Abstract
Objective:
We evaluated the separate and interactive associations of menopausal stage, menopausal symptoms, and HIV infection on cognition. We hypothesized that HIV-infected, perimenopausal women would show the greatest cognitive difficulties and that menopausal symptoms would be inversely associated with cognition.
Methods:
This cross-sectional study included 708 HIV-infected and 278 HIV-uninfected, pre-, peri-, or postmenopausal women (64% African-American; median age 44 years) from the Women’s Interagency HIV Study. Participants completed tests of verbal learning and memory, attention/processing speed, and executive function. We administered a menopausal symptom questionnaire that assessed anxiety, vasomotor, and sleep symptoms and obtained measures of depressive symptoms.
Results:
In multivariable regression analyses controlling for relevant covariates, HIV infection, but not menopausal stage, was associated with worse performance on all cognitive measures (p’s<0.05). Depressive symptoms were associated with lower cognitive performance on measures of verbal learning and memory, attention, and executive function (p’s<0.05); anxiety symptoms were associated with lower performance on measures of verbal learning and memory (p’s<0.05). Vasomotor symptoms were associated with worse attention (p<0.05). HIV and anxiety symptoms interacted to influence verbal learning (p’s<0.05); elevated anxiety was associated with worse verbal learning in HIV-infected women only.
Conclusion:
Vasomotor, depressive, and anxiety symptoms, but not menopausal stage, were associated with worse cognitive performance in both HIV-infected and uninfected women, although elevated anxiety symptoms were associated with verbal learning deficits more in HIV-infected women. Since cognitive problems can interfere with everyday functioning including treatment adherence, it may be important to screen and treat anxiety in HIV-infected women.
Keywords: HIV, Verbal Learning, Menopause, Mood, Anxiety, African American
INTRODUCTION
Approximately 52% of persons living with HIV/AIDS in the United States are aged 40-54 years1; for women, this coincides with the menopausal transition. Despite a growing literature on normative changes in cognition, depression, and anxiety associated with the menopausal transition, little is known about menopause in HIV-infected women, particularly with regard to cognitive function. Previous cross-sectional studies indicate that compared to HIV-uninfected women, HIV-infected women report more menopausal symptoms including vasomotor, sleep disturbances, and psychological symptoms2-5 and may have an earlier age at menopause3, 4, 6, 7. In the United States, smoking, low socioeconomic status, and drug use are relatively common among women with HIV; these are all factors associated with depression, anxiety, and vasomotor symptoms8, 9. No study has yet focused on the effects of menopausal stage and menopausal symptoms on cognition in HIV-infected women. Determining how reproductive aging might impact cognitive function in HIV is important because: 1) women comprise the majority of HIV-infected individuals globally10; 2) with HIV therapies, HIV-infected individuals now survive to middle and older age; 3) menopausal stage and symptoms impact cognition in healthy women11; 4) cognitive impairment is associated with specific functional deficits (e.g., non-adherence to treatment regimen) in HIV-infected individuals12; and 5) menopause-related cognitive issues might require new therapeutic approaches to HIV care.
Findings from large-scale longitudinal cohort studies in healthy women suggest that menopause-associated cognitive deficits are modest, limited to the perimenopausal stage, and rebound to normal in postmenopause. Specifically, perimenopausal women demonstrate greater cognitive impairment over time compared to premenopausal women11, 13, 14. For example in the Study of Women’s Health Across the Nation (SWAN), the largest ongoing multisite, community-based cohort study in the United States (n’s=1903-2362), perimenopausal but not postmenopausal, women demonstrated greater impairment in processing speed and verbal memory than premenopausal women11, 14. Cross-sectional studies have also been conducted, but have yielded disparate results possibly due to differences in menopausal staging criteria and differences in cognitive test batteries.
Two general mechanisms have been postulated to contribute to cognitive changes in the menopausal transition. First, cognitive performance may reflect alterations in the function of brain structures and circuits due to changes in sex steroid hormones. Both animal and human studies indicate that the hippocampus and prefrontal cortex, brain regions necessary for cognitive abilities such as verbal learning and memory, are influenced by estrogen15-17. Secondly, cognitive changes may be due to the indirect effects of menopausal symptoms including sleep disturbances, mood, anxiety, and/or vasomotor symptoms18, 19. Thus, the extent of cognitive changes during perimenopause may depend on symptom severity. Conversely, data from SWAN indicated that menopausal symptoms did not account for the finding that perimenopausal women were impaired on cognitive performance compared to premenopausal women11. In SWAN, cognition was associated with anxiety and depression but not with sleep disturbance or vasomotor symptoms.
The purpose of this study was to examine associations between cognition, menopausal stage, and menopausal symptoms including mood, anxiety, vasomotor, and sleep disturbances in HIV-infected and uninfected women from the WIHS. Our first hypothesis was that there would be greater cognitive difficulties on measures of processing speed and verbal memory in perimenopausal women compared to premenopausal women. Our second hypothesis was that severity of menopausal symptoms would be inversely associated with cognitive performance. Specifically, consistent with SWAN11, we predicted that depressive symptoms would be negatively associated with processing speed, whereas anxiety symptoms would be negatively associated with verbal memory. We also anticipated that sleep disturbances and vasomotor symptoms would be associated with worse overall cognitive performance, and the magnitude of these associations would be greater for HIV-infected compared to HIV-uninfected women.
METHODS
Study Population
All participants were enrolled in the WIHS, a national, multi-site study of women with and at-risk for HIV. Study methodology, standardized data collection, and training of interviewers have been previously reported20, 21. Briefly study visits are conducted every 6 months and include a detailed survey, brief physical exam and specimen collection. This study was approved by the IRB of all participating institutions and the WIHS Executive Committee with written informed consent provided by all participants. Analyses included WIHS participants who completed cognitive testing and survey measures of menopause and mood as part of their core assessment visits. Participants were enrolled at 6 sites (Chicago, Bronx, Brooklyn, Washington DC, San Francisco, Los Angeles) after institutional approval at each site, approval of the WIHS Executive Committee, and written informed consent provided by all participants. Study methodology, data collection, and training of interviewers have been previously reported20, 21.
Overall, 1901 women were assessed for some WIHS-related outcome and not necessarily for cognition during WIHS visit 25 (April 2007-April 2008). Of those women, 1329 met all of the following inclusionary criteria: a) valid and complete data on the Hopkins Verbal Learning Test-Revised (HVLT-R)(n=349 excluded); b) valid and complete data on menstrual cycle characteristics needed to stage menopause (n=254 excluded); c) valid and complete data on the SWAN menopausal symptom questionnaire (n=224 excluded); d) age 30 to 65 years (n=203 excluded); and e) valid and complete data on the Center for Epidemiologic Studies Depression Scale (CES-D)(n=22 excluded). Of the 1329 women meeting inclusion criteria (73% of the overall sample), 343 were excluded from the data analyses based on the following exclusionary criteria: a) primary language other than English (n=12); b) history of stroke/CVA (n=18); c) use of antipsychotic medication in the past 6 months (n=63); d) currently pregnant or breastfeeding (n=8); e) hysterectomy/bilateral oophorectomy (n=164); and/or f) use of hormone therapy/oral contraceptives within the past 6 months (n=117). In the “Results” section, we compare the characteristics of the women included in the analysis (n=986) and those who were excluded (n=912).
Measures
Neuropsychological Outcome Measures
Participants completed the HVLT-R (primary outcome measure) and the Comalli Stroop test. The HVLT-R is a 12-item list-learning test used to measure verbal episodic memory22, 23. The HVLT-R includes three learning trials, a delayed recall trial (20-25 minute delay), and a yes/no delayed recognition trial. Outcomes include total words recalled on Trial 1 (single trial learning), total words recalled across each of three learning trials (total learning), learning slope across the three trials (average number of new words recalled across trials), number of words recalled after a 25-minute delay (delayed recall), and number of words correctly identified on a yes/no recognition test (recognition). Recognition scores were calculated by subtracting the number of false positives (incorrectly responding ‘yes’ to a word not presented) from the number of hits (correctly responding ‘yes’ to a word that was presented). The Comalli Stroop Test includes three trials24 in which Trials 1 and 2 measure attention and processing speed; Trial 3 measures response inhibition/executive function. In Trial 1, participants name the colors of a series of squares; in Trial 2 they read a series of color names printed in black ink. In Trial 3, participants name the color of the ink but ignore the word (e.g., when shown the word “red” printed in blue ink, say “blue” rather than “red”)24. Completion times for all three trials were recorded. Outcomes included the average score on Trial 1 and 2 (r= 0.67, p<0.001) and Trial 3. All outcomes were right-skewed and therefore log-transformed. This was the first administration of the HVLT-R, the Stroop was administered previously to some participants (<1%: 0 exposures; 57%: 1 exposure; 40%: 2 exposures, 2%: 3 exposures).
Primary Predictors
Menopausal Stage
Questions pertaining to menopausal stage were administered during the interview. Responses were used to classify menopausal stage according to definitions used in the SWAN enabling us to compare our findings with those from the SWAN25-28. Definitions of menopausal stage were as follows: premenopausal (menses in the past 3 months with no changes in regularity); early perimenopausal (menses in the past 3 months with change in regularity); late perimenopausal (no menses within the past 3 months but some menstrual bleeding within the past 12 months); and postmenopausal (no menses within the past 12 months).
Depression and Anxiety Symptoms
Anxiety symptoms were ascertained using a questionnaire from the SWAN11, 29-31. Participants reported the frequency of four anxiety symptoms (irritability/grouchiness, tense/nervous, pounding/racing heart, feeling fearful for no reason) during the past two weeks on a scale from 1 (“not at all”, coded 0) to 5 (“every day”, coded 4). The anxiety score was the sum of the four symptom ratings11, 29-32 and anxiety symptoms were categorized as elevated when scores were 7 or higher which is consistent with SWAN11. Items were considered elevated if the symptom was experienced on six or more days in the past two weeks. Depressive symptoms were assessed by the CES-D33, a 20-item self-report measure of depressive symptoms over the past week. Depressive symptoms were considered elevated when they exceeded the scale’s established clinical cutoff of ≥16.
Vasomotor Symptoms and Sleep Disturbances
The frequency of three types of vasomotor symptoms (hot flashes, night sweats, cold sweats) during the previous two weeks were recorded as not at all, 1-5 days, 6-8 days, 9-13 days, or daily. Each of the three types of vasomotor symptoms were considered elevated if they occurred 6 or more days per week11. Overall vasomotor symptoms were categorized as elevated when women reported experiencing one or more of the three types of vasomotor symptoms as elevated. Given the high prevalence of sleep disturbances in our population34, sleep disturbances were considered elevated if two or more of the following symptoms were reported for three or more nights per week: trouble falling asleep, waking up during the night, waking up early.
Covariates
Socio-demographic variables and risk factors for cognitive impairment were selected based on previous literature14, 35-43 and included annual household income ≤$12,000; positive hepatitis C virus (HCV) infection (indicated by the presence of serum antibody to HCV); recent self-reported use (within 6 months) of marijuana, crack, cocaine, and/or heroin; smoking; heavy alcohol use (>7 drinks/week or ≥4 drinks in one sitting)43; antidepressants; past hormone therapy use (>6 months ago); and study site (of 6). Age, years of education, and race/ethnicity were covariates in a normative-based regression approach on each cognitive outcome (described below). Additional HIV-related clinical variables were combination antiretroviral therapy (cART) use (no cART therapy, cART therapy+<95% adherent, cART therapy+≥95% adherent); CD4 count <200cells/mm3; HIV viral load >10,000cp/ml; CD4 nadir <200cells/mm3; and duration of ART use.
Statistical analysis
Differences between HIV-infected and uninfected women in demographic characteristics were examined using independent sample t-tests for continuous variables and chi-square tests for categorical variables. A series of multivariable logistic regressions were conducted to assess and validate the influence of menopausal stage on menopausal symptoms adjusting for confounders shown to impact menopausal symptoms in prior studies including HIV status; age; race/ethnicity; education; marijuana use; crack, cocaine, and/or heroin use; smoking; heavy alcohol use; antidepressants; past hormone therapy use; HCV infection; income; and study site. There were no interactions between HIV status and menopausal stage.
In the absence of published cognitive tests norms for low-income minority women, we followed Heaton and colleagues44 and prior work within the WIHS38 by using a regression approach to estimate expected levels of function for the total sample based on scores of the comparison group (HIV-women) by regressing age, years of education, race/ethnicity, and the Wide Range Achievement Test–Reading (WRAT-R), a measure of reading ability45 and proxy for educational quality46, on each cognitive outcome. Next, the resulting unstandardized beta weights, constants, and standard errors were used to calculate predicted scores for each test that were then subtracted from each woman’s actual score and transformed to more interpretable scores with means of 50 and standard deviations of 10 that had the advantage of being the same across all cognitive outcomes. In the total sample, we used multivariable regression analyses to examine the separate and interactive effects of our three independent variables of interest: HIV-infection group, menopausal stage, and menopausal symptoms (depression, anxiety, sleep, vasomotor). The model adjusted for confounders shown to affect cognition in prior studies including marijuana use; crack, cocaine, and/or heroin use; smoking; alcohol use; antidepressants; past hormone therapy use; HCV infection; income; and study site. We also adjusted for number of prior exposures to the Stroop, although they were not significantly associated with Stroop performance (p’s>0.14). Interactions were retained in the final model (p’s<0.10); only HIV status x anxiety was significant at p<0.05. For significant interactions, the final model was re-run for HIV-infected women and included additional HIV specific covariates (recent CD4 count and viral load, CD4 nadir, cART use and adherence). All p values are two sided at a p<0.05 level of significance; p’s>0.05 and <0.10were considered trends. Analyses were performed using SAS (version 9.2, SAS Institute Inc, Cary, NC).
RESULTS
Sample Characteristics
Recent illicit substance use was reported significantly more frequently by HIV-uninfected than HIV-infected women, as was recent smoking, heavy alcohol use, and use of antidepressant medications (Table 1). HIV-infected women were older, less likely to report being Hispanic, and more likely to be HCV positive. All other differences were not significant. At the time of the cognitive assessment, SWAN staging indicated that 56% (n=550) of participants were premenopausal, 16% (n=158) early perimenopausal, 5% (n=46) late perimenopausal, and 24% (n=232) postmenopausal. As expected based on age distribution, a larger proportion of HIV-uninfected women were premenopausal, whereas there was a larger percentage of HIV-infected postmenopausal women (p’s<0.05). The prevalence of sleep disturbances, depressive, anxiety, and vasomotor symptoms ranged between 9% and 35% (Table 2). There was a trend for HIV-infected women to report more sleep disturbances compared to HIV-uninfected women (p=0.07).
Table 1.
Background Characteristics of Study Sample by HIV Status and for Total Group.
| HIV Status |
|||
|---|---|---|---|
| Characteristics, n (%) | Infected n = 708 |
Uninfected n = 278 |
p-value |
| Background Characteristics | |||
| Age, M (SD) | 44.56 (7.35) | 42.80 (7.51) | <0.001 |
| WRAT-R, M (SD) | 91.60 (17.89) | 91.08 (17.20) | 0.68 |
| Years of Education, M (SD) | 12.39 (2.94) | 12.53 (2.96) | 0.50 |
| Race/Ethnicity | 0.04 | ||
| Black, non-Hispanic | 455 (64) | 173 (62) | |
| White, non-Hispanic | 108 (15) | 29 (11) | |
| Hispanic | 118 (17) | 65 (23) | |
| Other | 27 (4) | 11 (4) | |
| Site | <0.001 | ||
| Bronx | 120 (17) | 68 (24) | |
| Brooklyn | 179 (25) | 47 (17) | |
| Washington DC | 121 (17) | 44 (16) | |
| Los Angeles | 75 (11) | 47 (17) | |
| San Francisco | 100 (14) | 45 (16) | |
| Chicago | 113 (16) | 27 (10) | |
| Hepatitis C virus antibody (HCV) | 225 (32) | 61 (22) | 0.002 |
| Recent1 | |||
| Annual household income, ≤$12,000 | 343 (48) | 131 (47) | 0.71 |
| Crack, cocaine, and/or heroin use | 81 (11) | 42 (15) | 0.08 |
| Marijuana use | 113 (16) | 67 (24) | 0.005 |
| Smoking | 309 (44) | 153 (55) | 0.02 |
| Heavy alcohol use† | 106 (15) | 74 (26) | <0.001 |
| Antidepressant medication use | 112 (16) | 28 (10) | 0.02 |
| Past2 | |||
| Crack, cocaine, and/or heroin use | 351 (50) | 139 (50) | 0.44 |
| Marijuana use | 413 (58) | 152 (55) | 0.47 |
| Smoking | 207 (29) | 61 (22) | 0.55 |
| Hormone therapy use | 95 (13) | 25 (9) | 0.06 |
| HIV-related Clinical Characteristics | |||
| CD4 nadir (cells/μl), M (SD) | 233 (178) | - | - |
| CD4 Count (cells/μl) | - | - | |
| > 500 | 307 (43) | ||
| ≥ 200 and < 500 | 298 (42) | ||
| < 200 | 103 (15) | ||
| Viral Load (HIV RNA (cp/ml)) | - | - | |
| Undetectable | 358 (51) | ||
| < 10,000 | 228 (32) | ||
| ≥ 10,000 | 122 (17) | ||
| Medication Use | - | - | |
| No cART | 247 (34) | ||
| cART+ < 95% medication compliance | 122 (17) | ||
| cART+ ≥ 95% medication compliance | 339 (48) | ||
| ART duration‡ (years), M (SD) | 9.05 (3.02) | - | - |
Note.
“Recent” refers to within 6 months of the most recent WIHS visit;
“Past” refers to any previous use but not within the past 6 months; WRAT-R = Wide Range Achievement Test Standard Score;
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommended limits for women are no more than three drinks per day and no more than seven drinks per week. Heavy alcohol use refers to amounts in excess of these limits or >7 drinks per week or ≥ 4 drinks at a sitting; cART = combination antiretroviral therapy; ART = antiretroviral therapy;
Reflects the mean for 458 HIV-infected women (89%) who started ART before current study.
Table 2.
Menopausal Stage, Reported Symptoms, and Neuropsychological Test Scores as a Function of HIV Status.
| HIV Status |
|||||
|---|---|---|---|---|---|
| Characteristics | n | Infected n = 708 |
Uninfected n = 278 |
p-value | Cohen’s d |
| Menopausal stage, n (%) | 0.009 | ||||
| Premenopausal | 986 | 378 (54) | 172 (62) | ||
| Early Perimenopausal | 986 | 109 (15) | 49 (18) | ||
| Late Perimenopausal | 986 | 35 (5) | 11 (4) | ||
| Postmenopausal | 986 | 186 (26) | 46 (16) | ||
| Prevalence of elevated menopausal symptoms, n (%) | |||||
| Depression | 986 | 249 (35) | 95 (34) | 0.77 | |
| Anxiety | 986 | 66 (9) | 25 (9) | 0.87 | |
| Sleep disturbance | 986 | 203 (29) | 64 (23) | 0.07 | |
| Vasomotor | 986 | 127 (18) | 50 (18) | 0.99 | |
|
| |||||
| Cognitive test (mean, SD) | |||||
| HVLT-R | |||||
| Trial 1 | 986 | 5.53 (1.64) | 5.74 (1.73) | 0.08 | 0.12 |
| Total learning | 986 | 21.31 (5.03) | 22.27 (4.89) | 0.007 | 0.19 |
| Learning slope | 986 | 1.45 (0.34) | 1.52 (0.29) | 0.007 | 0.22 |
| Delayed recall | 986 | 7.41 (2.55) | 7.85 (2.38) | 0.01 | 0.18 |
| Recognition | 984 | 10.09 (1.98) | 10.47 (1.84) | 0.006 | 0.20 |
| Stroop Test (time in seconds) | |||||
| Trials 1&2a | 919 | 63.15 (16.48) | 60.48 (12.84) | 0.02 | 0.18 |
| Trial 3a | 866 | 131.14 (36.14) | 124.12 (29.50) | 0.01 | 0.21 |
Note. HVLT-R = Hopkins Verbal Learning Test.
Unadjusted means are displayed such that higher is worse, but log-transformed scores were used in the statistical comparisons. See methods section for operational definitions of menopausal stage and elevated symptoms. Stroop Trials 1 and 2 represent the average across the 2 trials. Cohen’s d effect sizes: small effect = 0.2; medium effect = 0.5; large effect = 0.8.
Compared with the 912 women excluded from this analysis (see “Study Population”), the 986 women included in the analysis were more likely to be older (44.1 vs. 41.2 years, p<0.001); to be more educated (12.4 vs. 11.4 years, p<0.001);to be black non-Hispanic (64% vs. 51%, p<0.001); to smoke (47% vs. 40%, p=0.002); to recently use crack, cocaine, and/or heroin (12% vs. 8%, p=0.002); to be HCV positive (29% vs. 24%, p=0.01); and were less likely to have an annual income ≤12,000 (48% vs 53%, p=0.02).
Validation of Mood, Sleep, and Anxiety as Menopausal Symptoms
In multivariable analyses, early perimenopausal women were more likely to report depressive and anxiety symptoms compared to premenopausal women (p’s<0.05; Table 4). Postmenopausal women were more likely to report sleep disturbances and depressive symptoms compared to premenopausal women (p’s<0.05).
Table 4.
Results from Adjusted Analyses on the Impact of Menopausal Status on Elevated Menopausal Symptoms.
| Menopausal Stage |
|||
|---|---|---|---|
| Symptoms | Early Peri (vs Pre) ORa (95% CIb) |
Late Peri (vs Pre) OR (95%CI) |
Post (vs Pre) OR (95%CI) |
| Depression | 1.84 (1.23-2.74)** | 0.97 (0.48-1.96) | 1.61 (1.01-2.57)* |
| Anxiety | 1.94 (1.01-3.71)* | 1.27 (0.45-3.57) | 1.60 (0.75-3.42) |
| Sleep disturbance | 1.24 (0.80-1.91) | 0.81 (0.38-1.74) | 1.83 (1.13-2.97)* |
Note.
***p<0.001;
p<0.01;
p < 0.05.
OR=odds ratio.
CI=confidence interval. Pre = Premenopause. Peri = Perimenopause. Post = Postmenopause. See methods section for operational definitions of menopausal stage and elevated symptoms. All analyses adjusted for: HIV status; age; race/ethnicity; education; marijuana use; crack, cocaine, and/or heroin use; smoking; heavy alcohol use; antidepressants; past hormone therapy use; HCV infection; income; and study site.
Hopkins Verbal Learning Test-Revised (HVLT-R)
Table 2 shows the unadjusted neuropsychological test scores as a function of HIV status and Table 3 shows the unadjusted scores as a function of menopausal stage. In multivariable analyses, HIV-infected women demonstrated significantly worse function than HIV-uninfected women on all outcomes (p’s<0.05; ~0.20 standard deviation (SD) units different; Table 5). The cognitive differences observed between HIV-infected and HIV-uninfected women were equivalent to differences observed in midlife women who were 5 years different in age23. Menopausal stage was not significantly associated with any of the cognitive outcomes on the HVLT-R (p’s>0.06; see Table 5 for trends). Depressive and anxiety symptoms were inversely associated with cognition (p’s<0.05). Specifically, women with elevated depressive symptoms performed worse than women without elevated depressive symptoms on all but one HVLT-R outcome —learning slope (~0.20 SD units different). Women with elevated anxiety symptoms also performed worse than women without elevated anxiety symptoms on all but one HVLT-R outcome—Trial 1 learning (~0.30 SD units different). Notably, anxiety symptoms interacted with HIV status to significantly influence Trial 1, learning slope, and total learning (Table 5/Figure 1). Among the HIV-infected women, those with elevated anxiety symptoms performed significantly worse than those without elevated anxiety symptoms on verbal learning outcomes (p’s<0.05). The cognitive differences observed between HIV-infected with and without elevated anxiety symptoms were equivalent to differences observed in midlife women who were 20 years different in age23. In contrast, among the HIV-uninfected women, there were no significant differences in cognitive performance between individuals with and without elevated anxiety symptoms. There were no significant interactions between menopausal stage or additional menopausal symptoms (depressive, vasomotor, sleep symptoms) and HIV status on any cognitive outcome.
Table 3.
Neuropsychological Test Scores as a Function of Menopausal Status.
| Menopausal Stage |
Tukey Post Hoc | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive Test | n | Pre (n=550) M (SD) |
Early Peri (n=158) M (SD) |
Late Peri (n=46) M (SD) |
Post (n=232) M (SD) |
Omnibus p-value |
Early Peri vs. Pre Cohen’s d |
Late Peri vs. Pre Cohen’s d |
Post vs. Pre Cohen’s d |
| HVLT-R | |||||||||
| Trial 1 | 986 | 5.70 (1.66) | 5.54 (1.66) | 5.80 (1.84) | 5.33 (1.65) | 0.02 | ns | ns | 0.22* |
| Total learning | 986 | 22.02 (4.87) | 21.66 (5.22) | 21.78 (5.30) | 20.46 (4.95) | 0.001 | ns | ns | 0.32* |
| Learning slope | 986 | 1.50 (0.31) | 1.48 (0.34) | 1.46 (0.33) | 1.40 (0.34) | <0.001 | ns | ns | 0.12* |
| Delayed recall | 986 | 7.71 (2.43) | 7.79 (2.56) | 7.00 (3.04) | 7.04 (2.47) | 0.001 | ns | ns | 0.27* |
| Recognition | 984 | 10.23 (1.95) | 10.26 (1.92) | 9.61 (2.66) | 10.19 (1.80) | 0.21 | - | - | - |
| Stroop Test‡ | |||||||||
| Trials 1&2a | 919 | 59.95 (13.18) | 63.74 (15.39) | 62.77 (10.55) | 67.27 (20.07) | <0.001 | 0.27* | ns | 0.44* |
| Trial 3a | 866 | 123.81 (31.71) | 129.54 (32.76) | 138.46 (42.01) | 140.20 (37.61) | <0.001 | ns | ns | 0.47* |
Note.
p<0.05.
Time in seconds. Pre = Premenopause. Peri = Perimenopause. Post = Postmenopause. HVLT-R = Hopkins Verbal Learning Test. ns=not significant.
Unadjusted means are displayed such that higher is worse, but log-transformed scores were used in the statistical comparisons. See methods section for operational definitions of menopausal stage and elevated symptoms. Cohen’s d effect sizes: small effect = 0.2; medium effect = 0.5; large effect = 0.8. Stroop Trials 1 and 2 represent the average across the 2 trials.
Table 5.
Multivariable Linear Regression Analyses Predicting the Effects of HIV Status, Menopausal Stage, and Elevated Symptoms on Cognitive Test Performance.
| Cognitive Test |
|||||||
|---|---|---|---|---|---|---|---|
| HVLT-R | Stroop Test | ||||||
|
|
|||||||
| Trial 1 | Total learning | Learning Slope |
Delayed Recall |
Recognition | Trials 1&2 | Trial 3 | |
|
|
|||||||
| B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | |
| Main Effects | |||||||
| HIV Status | |||||||
| HIV+ (vs. HIV−) | −1.56 (0.73)* | −2.31 (0.75)** | −2.18 (0.81)** | −1.74 (0.75)* | −1.97 (0.81)* | −1.98 (0.82)* | −1.82 (0.85)* |
| Menopausal Stage (vs. pre) | |||||||
| Early Peri | −0.46 (0.88) | −0.06 (0.91) | −0.05 (0.98) | 1.11 (0.92) | 0.49 (1.02) | −1.70 (1.03)⌉ | 0.53 (1.07) |
| Late Peri | 1.51 (1.52) | 1.14 (1.56) | 0.16 (1.68) | −1.69 (1.58) | −3.00 (1.74)‡ | 0.46 (1.74) | −0.44 (1.81) |
| Post | −0.56 (0.86) | −1.17 (0.88) | −1.72 (0.95)† | −0.59 (0.89) | 0.97 (0.99) | −0.83 (1.00) | 1.49 (1.05) |
| Elevated Symptoms | |||||||
| Depression | −1.88 (0.74)* | −1.66 (0.75)* | −0.93 (0.81) | −1.79 (0.76)* | −1.97 (0.84)* | −2.19 (0.86)** | −2.47 (0.89)** |
| Anxiety | −1.74 (1.19) | −2.75 (1.22)* | −3.30 (1.32)** | −3.49 (1.24)** | −3.22 (1.36)* | −0.33 (1.40) | −2.42 (1.48) |
| Sleep Disturbance | 0.27 (0.76) | 0.11 (0.78) | 0.33 (0.84) | 1.21 (0.79) | 1.07 (0.84) | −0.47 (0.89) | 1.16 (0.92) |
| Vasomotor | −0.29 (0.86) | −0.23 (0.88) | 0.17 (0.95) | 0.85 (0.89) | 0.91 (0.99) | −2.49 (1.01)* | −1.88 (1.06) ‡ |
| Interaction | |||||||
| HIV Status x Anxiety | −4.72 (2.36)* | −6.48 (2.42)*** | −5.22 (2.62)* | −4.02 (2.45)⌉ | |||
| HIV−: Persistent (vs. not) | 1.54 (2.09) | 1.76 (2.14) | 0.34 (2.32) | −0.68 (2.17) | |||
| HIV+: Persistent (vs. not) | −3.18 (1.35)* | −4.72 (1.39)*** | −4.88 (1.50)** | −4.70 (1.40)*** | |||
Note.
p<0.01.
p<0.05,
p=0.07;
p=0.08;
p=0.09. B = parameter estimates for each factor and SE = standard error from the multivariable linear regression analyses. HVLT-R = Hopkins Verbal Learning Test. Pre = Premenopause. Peri = Perimenopause. Post = Postmenopause. All models are adjusted for site, crack, cocaine, and/or heroin use, marijuana use, smoking, heavy alcohol use, antidepressant medication use, income, HCV status, and past hormone therapy use. For Stroop we also controlled for the number of times a woman was exposed to the test (range 0-3 times). See methods section for operational definitions of menopausal stage and elevated symptoms. Stroop Trials 1 and 2 represent the average across the 2 trials.
Figure 1.
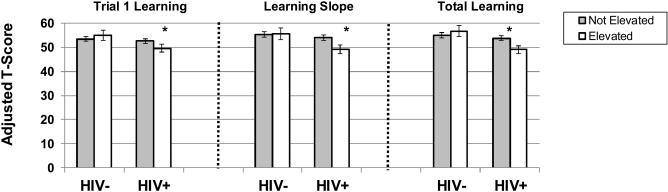
Note. ***p<0.001; *p<0.05. HVLT-R=Hopkins Verbal Learning Test. There was a significant interaction between anxiety symptoms and HIV status on trial 1 learning (p<.05), learning slope (p<0.05), and total learning (p<0.01). Among the HIV-infected, individuals with elevated anxiety symptoms performed worse than those without elevated anxiety symptoms on trial 1 learning (p<0.05), learning slope (p=0.01), and total learning (p<0.001). All models are adjusted for site, depressive symptoms, vasomotor symptoms, sleep disturbances, crack, cocaine, and/or heroin use, marijuana use, smoking, heavy alcohol use, antidepressant medication use, past hormone therapy use, HCV status, and income.
In multivariable analyses limited to HIV-infected women, elevated anxiety symptoms were significantly associated with Trial 1, (B=−3.37, SE=1.38, p=0.01), total learning (B=−4.40, SE=1.42, p=0.002), and learning slope (B=−4.31, SE=1.57, p=0.006) even after controlling for HIV morbidity indicators. Analyses focused on individual anxiety items indicated that symptoms of feeling tense/nervousness were significantly associated with worse performance on total learning (B=−3.50, SE=1.22, p=0.004); trend on learning slope (B=−2.70, SE=1.41, p=0.05). Greater feelings of fearfulness for no reason were also significantly associated with learning slope (B=-4.50, SE=1.87, p=0.01).
Stroop Test
HIV-infection was associated with diminished performance on Stroop Trials 1 & 2 and Trial 3 (Table 5). Menopausal stage was not significantly associated with any Stroop outcome (p’s>0.08; see Table 5 for trends). Depressive, but not anxiety, symptoms were associated with worse performance on each Stroop outcome (p’s<0.05; ~0.20 SD units different). Elevated vasomotor symptoms were associated with slower performance only on Stroop Trials 1 & 2 (p<0.05; 0.25 SD unit different; see Table 5 for trend on Stroop Trial 3). This association was driven by hot flashes (B=−2.75, SE=1.25, p=0.03) and not night sweats. There were no significant interactions between menopausal stage or menopausal symptoms and HIV status on the Stroop.
DISCUSSION
This study examined associations between menopausal stage and menopausal symptoms and performance of measures of verbal memory, processing speed, and response inhibition in HIV-infected and uninfected women. We found no support for our first hypothesis that menopausal stage would be associated with worse performance in the cognitive domains of processing speed and verbal memory. We did, however, find support for our second hypothesis that menopausal symptoms would be related to worse cognitive performance. Specifically, after adjusting for HIV status, depression, anxiety, and vasomotor symptoms were each independently associated with worse cognitive performance. We also found some support for our third hypothesis that menopausal symptoms would differentially be associated with cognitive performance in HIV-infected women. Specifically, there was an interaction between HIV status and anxiety such that the association between elevated anxiety and worse verbal learning was only evident in HIV-infected women and remained significant even after adjusting for HIV-related clinical indicators.
In contrast to previous studies, we found no evidence that cognitive performance, particularly in the domains of processing speed and verbal memory, was lowered in the perimenopausal stage. At least two general factors might have contributed to the lack of an association between cognition and menopausal stage in the present study. First is the cross-sectional design of this investigation. Cross-sectional analyses reveal no significant association between menopausal stage and cognitive performance in the SWAN47, whereas longitudinal analyses show significant associations11. Second, socio-demographic and mental health factors might have obscured an effect of menopausal stage in WIHS participants. The majority of women in WIHS are HIV-infected African Americans with annual incomes below ≤12,000. Therefore, compared to SWAN, the WIHS cohort is less healthy, more economically disadvantaged, lower educated, and more often drug abusing. Similarly, the prevalence of elevated depressive symptoms was 35% in WIHS versus 23% in SWAN. It may be that the negative effect of socio-demographic factors and depressive symptoms on cognition obscured any smaller effect of menopausal stage.
Menopausal symptoms – vasomotor symptoms, depressive symptoms, and anxiety – were associated with lowered cognitive function across HIV-infected and uninfected women in the WIHS. Vasomotor symptoms were related to slower processing speed on simple processing tasks requiring reading words aloud and identifying the color of blocks. Simple processing speed predicts driving behaviors and medication adherence in HIV-infected individuals, though the magnitude of the association of vasomotor symptoms on simple processing speed was small in this study. Thus, the clinical significance of the effect is likely to be minimal given that an average t-score is 50 and the average t-scores for women with elevated vasomotor symptoms was 47.7 and for those reporting no elevated vasomotor symptoms was 5012, 48. Simple processing speed was not measured in the SWAN, but both SWAN and WIHS found no association between performance on a more complex measure of processing speed – the Digit Symbol Modalities Test – and vasomotor symptoms. Depressive symptoms in WIHS participants were associated with decreased verbal memory, simple processing speed, and executive function. SWAN similarly found that depressive symptoms were negatively associated with processing speed11; however, other studies have not reported an influence of depressive symptoms on cognition49. Consistent with SWAN, anxiety symptoms in WIHS participants were associated with lower verbal learning11.
Of all of the symptoms investigated in this study, only anxiety was found to have a stronger association with lowered cognitive performance in HIV-infected compared to HIV-uninfected women, and this association was evident on measures of verbal learning. Notably, of all symptoms measured, anxiety had the largest association with cognitive performance across participants, and the magnitude of the association between anxiety and cognition was generally larger than the magnitude of the association between HIV and cognition. The test of the interaction between HIV status and anxiety symptoms (high versus low) on verbal memory revealed a significant association only among HIV-infected women, suggesting that the effects in the combined group were driven largely by the HIV-infected women. It is not the case that the association between elevated anxiety and cognition is due to a high prevalence of anxiety symptoms in the WIHS. In fact, elevated anxiety symptoms were less prevalent in WIHS (9%) compared to SWAN (~30%). Although the low rates of anxiety in the WIHS may be surprising, studies of HIV-infected individuals show that African-Americans are at a lower risk for anxiety and other psychiatric disorders compared to other ethnicities50-52 perhaps due to reporting biases. One possible mechanism linking anxiety to decreased verbal memory in HIV-infected women is a female vulnerability to the negative effects of stress hormones on verbal memory. For example, in a community-based study of older adults, 12-hour free cortisol excretion was associated with poorer delayed verbal recall in women, but not in men53. Moreover, increases in cortisol over a 2.5-year period were associated with declines in memory in women, but not in men53. In studies involving laboratory-induced stress, stress-induced increases in cortisol levels correlated negatively with memory in women, but not in men54.
Our study has several limitations. First, the cross-sectional design limited our ability to detect an effect of menopausal stage on cognition. The cross-sectional design also precludes the possibility of examining causality. We presume that anxiety and depressive symptoms lead to decreased cognitive functioning; however, it is possible that cognitive difficulties may precede anxiety and depressive symptoms for at least some women. A longitudinal investigation is underway in the WIHS. Second, elevated anxiety symptoms were measured using a widely-used menopause symptom inventory rather than a validated anxiety inventory. However, we used the same anxiety measure and cutoff as the SWAN allowing for comparisons with the largest investigation of menopause stage and symptoms on cognition11. Other studies also show that the anxiety scale used in this study is highly correlated with the Generalized Anxiety Disorder-7 scale (Spearman r = 0.71)32. Third, although depressive and anxiety symptoms increased in the menopausal transition in this study, these symptoms are not menopause-specific. Nevertheless, these symptoms are elevated in the perimenopause and relate to cognition in general11. Depressive symptoms have been shown to double the odds of cognitive impairment in a WIHS substudy40. Fourth, only two cognitive tests were administered so we could not evaluate associations across a broader spectrum of cognitive domains. Notably, however, we did include a test of verbal memory, a cognitive domain previously shown to be particularly sensitive to menopausal symptoms and stage55.
CONCLUSION
At a general level, the present results extend findings from cohort studies of more economically-advantaged women to a sample of economically-disadvantaged women with a high prevalence of HIV. Our findings suggest that elevated depression, anxiety, and vasomotor symptoms are associated with lower cognitive function in HIV-infected and HIV-uninfected women. All three symptoms are elevated in the perimenopause. Anxiety was differentially associated with worse verbal learning in HIV-infected women only. Our results suggest that screening and treating anxiety symptoms in HIV-infected women might confer benefits to cognitive functions that are negatively impacted by HIV. Longitudinal studies of the menopausal transition and menopause-associated affective symptoms are underway using a more comprehensive cognitive test battery.
Acknowledgments
Source of financial support: Dr. Rubin’s effort was supported by grant number 1K01MH098798-01 from the National Institute of Mental Health (NIMH) and K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women's Health (ORWH). Data in this manuscript were collected by the Women's Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest/Financial Disclosure: Pauline M. Maki was a consultant for Depomed pharmaceuticals. Kathyrn Anastos was a consultant for Bristol Meyers Squibb.
Preliminary data presented: North American Menopause Society, San Diego, CA, Oct 1-3, 2009 (poster); Ninth Annual Interdisciplinary Women's Health Research Symposium, Washington, DC, Nov 15, 2012 (invited talk).
REFERENCES
- 1.CDC HIV surveillance Report. 2012 2010. [Google Scholar]
- 2.Miller SA, Santoro N, Lo Y, et al. Menopause symptoms in HIV-infected and drug-using women. Menopause. 2005;12(3):348–56. doi: 10.1097/01.gme.0000141981.88782.38. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 3.Ferreira CE, Pinto-Neto AM, Conde DM, Costa-Paiva L, Morais SS, Magalhaes J. Menopause symptoms in women infected with HIV: prevalence and associated factors. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2007;23(4):198–205. doi: 10.1080/09513590701253743. [DOI] [PubMed] [Google Scholar]
- 4.Boonyanurak P, Bunupuradah T, Wilawan K, et al. Age at menopause and menopause-related symptoms in human immunodeficiency virus-infected Thai women. Menopause. 2012;19(7):820–4. doi: 10.1097/gme.0b013e31824cfc0f. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 5.Looby SE, Shifren J, Corless I, et al. Increased hot flash severity and related interference in perimenopausal human immunodeficiency virus-infected women. Menopause. 2013 doi: 10.1097/GME.0b013e31829d4c4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenbaum EE, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;41(10):1517–24. doi: 10.1086/497270. [DOI] [PubMed] [Google Scholar]
- 7.Kanapathipillai R, Hickey M, Giles M. Human immunodeficiency virus and menopause. Menopause. 2013;20(9):983–90. doi: 10.1097/GME.0b013e318282aa57. [DOI] [PubMed] [Google Scholar]
- 8.Kojic EM, Wang CC, Cu-Uvin S. HIV and menopause: a review. J Womens Health (Larchmt) 2007;16(10):1402–11. doi: 10.1089/jwh.2007.0345. [DOI] [PubMed] [Google Scholar]
- 9.Santoro N, Fan M, Maslow B, Schoenbaum E. Women and HIV infection: the makings of a midlife crisis. Maturitas. 2009;64(3):160–4. doi: 10.1016/j.maturitas.2009.09.001. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Women and health: Today's evidence Tomorrow's Agenda. WHO Press. 2009 [Google Scholar]
- 11.Greendale GA, Wight RG, Huang MH, et al. Menopause-associated symptoms and cognitive performance: results from the study of women's health across the nation. Am J Epidemiol. 2010;171(11):1214–24. doi: 10.1093/aje/kwq067. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev. 2009;19(2):186–203. doi: 10.1007/s11065-009-9095-0. [Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuh JL, Wang SJ, Lee SJ, Lu SR, Juang KD. A longitudinal study of cognition change during early menopausal transition in a rural community. Maturitas. 2006;53(4):447–53. doi: 10.1016/j.maturitas.2005.07.009. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 14.Greendale GA, Huang MH, Wight RG, et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology. 2009;72(21):1850–7. doi: 10.1212/WNL.0b013e3181a71193. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann N Y Acad Sci. 2001;949:203–14. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- 16.Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: a review of neuroimaging studies. Neuroimage. 2001;14(4):789–801. doi: 10.1006/nimg.2001.0887. [Review] [DOI] [PubMed] [Google Scholar]
- 17.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm Behav. 1998;34(2):171–82. doi: 10.1006/hbeh.1998.1476. [Clinical Trial] [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279(9):688–95. doi: 10.1001/jama.279.9.688. [Meta-Analysis Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 19.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288(7):872–81. doi: 10.1001/jama.288.7.872. [Research Support, U.S. Gov't, Non-P.H. Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 20.Bacon MC, von Wyl V, Alden C, et al. The Women's Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–9. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women's Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–25. [PubMed] [Google Scholar]
- 22.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test - Revised: Normative Data and Analysis of Inter-Form and Test-Retest Relability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- 23.Brandt J, Benedict R. Hopkins Verbal Learning Test-Revised: Professional Manual. Psychological Assessment Resources; Florida: 2001. [Google Scholar]
- 24.Comalli PE, Jr., Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- 25.Bromberger JT, Assmann SF, Avis NE, Schocken M, Kravitz HM, Cordal A. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158(4):347–56. doi: 10.1093/aje/kwg155. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 26.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103(1-3):267–72. doi: 10.1016/j.jad.2007.01.034. [Multicenter Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women's Health Across the Nation (SWAN) Psychol Med. 2011;41(9):1879–88. doi: 10.1017/S003329171100016X. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromberger JT, Schott LL, Kravitz HM, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women's Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67(6):598–607. doi: 10.1001/archgenpsychiatry.2010.55. [Comparative Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: study of women's health across the nation. Am J Public Health. 2006;96(7):1226–35. doi: 10.2105/AJPH.2005.066936. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurston RC, Bromberger JT, Joffe H, et al. Beyond frequency: who is most bothered by vasomotor symptoms? Menopause. 2008;15(5):841–7. doi: 10.1097/gme.0b013e318168f09b. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson CJ, Bromberger JT, Weiss GE, Thurston RC, Sowers M, Matthews KA. Negative attitudes and affect do not predict elective hysterectomy: a prospective analysis from the Study of Women's Health Across the Nation. Menopause. 2011;18(5):499–507. doi: 10.1097/gme.0b013e3181f9fa35. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bromberger JT, Kravitz HM, Chang Y, et al. Does risk for anxiety increase during the menopausal transition? Study of Women’s Health Across the Nation. Menopause. 2013;20(5):488–95. doi: 10.1097/GME.0b013e3182730599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 34.Jean-Louis G, Weber KM, Aouizerat BE, et al. Insomnia symptoms and HIV infection among participants in the Women's Interagency HIV Study. Sleep. 2012;35(1):131–7. doi: 10.5665/sleep.1602. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherner M, Letendre S, Heaton RK, et al. Hepatitis C augments cognitive deficits associated with HIV infection and methamphetamine. Neurology. 2005;64(8):1343–7. doi: 10.1212/01.WNL.0000158328.26897.0D. [DOI] [PubMed] [Google Scholar]
- 36.Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol. 2001;23(2):149–63. doi: 10.1076/jcen.23.2.149.1211. [DOI] [PubMed] [Google Scholar]
- 37.Letendre SL, Cherner M, Ellis RJ, et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS. 2005;19(Suppl 3):S72–8. doi: 10.1097/01.aids.0000192073.18691.ff. [DOI] [PubMed] [Google Scholar]
- 38.Manly JJ, Smith C, Crystal HA, et al. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV-women: the Women's Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol. 2011;33(8):853–63. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin EM, Novak RM, Fendrich M, et al. Stroop performance in drug users classified by HIV and hepatitis C virus serostatus. J Int Neuropsychol Soc. 2004;10(2):298–300. doi: 10.1017/S135561770410218X. [DOI] [PubMed] [Google Scholar]
- 40.Richardson JL, Nowicki M, Danley K, et al. Neuropsychological functioning in a cohort of HIV- and hepatitis C virus-infected women. AIDS. 2005;19(15):1659–67. doi: 10.1097/01.aids.0000186824.53359.62. [DOI] [PubMed] [Google Scholar]
- 41.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62(6):957–62. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valcour V, Maki P, Bacchetti P, et al. Insulin Resistance and Cognition Among HIV-Infected and HIV-Uninfected Adult Women: The Women's Interagency HIV Study. AIDS Res Hum Retroviruses. 2011 doi: 10.1089/aid.2011.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. American family physician. 2009;80(1):44–50. [Review] [PubMed] [Google Scholar]
- 44.Heaton RK, Grant I, Matthews CG. Psychological Assessment Resources; Odessa, FL: 1991. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. [Google Scholar]
- 45.Jastak S, Wilkinson GS, Jastak J. Wide Range Achievement Test-Revised. Jastak Associates Inc; Indianapolis: 1984. [Google Scholar]
- 46.Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. 2002;8(3):341–8. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- 47.Luetters C, Huang MH, Seeman T, et al. Menopause transition stage and endogenous estradiol and follicle-stimulating hormone levels are not related to cognitive performance: cross-sectional results from the study of women's health across the nation (SWAN) J Womens Health (Larchmt) 2007;16(3):331–44. doi: 10.1089/jwh.2006.0057. [Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 48.Marcotte TD, Lazzaretto D, Scott JC, Roberts E, Woods SP, Letendre S. Visual attention deficits are associated with driving accidents in cognitively-impaired HIV-infected individuals. J Clin Exp Neuropsychol. 2006;28(1):13–28. doi: 10.1080/13803390490918048. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 49.Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013 doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parhami I, Fong TW, Siani A, Carlotti C, Khanlou H. Documentation of Psychiatric Disorders and Related Factors in a Large Sample Population of HIV-Positive Patients in California. AIDS Behav. 2012 doi: 10.1007/s10461-012-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–8. doi: 10.1001/archpsyc.58.8.721. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 52.Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States. J Acquir Immune Defic Syndr. 2006;42(3):298–306. doi: 10.1097/01.qai.0000219773.82055.aa. [Research Support, N.I.H., Extramural Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 53.Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J Clin Endocrinol Metab. 1997;82(8):2458–65. doi: 10.1210/jcem.82.8.4173. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 54.Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23(6):617–29. doi: 10.1016/s0306-4530(98)00032-8. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 55.Weber MT, Maki PM, McDermott MP. Cognition and mood in perimenopause: A systematic review and meta-analysis. J Steroid Biochem Mol Biol. 2013 doi: 10.1016/j.jsbmb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


