke volume) [48].
In summary, there is a clear body of evidence supporting the ability of GLP-1 to enhance cardiac performance, especially in the setting of ischemia and/or cardiac failure. However, these studies do not fully explain the physiologic or molecular mechanisms underlying these effects of GLP-1. A more thorough understanding of these effects will be essential in determining how best to apply GLP-1 based therapies in settings of cardiac dysfunction. GLP-1 has been demonstrated to enhance myocardial glucose uptake, as covered in detail in other sections of this collection. If GLP-1 agents act via Frank-Starling mechanisms rather than as direct inotropes, the augmentation of function may be energetically less demanding and this could prove advantageous. These speculations require systematic study and careful evaluation of the clinical effects of GLP-1 based therapies already in use.
GLP-1 EFFECTS ON TISSUE PERFUSION
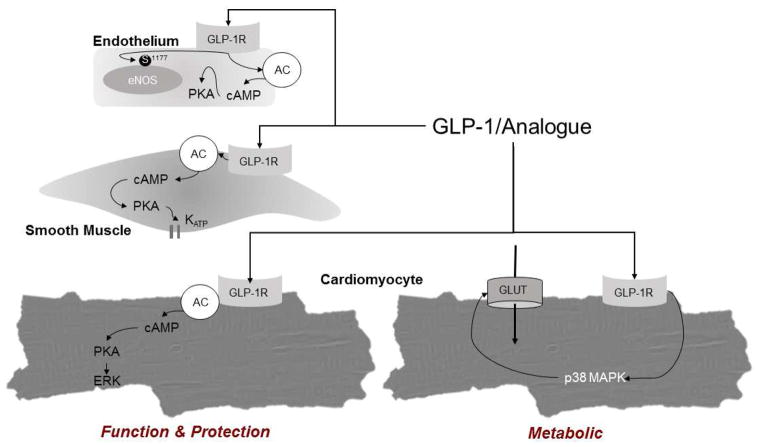
Initial studies designed to address the vascular effects of GLP-1 suggested that GLP-1R is expressed in endothelial [49–53] and vascular smooth muscle cells [12, 30, 33, 54]. Although there is reason to question immunodetection of GLP-1R expression using antibody-based methodologies available prior to 2013 [55], there is considerable evidence that GLP-1R is functionally expressed in the endothelium, as activation with GLP-1 (7-36), GLP-1R agonists, or DPP-4 inhibitors results in phosphorylation of endothelial nitric oxide synthase (eNOS) at Ser1177, production of nitric oxide, and suppression of endothelin-1 expression [53, 56–59]. Studies in isolated aorta [60, 61], pulmonary arteries [62], and rodent hearts [12, 16] also support that GLP-1 mediates vasodilation via an endothelial-dependent mechanism. Signaling pathways implicated in these endothelial effects of GLP-1 include AMPK [56] and cAMP/PKA-PI3K/Akt dependent activation of KATP channels (Summarized in Figure 1) [51, 59, 61, 63]. In contrast, in isolated rat femoral arteries GLP-1 produced endothelium-independent vasodilation [64], and other studies report little or no effect of GLP-1 (7-36) in isolated conduit coronary arteries [30]. These observations may highlight species differences, but may also reflect differing responsiveness in micro versus macro vessels.
Figure 1.
A graphical representation of putative pathways downstream from GLP-1 receptor activation in endothelial cells, vascular smooth muscle cells and cardiomyocytes. Pathways presented within this figure represent a compilation of data from multiple species.
There is little evidence to support prominent direct vasodilator effects in vivo in higher mammals; systemic administration of GLP-1 based therapeutics failed to significantly alter blood pressure in dogs or pigs [8, 14, 30, 33, 35]. In humans, as reviewed above, there is a modest effect to lower blood pressure; no studies have directly evaluated vasodilator function in large vessels in humans.
With regard to microvascular flow and tissue perfusion, GLP-1 has been shown to augment skeletal muscle microvascular blood flow in rats [59, 65] and to augment coronary blood flow in dogs and humans [14, 66], however, it is likely that the vasodilation observed in these experiments was directly related to increases in metabolism; i.e. mediated by local metabolic vasodilator influences [67]. This contention is supported by data demonstrating that GLP-1 mediated increases in coronary blood flow are accompanied by increases in myocardial oxygen consumption [14] and by experiments that found no effect of intracoronary GLP-1 administration (10 pM to 1 nM) on coronary blood flow or coronary venous PO2 [30]. Furthermore, other studies also report little or no effect of GLP-1 on skeletal muscle blood flow in humans [68] or on coronary blood flow in large animal models [8, 13, 33, 69] and humans [33]. GLP-1 was found to augment endothelium-dependent, but not endothelium-independent, vasodilation in the forearm of non-diabetic [70] and type 2 diabetic humans [49]. However, in the context of a more complex pathology (i.e. Metabolic Syndrome), GLP-1 was found to enhance both endothelium dependent and independent insulin mediated forearm vasodilator responses [71]. Taken together, these findings indicate that activation of GLP-1R can protect against endothelial dysfunction and therefore possibly be of benefit regarding the initiation and progression of vascular disease. However, GLP-1 has little effect on systemic vascular resistance or blood flow to skeletal or cardiac muscle. At present, many large clinical trials to address the efficacy of GLP-1 based therapies to attenuate atherosclerotic disease are underway but the only completed studies, both using DPP-4 inhibitors, failed to observe a net cardioprotective effect of this therapy [72, 73].
IMPLICATIONS FOR CARDIOVASCULAR RISK IN GLP-1 TREATED POPULATIONS
With the widespread adoption of GLP-1 mimetics and DPP-IV inhibitors into clinical practice for the treatment of diabetes mellitus there is now a worldwide population of individuals with pre-existing cardiovascular risk being exposed to these agents. As reviewed elsewhere in this collection and in the above review, there is reason to believe that these GLP-1 related agents may exert beneficial effects in the setting of ischemia. Similarly, the available animal data suggest that GLP-1 related therapeutics could produce beneficial effects on cardiac function through reductions in afterload, improvements in diastolic relaxation, and improved flow-metabolism matching in the myocardium. There are, however, two important caveats. First, the majority of the data evaluating the hemodynamic and anti-ischemic effects of GLP-1 related agents have been conducted in non-obese, non-diabetic animal models. The important and promising data that have been produced in humans [66] demonstrating a clear beneficial effect of exenatide to minimize myocardium at risk with infarction were collected in a majority non-diabetic population; this study is underpowered for subgroup analyses to evaluate effects specifically within diabetic patients. Data from our laboratory and others suggest that obesity and diabetes impair the beneficial effects of GLP-1 treatments in the heart, with the clear implication that the population who will most commonly be treated with GLP-1 related agents may not accrue the anticipated metabolic and anti-ischemic benefits [33, 66, 74]. This lack of effect in the setting of metabolic disease may help explain the observed lack of cardioprotective effects in the recently published large-scale clinical trials evaluating cardiovascular safety of aloglitpin and saxagliptin [72, 73].
SUMMARY AND FUTURE DIRECTIONS
Extensive data exist supporting a role for GLP-1 based therapies in mitigating cardiac damage and modulating cardiovascular behavior. However, despite an overwhelming body of evidence supporting cardiovascular actions of GLP-1 and its analogues, results are tremendously discrepant across species. Small animal models demonstrate significant dose-dependent pressor responses to GLP-1 with accompanying modifications of heart rate. These effects, which appear to be mediated largely through central actions, are absent in larger animal models and humans (See Figure 2). Future investigation must examine the origins of this discrepancy to establish whether these effects in small animals are informative regarding central control of hemodynamics, but the net physiologic effects are not adequately informative regarding human disease. Of particular interest will be investigations in the heretofore uninvestigated potential for central actions in large animals.
Figure 2.
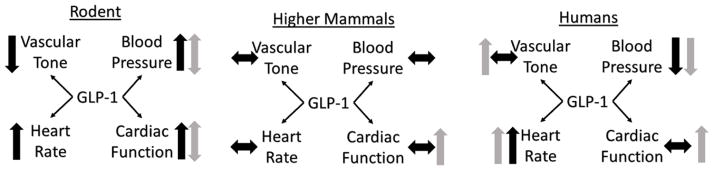
A schematic summary of cardiovascular outcomes resulting from GLP-1 based therapies in rodents (left), higher mammals (center) and humans (right). Black arrows reflect physiologic responses in healthy animals whereas gray arrows indicate responses measured with in animals with concurrent cardiovascular disease.
Regardless of animal model, GLP-1 mediated cardiovascular responses are heavily modified by the presence of overt cardiovascular disease. In large animals effects of GLP-1 have predominantly been investigated in models with cardiac disease (i.e. ischemia or dilated cardiomyopathies); only a limited dataset allows evaluation of healthy animal responses. There is a clear need for further studies in the setting of, metabolic disease such as obesity or type 2 diabetes. These investigations are key as obese subjects with type 2 diabetes are the primary populations that are prescribed these agents. Recent work by Moberly and colleagues has highlighted the potential for cardiac GLP-1 “resistance” in the setting of obesity and type 2 diabetes. Whether this phenomenon has a functional consequence with regard to cardiac performance is unknown.
Finally, load-independent assessments of changes cardiac contractility in response to exogenous GLP-1 based therapies are essential in determining the potential therapeutic value of GLP-1 and its analogues. While evidence clearly suggests effects to augment cardiac output and developed pressure, investigations of the underlying physiologic mechanisms both support and refute true inotropic effects of GLP-1 compounds. If GLP-1 acts to augment function via a Frank-Starling effect, this may prove to be energetically favorable compared to effects of direct inotropes and this may allow for a safer approach to treating myocardial dysfunction.
Acknowledgments
The authors gratefully acknowledge financial support from multiple agencies. This work was supported by a National Institutes of Health grant, HL117620 (J. Tune and K. Mather, PI). Dr. Goodwill was supported by American Heart Association 13POST1681001813 (A. Goodwill, PI). Mr. Conteh was supported by National Institutes of Health HL117620-S1 (J. Tune and K. Mather, PI). Mr. Sassoon was supported by grant number TL1 TR000162 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
Footnotes
CONFLICT OF INTEREST DISCLOSURE
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987;84:3434–8. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36:a physiological incretin in man. Lancet. 1987;2:1300–4. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 3.Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987;79:616–9. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorens B, Porret A, Buhler L, Deng SP, Morel P, Widmann C. Cloning and functional expression of the human islet GLP-1 receptor. Demonstration that exendin-4 is an agonist and exendin- (9-39) an antagonist of the receptor. Diabetes. 1993;42:1678–82. doi: 10.2337/diab.42.11.1678. [DOI] [PubMed] [Google Scholar]
- 5.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 6.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide- (1-38) J Biol Chem. 2003;278:22418–23. doi: 10.1074/jbc.M212355200. [DOI] [PubMed] [Google Scholar]
- 8.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 9.Edwards CM, Edwards AV, Bloom SR. Cardiovascular and pancreatic endocrine responses to glucagon-like peptide-1 (7-36) amide in the conscious calf. Exp Physiol. 1997;82:709–16. doi: 10.1113/expphysiol.1997.sp004059. [DOI] [PubMed] [Google Scholar]
- 10.Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide1- (7-36) amide in rats. Am J Physiol. 1994;266:E459–E466. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- 11.Barragan JM, Rodriguez RE, Eng J, Blazquez E. Interactions of exendin- (9-39) with the effects of glucagon-like peptide-1- (7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67:63–8. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- 12.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 13.Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, et al. Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail. 2010;3:512–21. doi: 10.1161/CIRCHEARTFAILURE.109.900282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–61. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 15.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 16.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–13. doi: 10.1124/jpet.106.100982. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A, et al. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol. 2010;9:76. doi: 10.1186/1475-2840-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsubara M, Kanemoto S, Leshnower BG, Albone EF, Hinmon R, Plappert T, et al. Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J Surg Res. 2011;165:38–45. doi: 10.1016/j.jss.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bojanowska E, Stempniak B. Effects of centrally or systemically injected glucagon-like peptide-1 (7-36) amide on release of neurohypophysial hormones and blood pressure in the rat. Regul Pept. 2000;91:75–81. doi: 10.1016/s0167-0115(00)00119-1. [DOI] [PubMed] [Google Scholar]
- 20.Gardiner SM, March JE, Kemp PA, Bennett T, Baker DJ. Possible involvement of GLP-1 (9-36) in the regional haemodynamic effects of GLP-1 (7-36) in conscious rats. Br J Pharmacol. 2010;161:92–102. doi: 10.1111/j.1476-5381.2010.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarters vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–9. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isbil-Buyukcoskun N, Gulec G. Effects of intracerebroventricularly injected glucagon-like peptide-1 on cardiovascular parameters; role of central cholinergic system and vasopressin. Regul Pept. 2004;118:33–8. doi: 10.1016/j.regpep.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1- (7-36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–E791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto H, Kishi T, Lee CE, Choi BJ, Fang H, Hollenberg AN, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–46. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardiner SM, March JE, Kemp PA, Bennett T. Autonomic nervous system-dependent and -independent cardiovascular effects of exendin-4 infusion in conscious rats. Br J Pharmacol. 2008;154:60–71. doi: 10.1038/bjp.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–13. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, et al. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380:44–9. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Yu M, Moreno C, Hoagland KM, Dahly A, Ditter K, Mistry M, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–35. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Moberly SP, Berwick ZC, Kohr M, Svendsen M, Mather KJ, Tune JD. Intracoronary glucagon-like peptide1 preferentially augments glucose uptaken in ischemic myocardium independent of changes in coronary flow. Exp Biol Med (Maywood) 2012;237:334–42. doi: 10.1258/ebm.2011.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaidis LA, Doverspike A, Hentosz T, Zourelias L, Shen YT, Elahi D, et al. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–8. doi: 10.1124/jpet.104.073890. [DOI] [PubMed] [Google Scholar]
- 33.Moberly SP, Mather KJ, Berwick ZC, Owen MK, Goodwill AG, Casalini ED, et al. Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic Res Cardiol. 2013;108:365. doi: 10.1007/s00395-013-0365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavianipour M, Ehlers MR, Malmberg K, Ronquist G, Ryden L, Wikstrom G, et al. Glucagon-like peptide-1 (7-36) amide prevents the accumulation of pyruvate and lactate in the ischemic and non-ischemic porcine myocardium. Peptides. 2003;24:569–78. doi: 10.1016/s0196-9781(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 35.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–10. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Kristensen J, Mortensen UM, Schmidt M, Nielsen PH, Nielsen TT, Maeng M. Lack of cardioprotection from subcutaneously and preischemic administered liraglutide in a closed chest porcine ischemia reperfusion model. BMC Cardiovasc Disord. 2009;9:31. doi: 10.1186/1471-2261-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–75. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez E, Martinez MD, Roncero I, Chowen JA, Garcia-Cuartero B, Gispert JD, et al. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798–806. doi: 10.1111/j.1471-4159.2004.02914.x. [DOI] [PubMed] [Google Scholar]
- 40.Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue; Novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014:en20131934. doi: 10.1210/en.2013-1934. [DOI] [PubMed] [Google Scholar]
- 41.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, et al. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–52. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 42.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sonne DP, Engstrom T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1 (9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243–9. doi: 10.1016/j.regpep.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89:445–52. doi: 10.1161/hh1701.095716. [DOI] [PubMed] [Google Scholar]
- 45.Ossum A, van DU, Engstrom T, Jensen JS, Treiman M. The cardioprotective and inotropic components of the postconditioning effects of GLP-1 and GLP-1 (9-36) a in an isolated rat heart. Pharmacol Res. 2009;60:411–7. doi: 10.1016/j.phrs.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Huisamen B, Genade S, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7-36) amide in the rat heart and their role in protection against ischaemia. Cardiovasc J Afr. 2008;19:77–83. [PMC free article] [PubMed] [Google Scholar]
- 47.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 48.Read PA, Hoole SP, White PA, Khan FZ, O’Sullivan M, West NE, et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4:266–72. doi: 10.1161/CIRCINTERVENTIONS.110.960476. [DOI] [PubMed] [Google Scholar]
- 49.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 50.Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem Biophys Res Commun. 2010;391:1405–8. doi: 10.1016/j.bbrc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 51.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–14. doi: 10.1161/ATVBAHA.110.206425. [DOI] [PubMed] [Google Scholar]
- 52.Ding L, Zhang J. Glucagon-like peptide-1 activates endothelial nitric oxide synthase in human umbilical vein endothelial cells. Acta Pharmacol Sin. 2012;33:75–81. doi: 10.1038/aps.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erdogdu O, Eriksson L, Nystrom T, Sjoholm A, Zhang Q. Exendin-4 restores glucolipotoxicity-induced gene expression in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2012;419:790–5. doi: 10.1016/j.bbrc.2012.02.106. [DOI] [PubMed] [Google Scholar]
- 54.Richards P, Parker HE, Adriaenssens AE, Hodgson JM, Cork SC, Trapp S, et al. Identification and characterisation of glucagon-like peptide-1 receptor expressing cells using a new transgenic mouse model. Diabetes. 2013 doi: 10.2337/db13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pyke C, Knudsen LB. The glucagon-like peptide-1 receptor--or not? Endocrinology. 2013;154:4–8. doi: 10.1210/en.2012-2124. [DOI] [PubMed] [Google Scholar]
- 56.Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, Kasai K, et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia. 2010;53:2256–63. doi: 10.1007/s00125-010-1831-8. [DOI] [PubMed] [Google Scholar]
- 57.Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol. 2011;55:2–9. doi: 10.1016/j.vph.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dai Y, Mehta JL, Chen M. Glucagon-like peptide-1 receptor agonist liraglutide inhibits endothelin-1 in endothelial cell by repressing nuclear factor-kappa B activation. Cardiovasc Drugs Ther. 2013;27:371–80. doi: 10.1007/s10557-013-6463-z. [DOI] [PubMed] [Google Scholar]
- 59.Dong Z, Chai W, Wang W, Zhao L, Fu Z, Cao W, et al. Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab. 2013;304:E222–E228. doi: 10.1152/ajpendo.00473.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozyazgan S, Kutluata N, Afsar S, Ozdas SB, Akkan AG. Effect of glucagon-like peptide-1(7-36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology. 2005;74:119–26. doi: 10.1159/000084277. [DOI] [PubMed] [Google Scholar]
- 61.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys. 2008;478:136–42. doi: 10.1016/j.abb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide- (7-36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–6. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 63.Erdogdu O, Nathanson D, Sjoholm A, Nystrom T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35. doi: 10.1016/j.mce.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 64.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–7. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 65.Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61:888–96. doi: 10.2337/db11-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gejl M, Sondergaard HM, Stecher C, Bibby BM, Moller N, Botker HE, et al. Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab. 2012;97:E1165–E1169. doi: 10.1210/jc.2011-3456. [DOI] [PubMed] [Google Scholar]
- 67.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol (1985) 2004;97:404–15. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 68.Bertin E, Arner P, Bolinder J, Hagstrom-Toft E. Action of glucagon and glucagon-like peptide 1- (7-36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J Clin Endocrinol Metab. 2001;86:1229–34. doi: 10.1210/jcem.86.3.7330. [DOI] [PubMed] [Google Scholar]
- 69.Dokken BB, Hilwig WR, Teachey MK, Panchal RA, Hubner K, Allen D, et al. Glucagon-like peptide-1 (GLP-1) attenuates post-resuscitation myocardial microcirculatory dysfunction. Resusciation. 2010;81:755–60. doi: 10.1016/j.resuscitation.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 70.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 71.Tesauro M, Schinzari F, Adamo A, Rovella V, Martini F, Mores N, et al. Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care. 2013;36:683–9. doi: 10.2337/dc12-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 73.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 74.Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does no attenuate development of atherosclerosis in diabetic male ApoE (−/−) mice. Endocrinology. 2013;154:127–39. doi: 10.1210/en.2012-1937. [DOI] [PubMed] [Google Scholar]