Abstract
Brief periods of cardiac ischemia and reperfusion exert a protective effect against subsequent longer ischemic periods, a phenomenon coined ischemic preconditioning. Similar, repeated brief episodes of coronary occlusion and reperfusion at the onset of reperfusion, called post-conditioning, dramatically reduce infarct sizes. Interestingly, both effects can be achieved by the administration of any volatile anesthetic. In fact, cardio-protection by volatile anesthetics is an older phenomenon than ischemic pre- or post-conditioning. Although the mechanism through which anesthetics can mimic ischemic pre- or post-conditioning is still unknown, adenosine generation and signaling are the most redundant triggers in ischemic pre- or postconditioning. In fact, adenosine signaling has been implicated in isoflurane-mediated cardioprotection. Adenosine acts via four receptors designated as A1, A2a, A2b, and A3. Cardioprotection has been associated with all subtypes, although the role of each remains controversial. Much of the controversy stems from the abundance of receptor agonists and antagonists that are, in fact, capable of interacting with multiple receptor subtypes. Recently, more specific receptor agonists and new genetic animal models have become available paving way towards new discoveries. As such, the adenosine A2b receptor was shown to be the only 1 of the adenosine receptors whose cardiac expression is induced by ischemia in both mice and humans and whose function is implicated in ischemic pre- or post-conditioning. In the current review, we will focus on adenosine signaling in the context of anesthetic cardioprotection and will highlight new discoveries, which could lead to new therapeutic concepts to treat myocardial ischemia using anesthetic preconditioning.
Introduction
Ischemic heart disease is a major cause of mortality and heart failure in western countries [1]. The prevalence of cardiovascular disease significantly affects the outcome of both cardiac and non-cardiac surgery, and perioperative cardiac morbidity is one of the leading causes of death following anesthesia and surgery [2]. In addition, as the average age of the surgical population increases, anesthesiologists have to treat older patients with known or suspected ischemic heart disease in the perioperative period [3]. Thus, developing novel drugs or interventions to improve the clinical outcomes of patients with ischemic heart disease is an imminent medical need.
Because myocardial infarct size determines acute and long-term prognosis in patients with acute myocardial infarction (AMI), reducing the size of the infarct is the therapeutic goal. Early reperfusion can prevent the ischemia-induced myocardial damage and reduce infarct size. This concept was quickly introduced for patients with AMI by the use of primary percutaneous coronary intervention and thrombolytic therapy. Although reperfusion can salvage myocardium after sustained ischemia, the reperfusion itself paradoxically induces myocardial injury named “reperfusion injury”, which attenuates the benefits of myocardial reperfusion [4].
Over 20 years ago, Murry et al. first demonstrated that brief episodes of nonlethal ischemia and reperfusion before sustained ischemia reduce myocardial infarct size, and it was termed “ischemic preconditioning” [5]. The infarct-size limiting effects of ischemic preconditioning have been consistently confirmed in many species and different models of ischemia and reperfusion (IR) injury [6–8]. Analogously, brief episodes of nonlethal ischemia and reperfusion at the onset of reperfusion also reduce myocardial infarct size, known as “ischemic postconditioning”. The therapeutic goal of ischemic postconditioning is to attenuate “reperfusion injury” only.
After these landmark studies, extensive basic research has elucidated some underlying mechanisms of ischemic preconditioning or postconditioning and led -in part- to their translation into a clinical setting [9–13]. In addition, many studies have shown that volatile anesthetics, which are used every day during surgery, may precondition the myocardium against ischemia and infarction as well. This phenomenon was termed the anesthetic-induced preconditioning. The cellular signaling of anesthetic-induced preconditioning involves many protein kinases and signaling molecules such as adenosine [14–27]. However, detailed studies on the mechanisms of anesthetic-induced preconditioning are still missing and therefore from the 4 adenosine receptors only the cardiac adenosine receptor A1 has been investigated so far [28]. Based on recent studies highlighting the importance of the adenosine A2b receptor for cardiac ischemic preconditioning [29, 30] or postconditioning [31, 32], a role for anesthetic-induced preconditioning is intriguing. Here, we will review the potential mechanisms of cardiac anesthetic-induced preconditioning and its relation to adenosine signaling, a core component of cardiac preconditioning.
Cardioprotection from bench to bedside: Pre- and Post-conditioning of the heart
Ischemic preconditioning can reduce infarct size, lethal arrhythmia and contractile dysfunction. Originally, Murry et al. hypothesized that ATP preservation during ischemia is the major cardioprotective mechanism underlying ischemic preconditioning [5, 33, 34]. Mechanistic studies identified different signaling molecules such as adenosine, bradykinin, opioids, or kinase signaling pathways that activate protein kinases through their respective receptors. Although these findings are consistently observed in experimental models, applying ischemic preconditioning in the clinical setting is restricted to scheduled cardiac operation and elective percutaneous coronary intervention. A meta-analysis showed that ischemic preconditioning may provide additional myocardial protection when compared to cardioplegia alone. However, cardiovascular surgeons do not like to repeatedly clamp and unclamp the aorta in patients with advanced atherosclerosis, resulting in decreased usage of the technique.
In 2003, Zhao et al. demonstrated that brief episodes of coronary occlusion and reperfusion at the onset of reperfusion, following 60 minutes of coronary occlusion, reduced myocardial infarct size by 40% in canine hearts. The protocols for ischemic postconditioning have been extensively investigated and the cardioprotective effects afforded by ischemic postconditioning have been confirmed in many species, including humans. Unlike preconditioning, the experimental design of postconditioning theoretically allows direct application to the clinical settings, especially during percutaneous transluminal coronary angioplasty. In this case, inflation and deflation of the angioplasty balloon after reopening of the coronary artery can mimic repetitive coronary artery clamping performed in postconditioning animal models. Several clinical studies already suggest that postconditioning by coronary angioplasty protects the human heart during acute myocardial infarction [9–13].
Bacterial cell-wall-fragment induced cardioprotection
Since Murry's initial observation on cardiac preconditioning, it has been reported that a variety of agents such as anesthetics, ligands of adenosine receptors, as well as ligands of toll-like receptors (TLR), such as lipolysaccharide (LPS) or lipoteichoic acid (LTA), are able to mediate preconditioning-like effects [35–43]. LPS is a wall fragment of gram-negative bacteria. Upon recognition by its receptor TLR4, an intracellular signaling cascade is activated leading to a systemic inflammatory response. LPS has proven cardioprotective effects in different species and models of IR [35, 39, 40]. LTA, a wall fragment of gram-positive bacteria and recognized by TLR2, can also elicit preconditioning effects on the myocardium. In an in vivo model of myocardial IR, LTA-treated rats showed a reduction in infarct size, a decreased expression of adhesion molecules, and subsequently an attenuated recruitment of polymorphonuclear neutrophils (PMNs)[35], which are generally considered to be the principal effectors of reperfusion injury. In fact, TLR antagonists are currently under clinical development but will still need to be tested in a clinical setting of myocardial ischemia and reperfusion injury [44]. Interestingly, increasing evidence indicates that various endogenous ligands that act as 'danger signals', also called danger-associated molecular patterns (DAMPs), are released upon injury and have been shown to act via TLRs on leukocytes and parenchymal cells. From a therapeutic perspective, DAMPs are attractive targets owing to their specific induction after injury [45]. However, if anesthetic-induced cardioprotection could potentially activate these endogenous protective pathways will require future studies.
Anesthetic-induced cardioprotection
The administration of several anesthetics produces a preconditioning-like effect, protecting the myocardium from the effects of myocardial infarction and myocardial dysfunction. Interestingly, the potential cardiac protective effects of volatile anesthetics were already recognized before the introduction of the concept of anesthetic-induced preconditioning. Warltier et al. described a better recovery of myocardial function after a 15-min coronary artery occlusion when a volatile anesthetic was administered before the occlusion [46]. In dogs anesthetized with isoflurane or halothane, myocardial function returned to baseline levels within 5 h after the start of reperfusion, whereas awake dogs that received the same treatment without anesthesia still had a 50% decrease in myocardial function at the same time point. Subsequent studies showed that anesthetic preconditioning, (i.e., administration of a volatile anesthetic before the period of myocardial ischemia) resulted in a similar degree of cardioprotection as observed after ischemic preconditioning, both for functional recovery and for protection from ischemic damage to the heart [47] and lungs [48]. Beneficial effects on myocardial stunning have been described for all commercially available volatile anesthetics. However, the exact mechanisms of anesthetic-induced preconditioning need to be elucidated. And, even though clinical studies show some degree of protection [49], anesthetic-induced preconditioning in a clinical setting is not fully established yet.
The role of adenosine in anesthetic cardioprotection
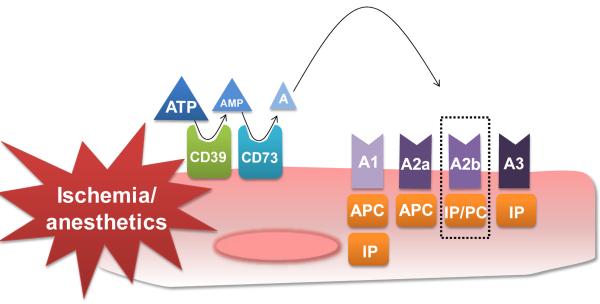
Studies directed at understanding extracellular metabolism of nucleotides in cell and tissue responses now suggest that a number of different cells can release ATP in an active manner, particularly when oxygen levels are limited [50, 51]. It is now accepted that the major pathway for extracellular hydrolysis of ATP/ADP or AMP is the ectonucleoside-triphosphatediphosphohydrolase 1 (ENTPDase 1/CD39) or Ecto-5'-nucleotidase (CD73) [52, 53] (see Figure 1). As a key enzyme, CD73 converts AMP into adenosine, which in turn can activate any of the four extracellular transmembrane adenosine receptors (ARs; A1AR, A2AAR, A2BAR, or A3AR) or can be internalized through equilibrative nucleoside transporters [54]. As indicated by several studies, the induction of CD73 and the A2b adenosine receptor (Adora2b) on endothelial cells leads to enhanced extracellular adenosine generation and signaling, thereby mediating barrier protection during conditions of limited oxygen availability [55–57]. Using a murine model of in situ myocardial ischemia and preconditioning [58, 59], we showed a pivotal role of extracellular adenosine generation via CD73 and signaling through the Adora2b in myocardial tissue protection [30]. In this study, we found that mice incapable of extracellular adenosine generation (Cd73−/− mice) show increased infarct sizes following ischemia and abolished cardioprotection by IP. As CD73 represents the key enzyme of extracellular adenosine generation, one would speculate that it might play an important role for anesthetic-induced preconditioning as well. In fact, in a very elegant study on the mechanisms of isoflurane preconditioning, the authors found a significant increase of CD73 activity after isoflurane preconditioning in patients undergoing elective CABG [60]. Moreover, several studies implicated adenosine receptor signaling in cardiac preconditioning through volatile anesthetics [28, 61, 62]. However, the exact role of the four adenosine receptors is not investigated yet. Based on recent findings on the importance of the Adora2b receptor for IP [29, 30, 63] or PC [32], systematic studies on the role of all four adenosine receptors in anesthetic-induced preconditioning are warranted (Figure 1).
Figure 1. Adenosine generation and signaling in cardioprotection.
Extracellular generation via CD39 (Apyrase) and CD73 (5'-Ectonucleotidase) lead to increased adenosine levels during conditions of limited oxygen availability such as hypoxia or ischemia. Signaling via all four adenosine receptors (A1, A2a, A2b, A3) has been associated with cardioprotection. The A2b (highlighted by dotted lines) is the only receptor that has been shown to be induced in mice and men during ischemia and being involved in pre- and postconditioning of the heart. The A1 is the only adenosine receptor investigated in anesthetic preconditioning so far. APC=anesthetic preconditioning, IP= ischemic preconditioning, PC= ischemic postconditioning.
New perspectives: The central role of the Adora2b in cardioprotection
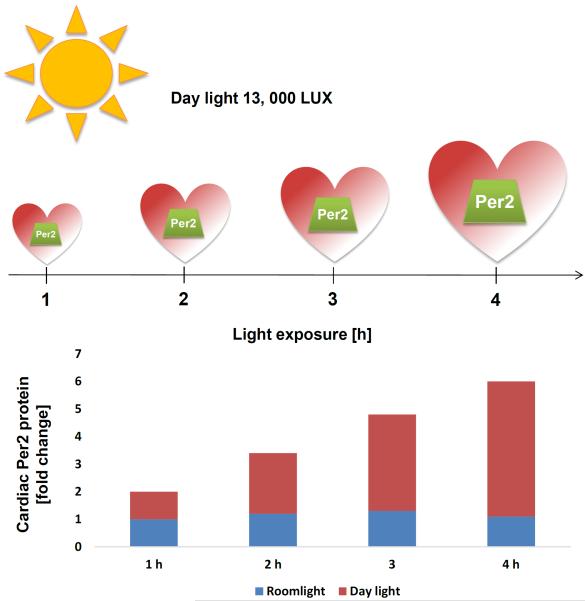
Despite it long being the focus of extensive studies, including its replication in humans, the fundamental mechanism of ischemic preconditioning remains unclear. So far, the Adora2b receptor is the only one of the four-adenosine receptors whose cardiac expression is induced by ischemia in both mice and humans and whose function is implicated in ischemic preconditioning and postconditioning. Moreover, when we compared all gene-targeted mice for the four ARs, we found that only in Adora2b−/− mice IP mediated cardioprotecion was completely abolished and Adora2b receptor agonist treatment (BAY 60–6583) recapitulated cardioprotection by ischemic preconditioning. Interestingly, following microarray studies in mice with deletion of Adora2b signaling pathways during in situ myocardial ischemia pointed us towards the circadian rhythm protein Period2 (Per2) [29]. Following up on these microarray studies, we observed that Per2−/− mice had larger infarct sizes compared to wild-type mice [29]. Metabolic in vivo studies with labeled tracers indicated a limited ability of Per2−/− mice to use carbohydrates for oxygen-efficient glycolysis during myocardial ischemia [29]. Consistent with the notion that Per2 is a light dependent protein, we found that intense light exposure (daylight, 13,000 LUX) was capable of increasing cardiac Per2 protein levels (Figure 2). Finally, exposing mice to intense light prior to the onset of ischemia provided induction of glycolytic enzymes and robust cardioprotection from myocardial ischemia. As Adora2b signaling increases Per2 expression by altering both Per2 transcription and Per2 protein stability, Per2 seems to be a strong candidate for adenosine elicited cardioprotection. Based on the similar mechanisms between ischemic preconditioning, ischemic postconditioning or anesthetic-induced preconditioning, the involvement of Adora2b induced circadian rhythm pathways in anesthetic-induced cardioprotection is an appealing thought, which will require future studies (Figure 3).
Figure 2. Light-elicited cardiac Period 2 stabilization.
A hallmark of the mammalian circadian pacemaker is its ability to be synchronized by light, thus allowing organisms to adapt to temporal variations of natural light conditions. Photic stimuli are transmitted from the retina to target neurons in the brain, where they are transduced to the molecular clockwork. Light activation of melanopsin receptors in the retinal ganglion cells lead to the transcriptional induction of Period 2 (Per2) and concomitant synchronization. Peripheral tissues display oscillations in Per2 expression similar to those of the brain, probably through secreted signaling molecules. Following exposure to 12 h of darkness, wildtype mice were exposed to indicated times of daylight (13,000 lux) and compared to controls that were maintained at room light (200 lux). Analysis of cardiac Per2 protein levels revealed a significant protein increase - in a time dependent manner - following daylight exposure when compared to room light.
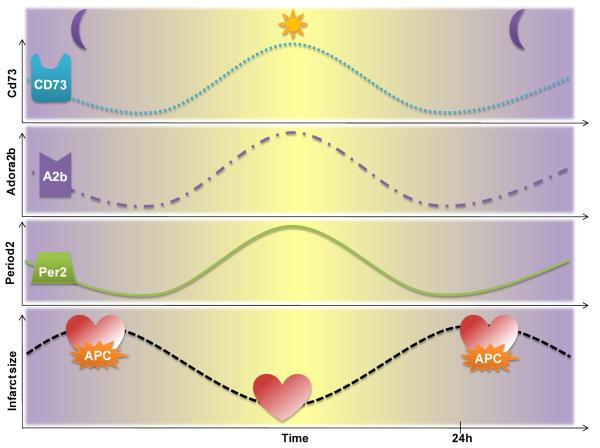
Figure 3. Diurnal variations of adenosine mediated cardioprotection.
CD73, the key enzyme of adenosine generation, the adenosine receptor A2b (Adora2b), and the Adora2b dependent circadian rhythm protein Period 2 (Per2) and infarct sizes follow a circadian pattern over a 24 h time period. Infarct sizes are lowest when Adora2b or Per2 protein levels are highest in the heart. Based on these findings anesthetic preconditioning could be most effective during time points where the heart is especially vulnerable to ischemia. APC=anesthetic preconditioning.
The impact of circadian rhythmes on infarct size
In 1985, a multicenter analysis of the limitations of infarct size observed circadian periodicity in the time of onset of the clinical manifestations of acute myocardial infarction, with a peak incidence between 6AM and noon. This increased morning incidence has been confirmed repeatedly in individual populations and in a meta-analysis of more than 60,000 patients. Other studies reported an increased incidence during early morning hours in unstable angina, for sudden death, stroke, ventricular arrhythmias, cardiogenic shock, aortic aneurysm rupture, stent thrombosis, and transient myocardial ischemia [64]. In fact, two recent studies even found that patients have larger infarct sizes in the early morning hours compared to other times of the day [65–67].
As cardiac metabolism is critical for the heart to adapt to ischemia, circadian variations in metabolism could explain the above findings. In order to adapt its metabolism, the cardiomyocyte has a number of important adaptive transcription factor pathways controlling cardiac energy metabolism, including the Hypoxia inducible factor-1 α (Hif1α) pathway [63, 68]. Hif1α is an important mediator of anaerobic glycolysis in mammalian cells. In our recent studies, Per2 stabilization was associated with the induction of Hif1α dependent anaerobic glycolysis, where hearts from Per2−/− mice were unable to produce lactate during ischemia. This `lactate deficiency' led to dramatically increased infarct sizes when compared to wildtype controls. Moreover, as Per2 but also Adora2b expression in the heart follows a circadian kinetic, infarct sizes were found to be time-of-day dependent [29] as shown in Figure 3. As myocardial ischemia seems to be worse at certain time points of the day in mice and men, therapy should also be adapted to the time-of-day. In addition, time-of-day may need to be considered when conducting future studies on ischemic pre-, postconditioning or anesthetic-induced preconditioning in a clinical setting (Figure 3).
Chronotherapeutics: Volatile anesthetics time of day dependent ?
Circadian rhythmicity of cardiac metabolism and susceptibility to ischemia suggests that the outcome of experiments at the bench, or pharmacotherapy and interventions in a clinical setting are time-of-day dependent. In fact, chronotherapeutics has already proven to be promising for improving the therapeutic index of several drugs [69]. Whether or not this could be true for drugs like adenosine A2b receptor agonists, Hif1a activators or interventions like anesthetic-induced preconditioning needs to be elucidated yet. As many studies in the past did not consider the time-of-day, this might explain why many interventions, found at the bench, did not find their way into clinical practice. The findings on a circadian adenosine-Per2-Hif1a axis [29], as endogenous cardioprotective mechanism strongly support this idea. Moreover, not only might anesthetic-induced preconditioning be time-of-day dependent, but also interventions such as heart surgery or angioplasty could have different outcomes when performed at specific times of the day.
Summary
Anesthetic-induced preconditioning of the heart represents an interesting and promising therapeutic strategy in a perioperative setting. While many mechanisms have been elucidated in the past, many detailed studies on mechanism are still missing. Adenosine signaling and circadian rhythms as important mechanisms in ischemic preconditioning could play an important role for the mechanism of anesthetic-induced preconditioning mediated cardioprotection. Time-of-day application of interventions such as anesthetic-induced preconditioning could improve their therapeutic index but this will require future studies.
Acknowledgments
Source of financial support for the work: National Heart, Lung, and Blood Institute (NIH-NHLBI) Grant 1K08HL102267-01 to T.E.
References
- [1].Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, on behalf of the American Heart Association Statistics C, Stroke Statistics S Heart Disease and Stroke Statistics--2013 Update: A Report From the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mangano DT, Browner WS, Hollenberg M, London MJ, Tubau JF, Tateo IM. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med. 1990;323:1781–8. doi: 10.1056/NEJM199012273232601. [DOI] [PubMed] [Google Scholar]
- [3].De Hert SG. Cardioprotection in anesthesia. Minerva Anestesiol. 2008;74:259–70. [PubMed] [Google Scholar]
- [4].Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T. Myocardial Ischemia Reperfusion Injury: From Basic Science to Clinical Bedside. Semin Cardiothorac Vasc Anesth. 2012 doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- [6].Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y. Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis. 2004;172:201–10. doi: 10.1016/S0021-9150(03)00238-7. [DOI] [PubMed] [Google Scholar]
- [7].Alsaddique AA. Ischemic preconditioning: the endogenous power--a review of the literature. Angiology. 2000;51:355–60. doi: 10.1177/000331970005100501. [DOI] [PubMed] [Google Scholar]
- [8].Reimer KA, Jennings RB. Ischemic preconditioning: a brief review. Basic Res Cardiol. 1996;91:1–4. doi: 10.1007/BF00788850. [DOI] [PubMed] [Google Scholar]
- [9].Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, Cung TT, Bonnefoy E, Angoulvant D, Aupetit JF, Finet G, Andre-Fouet X, Macia JC, Raczka F, Rossi R, Itti R, Kirkorian G, Derumeaux G, Ovize M. Long-term benefit of postconditioning. Circulation. 2008;117:1037–44. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- [10].Xue F, Yang X, Zhang B, Zhao C, Song J, Jiang T, Jiang W. Postconditioning the human heart in percutaneous coronary intervention. Clin Cardiol. 2010;33:439–44. doi: 10.1002/clc.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jin ZX, Zhou JJ, Xin M, Peng DR, Wang XM, Bi SH, Wei XF, Yi DH. Postconditioning the human heart with adenosine in heart valve replacement surgery. Ann Thorac Surg. 2007;83:2066–72. doi: 10.1016/j.athoracsur.2006.12.031. [DOI] [PubMed] [Google Scholar]
- [12].Darling CE, Solari PB, Smith CS, Furman MI, Przyklenk K. 'Postconditioning' the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res Cardiol. 2007;102:274–8. doi: 10.1007/s00395-007-0643-6. [DOI] [PubMed] [Google Scholar]
- [13].Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–8. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- [14].Zhou C, Liu Y, Yao Y, Zhou S, Fang N, Wang W, Li L. beta-Blockers and Volatile Anesthetics May Attenuate Cardioprotection by Remote Preconditioning in Adult Cardiac Surgery: A Meta-analysis of 15 Randomized Trials. J Cardiothorac Vasc Anesth. doi: 10.1053/j.jvca.2012.09.028. [DOI] [PubMed] [Google Scholar]
- [15].Van Allen NR, Krafft PR, Leitzke AS, Applegate Ii RL, Tang J, Zhang JH. The role of Volatile Anesthetics in Cardioprotection: a systematic review. Med Gas Res. 2:22. doi: 10.1186/2045-9912-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Hert SG. Cardioprotection by volatile anesthetics: what about noncardiac surgery? J Cardiothorac Vasc Anesth. 25:899–901. doi: 10.1053/j.jvca.2011.08.004. [DOI] [PubMed] [Google Scholar]
- [17].Jovic M, Krivokapic B, Babic M, Nezic D, Dukanovic B, Stevanovic P. Prospects of cardioprotection by volatile anesthetics. Med Pregl. 63:393–8. doi: 10.2298/mpns1006393j. [DOI] [PubMed] [Google Scholar]
- [18].Pagel PS. Cardioprotection by volatile anesthetics: established scientific principle or lingering clinical uncertainty? J Cardiothorac Vasc Anesth. 2009;23:589–93. doi: 10.1053/j.jvca.2009.07.001. [DOI] [PubMed] [Google Scholar]
- [19].Lorsomradee S, Cromheecke S, De Hert SG. Cardioprotection with volatile anesthetics in cardiac surgery. Asian Cardiovasc Thorac Ann. 2008;16:256–64. doi: 10.1177/021849230801600319. [DOI] [PubMed] [Google Scholar]
- [20].Landoni G, Fochi O, Zangrillo A. Cardioprotection by volatile anesthetics in noncardiac surgery? No, not yet at least. J Am Coll Cardiol. 2008;51:1321. doi: 10.1016/j.jacc.2007.12.020. author reply 1321-2. [DOI] [PubMed] [Google Scholar]
- [21].De Hert SG. Cardioprotection with volatile anesthetics: clinical relevance. Curr Opin Anaesthesiol. 2004;17:57–62. doi: 10.1097/00001503-200402000-00009. [DOI] [PubMed] [Google Scholar]
- [22].Pratt PF, Jr., Wang C, Weihrauch D, Bienengraeber MW, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics: new applications for old drugs? Curr Opin Anaesthesiol. 2006;19:397–403. doi: 10.1097/01.aco.0000236139.31099.b5. [DOI] [PubMed] [Google Scholar]
- [23].Kalenka A, Maurer MH, Feldmann RE, Kuschinsky W, Waschke KF. Volatile anesthetics evoke prolonged changes in the proteome of the left ventricule myocardium: defining a molecular basis of cardioprotection? Acta Anaesthesiol Scand. 2006;50:414–27. doi: 10.1111/j.1399-6576.2006.00984.x. [DOI] [PubMed] [Google Scholar]
- [24].Bienengraeber MW, Weihrauch D, Kersten JR, Pagel PS, Warltier DC. Cardioprotection by volatile anesthetics. Vascul Pharmacol. 2005;42:243–52. doi: 10.1016/j.vph.2005.02.005. [DOI] [PubMed] [Google Scholar]
- [25].De Hert SG, Turani F, Mathur S, Stowe DF. Cardioprotection with volatile anesthetics: mechanisms and clinical implications. Anesth Analg. 2005;100:1584–93. doi: 10.1213/01.ANE.0000153483.61170.0C. [DOI] [PubMed] [Google Scholar]
- [26].Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707–21. doi: 10.1097/00000542-200403000-00035. [DOI] [PubMed] [Google Scholar]
- [27].Patel HH, Ludwig LM, Fryer RM, Hsu AK, Warltier DC, Gross GJ. Delta opioid agonists and volatile anesthetics facilitate cardioprotection via potentiation of K(ATP) channel opening. FASEB J. 2002;16:1468–70. doi: 10.1096/fj.02-0170fje. [DOI] [PubMed] [Google Scholar]
- [28].Kersten JR, Orth KG, Pagel PS, Mei DA, Gross GJ, Warltier DC. Role of adenosine in isoflurane-induced cardioprotection. Anesthesiology. 1997;86:1128–39. doi: 10.1097/00000542-199705000-00017. [DOI] [PubMed] [Google Scholar]
- [29].Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18:774–82. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5'-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- [31].Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol. 2007;43:262–71. doi: 10.1016/j.yjmcc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006;70:308–14. doi: 10.1016/j.cardiores.2006.02.014. [DOI] [PubMed] [Google Scholar]
- [33].Reimer KA, Murry CE, Jennings RB. Cardiac adaptation to ischemia. Ischemic preconditioning increases myocardial tolerance to subsequent ischemic episodes. Circulation. 1990;82:2266–8. doi: 10.1161/01.cir.82.6.2266. [DOI] [PubMed] [Google Scholar]
- [34].Jennings RB, Murry CE, Reimer KA. Preconditioning myocardium with ischemia. Cardiovasc Drugs Ther. 1991;5:933–8. doi: 10.1007/BF00053555. [DOI] [PubMed] [Google Scholar]
- [35].Schober P, Oprea G, Mersmann J, Nebert A, Zacharowski K, Zacharowski PA. Lipoteichoic acid induces delayed myocardial protection in isolated rat hearts: a comparison with endotoxin. Resuscitation. 2008;79:311–5. doi: 10.1016/j.resuscitation.2008.06.001. [DOI] [PubMed] [Google Scholar]
- [36].Koch A, Boehm O, Zacharowski PA, Loer SA, Weimann J, Rensing H, Foster SJ, Schmidt R, Berkels R, Reingruber S, Zacharowski K. Inducible nitric oxide synthase and heme oxygenase-1 in the lung during lipopolysaccharide tolerance and cross tolerance. Crit Care Med. 2007;35:2775–84. doi: 10.1097/01.CCM.0000288122.24212.40. [DOI] [PubMed] [Google Scholar]
- [37].Kanczkowski W, Morawietz H, Ziegler CG, Funk RH, Schmitz G, Zacharowski K, Mohn CE, Ehrhart-Bornstein M, Bornstein SR. Pam3CSK4 and LTA-TLRs ligands associated with microdomains induce IL8 production in human adrenocortical cancer cells. Horm Metab Res. 2007;39:457–60. doi: 10.1055/s-2007-980189. [DOI] [PubMed] [Google Scholar]
- [38].Bornstein SR, Zacharowski P, Schumann RR, Barthel A, Tran N, Papewalis C, Rettori V, McCann SM, Schulze-Osthoff K, Scherbaum WA, Tarnow J, Zacharowski K. Impaired adrenal stress response in Toll-like receptor 2-deficient mice. Proc Natl Acad Sci U S A. 2004;101:16695–700. doi: 10.1073/pnas.0407550101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chatterjee PK, Zacharowski K, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. Lipoteichoic acid from Staphylococcus aureus reduces renal ischemia/reperfusion injury. Kidney Int. 2002;62:1249–63. doi: 10.1111/j.1523-1755.2002.kid580.x. [DOI] [PubMed] [Google Scholar]
- [40].Zacharowski K, Chatterjee PK, Thiemermann C. Delayed preconditioning induced by lipoteichoic acid from B. subtilis and S. aureus is not blocked by administration of 5-hydroxydecanoate. Shock. 2002;17:19–22. doi: 10.1097/00024382-200201000-00004. [DOI] [PubMed] [Google Scholar]
- [41].Zacharowski K, Berkels R, Olbrich A, Chatterjee PK, Cuzzocrea S, Foster SJ, Thiemermann C. The selective guanylate cyclase inhibitor ODQ reduces multiple organ injury in rodent models of Gram-positive and Gram-negative shock. Crit Care Med. 2001;29:1599–608. doi: 10.1097/00003246-200108000-00017. [DOI] [PubMed] [Google Scholar]
- [42].Zacharowski K, Olbrich A, Cuzzocrea S, Foster SJ, Thiemermann C. Membrane-permeable radical scavenger, tempol, reduces multiple organ injury in a rodent model of gram-positive shock. Crit Care Med. 2000;28:1953–61. doi: 10.1097/00003246-200006000-00044. [DOI] [PubMed] [Google Scholar]
- [43].Zacharowski K, Frank S, Otto M, Chatterjee PK, Cuzzocrea S, Hafner G, Pfeilschifter J, Thiemermann C. Lipoteichoic acid induces delayed protection in the rat heart: A comparison with endotoxin. Arterioscler Thromb Vasc Biol. 2000;20:1521–8. doi: 10.1161/01.atv.20.6.1521. [DOI] [PubMed] [Google Scholar]
- [44].Kats S, Schonberger JP, Brands R, Seinen W, van Oeveren W. Endotoxin release in cardiac surgery with cardiopulmonary bypass: pathophysiology and possible therapeutic strategies. An update. Eur J Cardiothorac Surg. 2011;39:451–8. doi: 10.1016/j.ejcts.2010.06.011. [DOI] [PubMed] [Google Scholar]
- [45].Eltzschig HK, Eckle T. Ischemia and Reperfusion - From Mechanism to Translation. Nat Med. 2011 doi: 10.1038/nm.2507. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Warltier DC, al-Wathiqui MH, Kampine JP, Schmeling WT. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology. 1988;69:552–65. doi: 10.1097/00000542-198810000-00016. [DOI] [PubMed] [Google Scholar]
- [47].Kersten JR, Brayer AP, Pagel PS, Tessmer JP, Warltier DC. Perfusion of ischemic myocardium during anesthesia with sevoflurane. Anesthesiology. 1994;81:995–1004. doi: 10.1097/00000542-199410000-00027. [DOI] [PubMed] [Google Scholar]
- [48].Liu R, Ishibe Y, Ueda M. Isoflurane-sevoflurane adminstration before ischemia attenuates ischemia-reperfusion-induced injury in isolated rat lungs. Anesthesiology. 2000;92:833–40. doi: 10.1097/00000542-200003000-00027. [DOI] [PubMed] [Google Scholar]
- [49].Landoni G, Fochi O, Bignami E, Calabro MG, D'Arpa MC, Moizo E, Mizzi A, Pappalardo F, Morelli A, Zangrillo A. Cardiac protection by volatile anesthetics in non-cardiac surgery? A meta-analysis of randomized controlled studies on clinically relevant endpoints. HSR Proc Intensive Care Cardiovasc Anesth. 2009;1:34–43. [PMC free article] [PubMed] [Google Scholar]
- [50].Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103–7. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Weissmuller T, Campbell EL, Rosenberger P, Scully M, Beck PL, Furuta GT, Colgan SP. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J Clin Invest. 2008;118:3682–92. doi: 10.1172/JCI35874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5'-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5'-nucleotidase (CD73) Purinergic Signal. 2006;2:351–60. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–96. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–35. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lennon PF, Taylor CT, Stahl GL, Colgan SP. Neutrophil-derived 5'-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J Exp Med. 1998;188:1433–43. doi: 10.1084/jem.188.8.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4:e6784. doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–40. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]
- [60].Belhomme D, Peynet J, Louzy M, Launay JM, Kitakaze M, Menasche P. Evidence for preconditioning by isoflurane in coronary artery bypass graft surgery. Circulation. 1999;100:II340–4. doi: 10.1161/01.cir.100.suppl_2.ii-340. [DOI] [PubMed] [Google Scholar]
- [61].Roscoe AK, Christensen JD, Lynch C., 3rd Isoflurane, but not halothane, induces protection of human myocardium via adenosine A1 receptors and adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2000;92:1692–701. doi: 10.1097/00000542-200006000-00029. [DOI] [PubMed] [Google Scholar]
- [62].Cope DK, Impastato WK, Cohen MV, Downey JM. Volatile anesthetics protect the ischemic rabbit myocardium from infarction. Anesthesiology. 1997;86:699–709. doi: 10.1097/00000542-199703000-00023. [DOI] [PubMed] [Google Scholar]
- [63].Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- [64].Braunwald E. On circadian variation of myocardial reperfusion injury. Circ Res. 2012;110:6–7. doi: 10.1161/CIRCRESAHA.111.260265. [DOI] [PubMed] [Google Scholar]
- [65].Reiter R, Swingen C, Moore L, Henry TD, Traverse JH. Circadian Dependence of Infarct Size and Left Ventricular Function After ST Elevation Myocardial Infarction. Circ Res. 2012;110:105–10. doi: 10.1161/CIRCRESAHA.111.254284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Suarez-Barrientos A, Lopez-Romero P, Vivas D, Castro-Ferreira F, Nunez-Gil I, Franco E, Ruiz-Mateos B, Garcia-Rubira JC, Fernandez-Ortiz A, Macaya C, Ibanez B. Circadian variations of infarct size in acute myocardial infarction. Heart. 2011 doi: 10.1136/hrt.2010.212621. [DOI] [PubMed] [Google Scholar]
- [67].Ibanez B, Suarez-Barrientos A, Lopez-Romero P. Circadian variations of infarct size in STEM1. Circ Res. 2012;110:e22. doi: 10.1161/CIRCRESAHA.111.262816. author reply e23. [DOI] [PubMed] [Google Scholar]
- [68].Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130–40. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- [69].Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–5. doi: 10.1038/nm0106-54. discussion 55. [DOI] [PubMed] [Google Scholar]