Abstract
Background
Environmental fungi have been linked to T helper type 2 (Th2) cell-related airway inflammation and the Th2-associated chronic airway diseases asthma, chronic rhinosinusitis with nasal polyps (CRSwNP) and allergic fungal rhinosinusitis (AFRS), but whether these organisms participate directly or indirectly in disease pathology remains unknown.
Objective
To determine the frequency of fungus isolation and fungus-specific immunity in Th2-associated and non-associated airway disease patients.
Methods
Sinus lavage fluid and blood were collected from sinus surgery patients (n=118) including CRS patients with and without nasal polyps and AFRS and non-CRS/non-asthmatic control patients. Asthma status was deteremined from medical history. Sinus lavage fluids were cultured and directly examined for evidence of viable fungi. Peripheral blood mononuclear cells were restimulated with fungal antigens in an enzyme linked immunocell spot (ELISpot) assay to determine total memory fungus-specific IL-4-secreting cells. These data were compared to fungus-specific IgE levels measured from plasma by ELISA.
Results
Filamentous fungi were significantly more commonly cultured from Th2-associated airway disease subjects (asthma, CRSwNP, or AFRS: n=68) compared to non-Th2-associated control patients (n=31); 74% vs 16% respectively, p<0.001. Both fungus-specific IL-4 ELISpot (n=48) and specific IgE (n=70) data correlated with Th2-associated diseases (sensitivity 73% and specificity 100% vs. 50% and 77%, respectively).
Conclusions
The frequent isolation of fungi growing directly within the airways accompanied by specific immunity to these organisms only in patients with Th2-associated chronic airway diseases suggests that fungi participate directly in the pathogenesis of these conditions. Efforts to eradicate airway fungi from the airways should be considered in selected patients.
Clinical Implications
Airway fungi may contribute to the expression of sinusitis with nasal polyps and asthma, suggesting that efforts to eradicate fungi from the airways and environments of these patients should be considered.
Keywords: Allergic, Airway, Mycosis, Fungal, Asthma, Chronic Rhinosinusitis, Th2-associated airway disease
INTRODUCTION
Chronic rhinosinusitis (CRS) is an inflammatory process of the nasal and paranasal sinus mucosa that affects more than 30 million people annually and results in over $6 billion in direct healthcare costs1. CRSsNP and CRSwNP are associated with Th1 and Th2-associated cytokine responses, respectively2,3. Many studies have implicated infectious agents including fungi, bacteria and viruses in the pathogenesis of CRS, but the precise relationship of infectious agents to the expression of Th2-associated diseases is not known4-6.
Similar to CRS with nasal polyps, allergic asthma is characterized by the presence of Th2 cells, eosinophils, and mast cells in the lower airways. Asthma and CRS are epidemiologically linked, with over 40% of CRSwNP patients having allergic asthma and 80% of asthmatic patients showing some imaging data suggestive of nasal and/or paranasal sinus inflammation 7,8. The physical link between the upper and lower airways, shared environmental exposures, and similar histology, epidemiology, and Th2-associated immunopathology strongly suggest that allergic asthma and CRSwNP are linked by a common pathophysiology. Allergic fungal rhinosinusitis (AFRS) is similar to CRSwNP, but afflicted patients demonstrate IgE reactivity to one or more fungi and often manifest more advanced disease.
Fungal sensitization as assessed by skin prick testing or specific IgE measurements has been associated with worsening asthma in humans; however, the relationship of fungal sensitization to the pathophysiology of asthma remains uncertain9. Of the many agents commonly linked to asthma pathogenesis, including dust mites, pollens, and fungi, only fungi are capable of growing within the mammalian airway. Two successful clinical trials with oral antifungals in severe asthmatics with fungal sensitization suggest that fungal infection could underlie disease in a subset of asthmatic patients10,11. Unresolved with these studies, however, is whether the salutary effect of oral antifungals in asthma reflects their anti-inflammatory12 or antifungal activities. Indeed, clear evidence of fungi growing within the airways of patients with a variety of Th2-associated and non-associated diseases is lacking.
Prior studies from our laboratories have established that fungal sensitization in CRS is very common and that fungal growth within the airways is essential to the pathophysiology of experimental fungal allergic airway disease in mice13-15. We have further shown that fungal proteinases are intimately linked to both anti-fungal immunity and the expression of experimental asthma through the activation of fibrinogen and TLR416. We therefore hypothesized that fungal growth directly within the airways could potentially contribute to the expression of a variety of Th2-associated airway diseases in humans. To test this, we determined the frequency of both fungal isolation from sinus lavage specimens and the presence of fungus-specific immunity in patients undergoing sinus surgery with and without asthma and CRS. We further examined sinus lavage material for direct evidence of fungal growth.
METHODS: (see supplementary methods)
Patients
The research protocol for this study was approved by the Institutional Review Board of The University of Texas Health Science Center and Baylor College of Medicine, Houston, TX. Patients (n=118) scheduled to undergo endoscopic sinus surgery who met inclusion and exclusion criteria (see Table S1 in Supplementary Appendix) were consented and de-identified for the collection of peripheral blood and/or sinus lavage fluid. Clinical definitions are defined in Table S2 and patient characteristics are summarized in Table S3 in the Supplementary Appendix.
Collection and fungal culture of sinus lavage fluid
Sinus lavage fluid was collected mainly from the maxillary sinuses using an endoscope with sterile saline and aseptic technique. The collected sinus fluid was processed with ACK for red blood cell lysis, 50 mM dithiothreitol for mucolysis, and trypsin for tissue disaggregation as needed, and then cultured as previously described17. Select common, mature fungal colonies were isolated for microscopic identification at the Fungus Testing Laboratory at The University of Texas Health Science Center (San Antonio, Texas, USA).
Fungal antigen preparation and ELISpot assay
Fungal allergens were prepared from pure cultured hyphal mats using colloidal grinding in a planetary ball mill (Retsch/Verder Scientific, Newtown, PA) with 2 mm zirconium oxide balls in PBS. Peripheral blood mononuclear cells (PBMCs) were isolated by ficoll-hypaque gradient centrifugation, and co-incubated with and without fungal allergen from Aspergillus niger, Aspergillus fumigatus, Aspergillus flavus, Alternaria alternata, Curvularia lunata, Penicillium oxalicum, Paecilomyces variotii, Candida albicans, and Bipolaris spicifera or from fungal allergens prepared from microscopically unidentifiable (non-maturing) fungal colonies isolated specifically from some control and CRSsNP patients in an IL-4 ELISpot assay. Based on receiver operating characteristic (ROC) analyses, a cutoff of 50 cells per million cells induced to secrete IL-4 in response to fungal allergen was considered positive. Data are reported as the number of cells induced above the media control for each allergen. Cell viability and responsiveness were confirmed by phytohemagglutinin challenge.
ELISA fungal specific IgE assay
Patient plasma was incubated in fungal allergen (A. niger, A. fumigatus, or A. alternata) coated ELISA plates. Fungal allergen binding IgE was detected using HRP conjugated anti-human IgE (ICL Labs, Stuart, Florida) and compared to a standard curve prepared using an IgE sandwich ELISA with a lower limit of detection of 6 kIU/L which was also the optimal cutoff as detemined by ROC analyses.
Statistics
Summary statistics are presented as proportions, means (with standard error of the mean (SEM)). Bivariate associations were examined using Mann-Whitney and Fisher’s exact tests, as appropriate. The discriminatory ability of IgE and IL-4 ELISpot were characterized by the area under the ROC curve. Optimal disease associated cutoffs for ELISpot and IgE analyses were also determined during ROC analyses. All analyses were performed using Prism v6.0 (GraphPad Software, San Diego, CA) or Stata v12.0 software (StataCorp, College Station, TX).
RESULTS
High rate of fungal isolation from airways of patients with CRSwNP and asthma
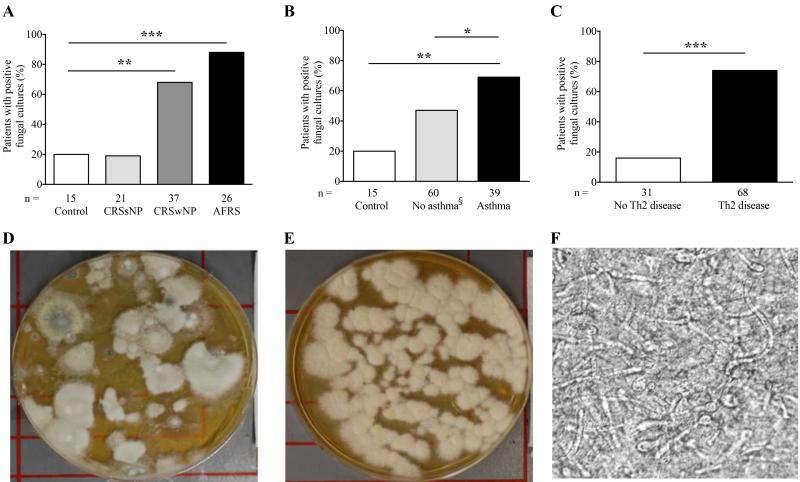
We first determined the frequency with which fungi could be cultured from sinus lavage fluids collected at the time of surgery from patients without (control, CRSsNP without asthma) and with (CRSwNP, AFRS, asthma) Th2-associated chronic airway disease undergoing sinus surgery. AFRS patients were treated as a distinct group from CRSwNP in our analyses. Patients were separated into with or without asthma groups based on medical history. Relative to control patients, fungi were significantly more frequently cultured from patients with CRSwNP, AFRS, or asthma (p<0.01, p<0.001, and p<0.01, respectively; Fig. 1A, B, Supplementary Table S4). Fungal isolation differences were most dramatically enhanced in patients categorized based on the presence of any Th2-associated disease as compared to non-Th2-associated patients. Sixteen percent of non-allergic disease patients had a positive fungal culture while 74% of patients with any allergic disease had a positive fungal culture (Fig. 1C; p<0.001). Typical specimens from patients with Th2-associated upper airway disease produced robust fungal growth on culture plates and plain microscopy revealed fungal hyphae, often as part of extensive networks, in 95% (n=20) of patient specimens (Fig. 1 D-F). These findings demonstrate that fungi can be directly observed in and grown from the sinuses more often in patients with chronic Th2-associated airway disease and further document in situ fungal growth within the sinuses. Tables S4-S6 summarize data for all patient diagnostic combinations. All fungal species identified in this study are listed in Table S7.
Figure 1.
Fungal recovery from sinus lavage is more likely in the context of Th2-associated airway disease. (A-C) Percent of patients yielding positive fungal cultures from sinus lavage comparing (A) control patients to CRS without nasal polyps, CRS with nasal polyps, and AFRS patients; B) control patients and non-asthmatics to asthmatics; and C) Non-Th2-associated to Th2-associated patients. * p<0.05, ** p<0.01, *** p<0.001. (D, E) Representative Sabouraud’s plate fungal cultures of sinus lavage fluid from two subjects. (F) Photomicrograph of unstained sinus lavage mucus from a representative AFRS patient showing extensive hyphal network (400×). §Control is a subset of all non-asthmatics.
Both IL-4 reactivity and specific IgE detect fungal immune memory in Th2-associated airway disease
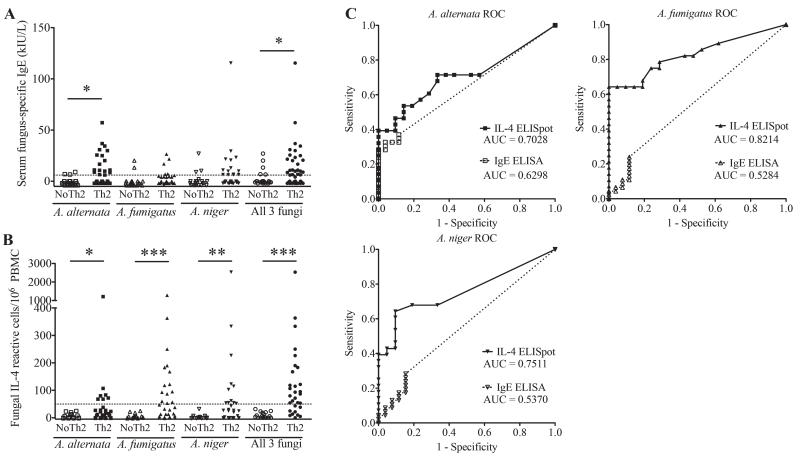
In addition to fungal cultures, we sought additional evidence of the physiological impact of airway fungi in Th2-associated airway diseases. Although a large number fungal species were recovered in this study overall (>23), we utilized whole organism lysates from the three most commonly isolated fungi (A. alternata, A. fumigatus, and A. niger) to compare fungal-specific immunity detection using two methods, an IgE ELISA and an IL-4 ELISpot. We chose IL-4 for its importance as a canonical marker of the presence of Th2 cells and growth factor for IgE-secreting B cells18,19. Fungal specific IgE was assessed in 22 non-Th2-associated and 48 Th2-associated airway disease patients using an ELISA developed in our laboratory as commercial assays cannot assess reactivity against the specific fungal strains we isolated. Clinically, patients are considered fungus sensitive if they are reactive against any fungus as assessed by skin prick or have elevated specific IgE. Specific IgE against A. alternata, A. fumigatus and A. niger was found in a minority of patients both with and without Th2-associated disease, but only IgE against A. alternata was significantly more commonly present in the Th2-associated disease group (P < 0.05; Fig 2A). A positive response to any fungus as determined by the highest fungus-specific IgE titer of the three tested that exceeded the assay’s lower limit of detection (6kIU/L IgE) was found in 50% of Th2-associated disease patients and was again significantly more often present in those with compared to those without Th2-associated disease (P < 0.05; Fig 2A).
Figure 2.
Fungus-specific IL-4 and IgE responses predict Th2-associated disease. Subjects with and without Th2-associated disease (Th2 and no Th2, respectively) were assessed for (A) specific serum IgE and (B) IL-4 memory recall responses from PBMC against whole cell antigens derived from the fungi A. alternata, A. fumigatus, and A. niger, both individually and combined. For the combined analysis, the largest positive response obtained with any of the three fungal antigens was plotted. Values above dotted lines represent positive responses. (C) Relative Operating Characteristic (ROC) curve analyses for both IgE and IL-4 reactivity using optimal IgE & IL-4 cutoff values of 6 kIU/L and 50 cells/106 PBMCs, respectively. AUC: area under the curve.* p<0.05, ** p<0.01, *** p<0.001.
We further performed ELISpot assays to measure the net number of IL-4-secreting cells induced by fungal antigen exposure above media stimulation. In contrast to the IgE analysis, positive responses in this assay to each of the three fungi and the highest response to any fungus readily distinguished Th2 associated from non-Th2 associated disease patients. Moreover, fungus-specific IL-4 responses were observed only in the Th2-associated disease group (Fig. 2B). ROC curve analyses were further used to compare the performance of the two assays for each antigen. The ROC analyses were limited to patients in which both IgE and IL-4 ELISpot analyses were performed. The IL-4 ELISpot assay showed superior performance in discerning subjects with (n=30) and without (n=18) Th2-associated airway disease compared to the IgE ELISA as noted by the higher area under the curve (AUC) values (Fig. 2C). Based on these analyses, we confirmed the optimal performance threshold of the ELISpot assay as an induction of 50 IL-4-secreting cells per million PBMCs, providing 100% specificity and 73% sensitivity. In contrast, the cutoff of the IgE ELISA, independently determined as the lower limit of detection of the assay (6kIU/L IgE), yielded a specificity of 77% and 50% sensitivity. Fungus-specific IgE data are expressed in relation to patient diagnostic category in Figure S1. As expected, fungal specific IgE positivity was most clearly associated with AFRS.
Enhanced fungus-specific IL-4 memory recall in patients with CRS with nasal polyps and asthma
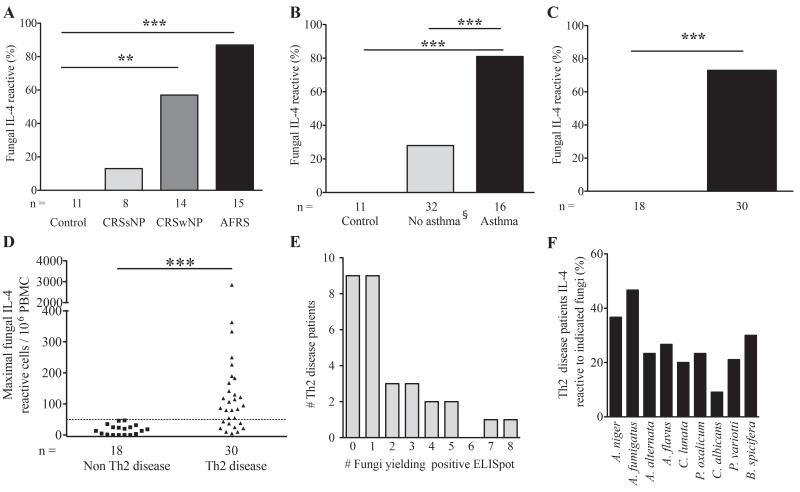
Given the more robust specificity of the IL-4 ELISpot assay relative to IgE for the three fungi, we expanded it to include allergens derived from nine commonly identified fungi from subjects’ airways to further enhance the sensitivity of the assay. Analyses were also performed using allergens from non-maturing, unidentifiable, fungi isolated from healthy control patients due to the rarity of fungal growth in this subset. Despite isolating fungi from ~16% of control and CRSsNP patients, only one patient with CRSsNP, who had concomitant asthma, exhibited a positive IL-4 ELISpot response (Fig. 3A and Supplementary Table S5). In contrast, patients with Th2-associated sinus disease (CRSwNP and AFRS) had a significant increase in positive fungus-specific IL-4 ELISpot (p<0.01 and p<0.001, respectively; Fig 3A). Similarly, we found a markedly higher percentage of asthmatic patients with positive IL-4 ELISpot results as compared to both control and all non-asthmatic patients (80% versus 0% and 27%, respectively; p<0.001 for both; Fig 3B). Note that the non-asthmatic group includes the subgroup of healthy control subjects plus non-asthmatic CRS subjects. Again, the most polarized data were seen when patients were categorized by the absence or presence of any Th2-associated airway disease, where no patient without Th2-associated airway disease and 73% of patients with Th2-associated airway disease exhibited positive fungal specific IL-4 ELISpot reactivity (p<0.001) (Fig. 3C). Maximal fungus induced ELISpot values for Th2-associated disease patients ranged from 51 to ~3000 IL-4-secreting cells/million PBMC (Fig. 3D), with higher values tending to occur in patients with AFRS (data not shown). Further analysis of the patient data indicated that most Th2-associated disease patients reacted to only one or two fungi of the nine tested against, but two patients with AFRS reacted against most fungal antigens (Fig. 3E), demonstrating that pan-fungal reactivity was the exception rather than the rule in our patient sample. ELISpot reactivity was found against the standard nine antigen panel, with the highest rate of reactivity observed against A. fumigatus (47%) and the least against C. albicans (9%; Fig. 3F).
Figure 3.
Fungus-specific PBMC IL-4 responses occur in the majority of Th2-associated disease patients. (A-C) PBMC isolated from the indicated subject populations were cultured in media alone or supplemented with 5 mg of antigen derived from nine fungi and IL-4 secretion by ELISpot analysis was performed 24 hours later. A positive reaction to any antigen was >50 IL-4-secreting cells/106 PBMC above media control. Th2-associated airway disease patients have either CRS with nasal polyps or asthma or both D) Maximal fungus specific IL-4 ELISpot responses comparing Th2-associated and non-associated disease subjects. E) Distribution of the number of distinct fungi inducing positive IL-4 ELISpot responses in subjects with allergic airway disease. F) Percentage of Th2-associated disease patients reacting to the indicated fungal allergens. * p<0.05, ** p<0.01, *** p<0.001. §Control is a subset of all non-asthmatics.
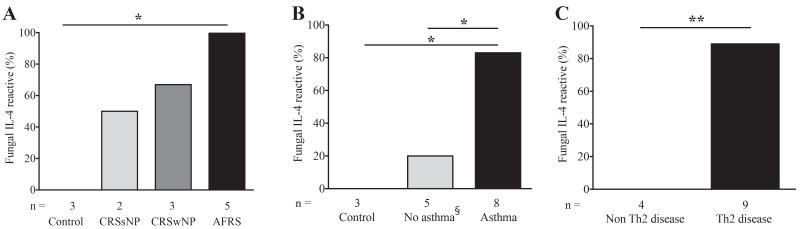
These findings indicated that fungal antigen-specific IL-4 secretion from PBMC is strongly linked to the presence of concurrent Th2-associated airway disease, but the relevance of this observation is potentially limited because the fungi eliciting IL-4 responses and those cultured from the airway often differed. To further refine these observations, we next determined if IL-4 memory responses also correlated with the presence of Th2-associated airway disease when the fungal antigen was matched specifically to the fungus cultured from the patient’s airway. In nine additional patients (Fig. 4A-C and Supplementary Table S6), we found a much higher correlation (89%) between fungus-specific IL-4 responses and Th2-associated disease status compared to similar patients tested against the panel of nine fungal allergens (Fig. 3 and Supplementary Table S5). Again, however, none of the four patients without Th2-associated airway disease reacted positively in the ELISpot assay to the fungus grown from their airway fluids (p<0.01; Fig. 4C). Thus, a positive IL-4 ELISpot response to a concomitantly isolated airway fungus was especially strongly associated with the presence of Th2-associated airway disease. A similarly strong association was seen with AFRS patients in which fungus specific IgE was matched to the actual fungus cultured (Figure S1D).
Figure 4.
Fungal-specific IL-4 responses are highly concordant with the species isolated. (A-C) IL-4 ELISpot data were determined using antigens matched to fungi derived from the same patient and were otherwise collected as in Figure 3 * p<0.05, ** p<0.01, *** p<0.001. §Control is a subset of all non-asthmatics.
DISCUSSION
Using a sterile, surgical approach in patients presenting for medically necessary sinus surgery, we have shown that patients with chronic Th2 associated airway disease carry a much higher airway fungal burden relative to patients free of Th2 associated airway disease as assessed by the higher probability of isolating fungi from the sinuses and lower respiratory tract. Our studies further revealed the presence of mature fungal elements, especially hyphae, from sinus lavage samples in the vast majority of patients with Th2 associated airway diseases, indicating fungal growth within the sinuses. Moreover, IL-4- and IgE-specific reactivity to airway fungi were detected in the majority of patients with Th2-associated chronic airway diseases, but in none of our non-Th2-associated disease patients. Combined with complementary prior observations, these findings provide robust evidence that environmental fungi directly contribute to the pathogenesis of chronic Th2-associated airway diseases.
Asthma symptoms worsen after enhanced exposure to aerosolized fungal spores20, an event especially seen in communities affected by environmental disturbances such as thunderstorms and hurricanes that favor spore dispersal21-24. Indoor mold exposure and spore counts are further linked to asthma severity25, and a higher prevalence of fungal sensitivity is associated with increased hospitalizations and intensive care unit admissions for acute asthma26. A new asthma phenotype termed severe asthma with fungal sensitization (SAFS) describes the relationship between fungal sensitivity and asthma severity. Based on a meta-analysis limited to Aspergillus sensitization, the prevalence of SAFS is estimated at 30% or greater9. In our study, sensitization to a much broader range of fungi in the asthmatic subgroup based on fungus-specific IL-4 responses was markedly higher at 75%, suggesting that many fungi can contribute to the expression of Th2-associated airway disease.
This observation is further supported by studies from animal systems. Both Aspergillus and Alternaria spp. introduced repeatedly intranasally incite increased goblet cell hyperplasia, mucus production and enhanced IL-4 secretion consistent with Th2 cell-dependent sinus inflammation in mice27,28. Similarly, we have previously shown that hallmarks of asthma including airway hyperreactivity, airway eosinophil infiltration, and goblet cell metaplasia can be induced in mice challenged repeatedly intranasally with diverse fungi, including many of those identified in our patients13,14. We and others have further demonstrated that many of the cellular and molecular components of Th2-associated inflammation, including eosinophils, Th2 cells themselves, IL-5, and IL-13, have fungistatic activity13,14,29. Persistent, moderately high-level exposures to airway fungi lead to asthma-like airway disease even in normal mice through airway infection14. Consequently, chronic Th2-associated inflammatory responses of the airway may represent complex anti-fungal immune responses that are directed at airway fungi to preclude epithelial invasion and potentially lethal dissemination30.
Despite evidence of a direct role of fungi in Th2-associated diseases, efforts to eradicate airway fungi in both asthma and chronic rhinosinusitis have not yielded consistent findings. In a recent meta-analysis including 6 randomized controlled trials in CRS patients, evidence was against the effectiveness of either topical or systemic antifungal therapy31. However, in a select subgroup of AFRS patients, oral itraconazole and ketoconazole had a more positive response, although most of these studies were uncontrolled32. Oral itraconazole yielded positive effects in a subset of asthmatic patients with fungal sensitivity10 and the majority of asthma or ABPA patients that cannot tolerate itraconazole appear to benefit from either posaconazole or voriconazole11. In contrast, findings with voriconazole could not be replicated in a prospective study33. Although the reasons for the inconsistent performance of anti-fungal antibiotics are not entirely clear, negative studies with voriconazole have been used to argue that positive results with other antifungal antibiotics, especially itraconazole, are due to their ability to promote corticosteroid activity. However, given the very strong link between airway fungi and Th2-associated allergic airway diseases as shown here, we believe that a reduction in airway fungal burden achieved in some, but perhaps not all studies, more likely accounts for the findings. Our study therefore warrants future clinical studies that dissect the relative importance of steroid promoting versus anti-fungal effects of anti-fungal antibiotics in Th2-associated diseases.
There are several limitations to our study. The patients studied were all from a single enrollment site and all had complex and severe disease that may not be representative of asthma or CRS in the general population. Specifically, the increased fungal burdens and enhanced type-2 immunity observed in these subjects suggests that modest defects in anti-fungal immunity might drive these more severe phenotypes. For clarity, we limited our microbial evaluation to fungi, but our findings do not rule out the potentially important contributions of bacteria and viruses to subject phenotypes34,35. In order to assess IgE responses against fungal strains that may not be available through commercial testing (ImmunoCAP) we used our standard laboratory IgE assay. As this assay (lower limit of detection 6kIU/L) is less sensitive than the ImmunoCAP assay (lower limit of detection ~0.35kIU/L), it is possible that the IL-4 ELISpot assay offers sensitivity that is comparable to the ImmunoCAP assay in detecting fungal sensitization. Direct comparison of the ImmunoCAP and ELISpot methods is warranted as part of future studies. Finally, our study did not address the numerous environmental, genetic, and anatomic factors that potentially contribute to Th2-associated disease expression.
In summary, our findings demonstrate that culturable filamentous fungi from sinus lavage samples, microscopic evidence of fungal growth, and fungus-specific IL-4 and IgE immune reactivity were defining features of the subset of patients with Th2-associated airway disease, suggesting that fungi contribute directly to the pathogenesis of these conditions. Especially in light of the ambiguous role of anti-fungal therapy in these conditions, our findings warrant additional, carefully conducted clinical studies to clarify the utility of airway fungal eradication in Th2-associated airway diseases.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Faramarz Ashoori for exceptional technical support; Ms. Luz Roberts and Drs. Seth Isaacs, Li-Xing Man, and Paul Lee for assistance with human specimen collection; and Drs. Frank Orson, Roger Rossen, and William Shearer for critical review of the manuscript.
Funding Declarations: Supported by the Papadopoulos Family Fund for Allergic Disease Research from the Biology of Inflammation Center (to D.B.C.), NIH grants U19AI070973 (to D.B.C. and F.K.)
Abbreviations
- CRS
Chronic Rhinosinusitis
- CRSwNP
Chronic Rhinosinusitis with Nasal Polyps
- CRSsNP
Chronic Rhinosinusitis without Nasal Polyps
- AFRS
Allergic Fungal Rhinosinusitis
- Th1
T helper cell type 1
- Th2
T helper cell type 2
- IL
Interleukin
- ELISA
Enzyme Linked Immunosorbent Assay
- ELISpot
Enzyme Linked Immunocell Spot Assay
- PBMC
Peripheral Blood Mononuclear Cells
- SEM
Standard Error of the Mean
- SAFS
Severe Asthma with Fungal Sensitization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707. doi: 10.1016/j.jaci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 3.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–32. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Fokkens WJ, Ebbens F, van Drunen CM. Fungus: a role in pathophysiology of chronic rhinosinusitis, disease modifier, a treatment target, or no role at all? Immunol Allergy Clin North Am. 2009;29:677–88. doi: 10.1016/j.iac.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Wood AJ, Antoszewska H, Fraser J, Douglas RG. Is chronic rhinosinusitis caused by persistent respiratory virus infection? Int Forum Allergy Rhinol. 2011;1:95–100. doi: 10.1002/alr.20030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staikuniene J, Vaitkus S, Japertiene LM, Ryskiene S. Association of chronic rhinosinusitis with nasal polyps and asthma: clinical and radiological features, allergy and inflammation markers. Medicina. 2008;44:257–65. [PubMed] [Google Scholar]
- 8.Bresciani M, Paradis L, Des Roches A, et al. Rhinosinusitis in severe asthma. J Allergy Clin Immunol. 2001;107:73–80. doi: 10.1067/mai.2001.111593. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R. Severe asthma with fungal sensitization. Curr Allergy Asthma Rep. 2011;11:403–13. doi: 10.1007/s11882-011-0217-4. [DOI] [PubMed] [Google Scholar]
- 10.Denning DW, O’Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–8. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 11.Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012;49:423–33. doi: 10.3109/02770903.2012.662568. [DOI] [PubMed] [Google Scholar]
- 12.Wark PAB, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol. 2003;111:952–7. doi: 10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]
- 13.Porter P,AF, Qian Y, et al. Necessary and sufficient role for T helper cells to prevent fungal dissemination during mucosal airway infection. Infect Immun. 2011;79:4459–71. doi: 10.1128/IAI.05209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter P, Susarla SC, Polikepahad S, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–17. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23:281–7. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 16.Millien VO, Lu W, Shaw J, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–6. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mak G, Porter PC, Bandi V, Kheradmand F, Corry DB. Tracheobronchial Mycosis in a Retrospective Case-Series Study of Five Status Asthmaticus Patients. Clin Immunol. 2012;146:73–83. doi: 10.1016/j.clim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holter W. Regulation of interleukin 4 production and of interleukin 4-producing cells. Int Arch Allergy Immunol. 1992;98:273–8. doi: 10.1159/000236198. [DOI] [PubMed] [Google Scholar]
- 19.Paul WE. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Found Symp. 1997;204:208–16. doi: 10.1002/9780470515280.ch14. [DOI] [PubMed] [Google Scholar]
- 20.Salvaggio J, Seabury J, Schoenhardt FA. New Orleans asthma. V. Relationship between Charity Hospital asthma admission rates, semiquantitative pollen and fungal spore counts, and total particulate aerometric sampling data. J Allergy Clin Immunol. 1971;48:96–114. doi: 10.1016/0091-6749(71)90091-1. [DOI] [PubMed] [Google Scholar]
- 21.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120:610–7. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Marks GB, Colquhoun JR, Girgis ST, et al. Thunderstorm outflows preceding epidemics of asthma during spring and summer. Thorax. 2001;56:468–71. doi: 10.1136/thorax.56.6.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dales RE, Cakmak S, Judek S, et al. The role of fungal spores in thunderstorm asthma. Chest. 2003;123:745–50. doi: 10.1378/chest.123.3.745. [DOI] [PubMed] [Google Scholar]
- 24.Rath B, Donato J, Duggan A, et al. Adverse health outcomes after Hurricane Katrina among children and adolescents with chronic conditions. J Health Care Poor Underserved. 2007;18:405–17. doi: 10.1353/hpu.2007.0043. [DOI] [PubMed] [Google Scholar]
- 25.Douwes J, Pearce N. Invited commentary: is indoor mold exposure a risk factor for asthma? Am J Epidemiol. 2003;158:203–6. doi: 10.1093/aje/kwg149. [DOI] [PubMed] [Google Scholar]
- 26.Menzies D, Holmes L, McCumesky G, Prys-Picard C, Niven R. Aspergillus sensitization is associated with airflow limitation and bronchiectasis in severe asthma. Allergy. 2011;66:679–85. doi: 10.1111/j.1398-9995.2010.02542.x. [DOI] [PubMed] [Google Scholar]
- 27.Khalid AN, Woodworth BA, Prince A, et al. Physiologic alterations in the murine model after nasal fungal antigenic exposure. Otolaryngol Head Neck Surg. 2008;139:695–701. doi: 10.1016/j.otohns.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Ahn BH, Park YH, Shin SH. Mouse model of Aspergillus and Alternaria induced rhinosinusitis. Auris, Nasus, Larynx. 2009;36:422–6. doi: 10.1016/j.anl.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Matsuwaki Y, Wada K, White TA, et al. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J Immunol. 2009;183:6708–16. doi: 10.4049/jimmunol.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter PC, Ongeri V, Luong A, Kheradmand F, Corry DB. Seeking common pathophysiology in asthma, atopy and sinusitis. Trends Immunol. 2011;32:43–9. doi: 10.1016/j.it.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacks PL, Harvey RJ, Rimmer J, Gallagher RM, Sacks R. Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis. Cochrane Database Syst Rev. 2011:8. doi: 10.1002/14651858.CD008263.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Thanasumpun T, Batra PS. Oral antifungal therapy for chronic rhinosinusitis and its subtypes: a systematic review. Int Forum Allergy Rhinol. 2011;1:382–9. doi: 10.1002/alr.20088. [DOI] [PubMed] [Google Scholar]
- 33.Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of Aspergillus fumigatus-associated asthma (EVITA3 study) J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 34.Osur SL. Viral respiratory infections in association with asthma and sinusitis: a review. Ann Allergy Asthma Immunol. 2002;89:553–60. doi: 10.1016/S1081-1206(10)62101-1. [DOI] [PubMed] [Google Scholar]
- 35.Pastacaldi C, Lewis P, Howarth P. Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy. 2011;66:549–55. doi: 10.1111/j.1398-9995.2010.02502.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.