Summary
Monoamine neurotransmitter deficiency has been implicated in the etiology of neuropsychiatric symptoms associated with chronic hyperphenylalaninemia in phenylketonuria (PKU). Two proposed explanations for neurotransmitter deficiency in PKU include first, that chronically elevated blood L-phenylalanine (Phe) inhibits the transport of L-tyrosine (Tyr) and L-tryptophan (Trp), the substrates for dopamine and serotonin synthesis respectively, into brain. In the second hypothesis, elevated Phe competitively inhibits brain tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) activities, the rate limiting steps in dopamine and serotonin synthesis. Dietary supplementation with large neutral amino acids (LNAA) including Tyr and Trp has been recommended for individuals with chronically elevated blood Phe in an attempt to restore amino acid and monoamine homeostasis in brain. As a potential alternative treatment approach, we demonstrate that pharmacologic inhibition of Tyr degradation through oral administration of nitisinone (NTBC) yielded sustained increases in blood and brain Tyr, decreased blood and brain Phe, and consequently increased dopamine synthesis in a murine model of PKU. Our results suggest that Phe-mediated inhibition of TH activity is the likely mechanism of impaired dopamine synthesis in PKU. Pharmacologic inhibition of Tyr degradation may be a promising adjunct therapy for CNS monoamine neurotransmitter deficiency in hyperphenylalaninemic individuals with PKU.
Introduction
Phenylketonuria (PKU, OMIM # 261600), one of the most common human inborn errors of metabolism, is caused by recessively inherited phenylalanine hydroxylase (PAH, EC 1.14.16.1) deficiency (Figure 1). PAH deficiency leads to chronically elevated L-phenylalanine (Phe) in blood and brain, and if left untreated, causes profound cognitive disability. Much of the world’s population is screened for hyperphenylalaninemia shortly after birth and early introduction of a Phe-restricted diet largely prevents the major manifestations of the disorder. However, PKU therapy requires lifelong strict adherence to an unpalatable and complicated diet, and mild cognitive deficits still persist in some treated children (Azen et al. 1991). Additionally, non-adherence to this difficult diet is common in adolescents and adults. Up to 75% of adolescents in some PKU treatment centers are unable to maintain blood Phe levels within the recommended target range (Walter and White 2004). Chronically elevated blood Phe is frequently associated with disturbed executive functioning (VanZutphen et al. 2007; Christ et al. 2010), and in extreme cases can be associated with adult onset white matter degeneration, ataxia and seizures (Thompson et al. 1990). Novel therapeutic approaches to PKU that do not rely solely upon dietary manipulation continue to be sought (Harding 2008), including novel therapies targeting selective restriction of brain Phe accretion (Vogel et al. 2013).
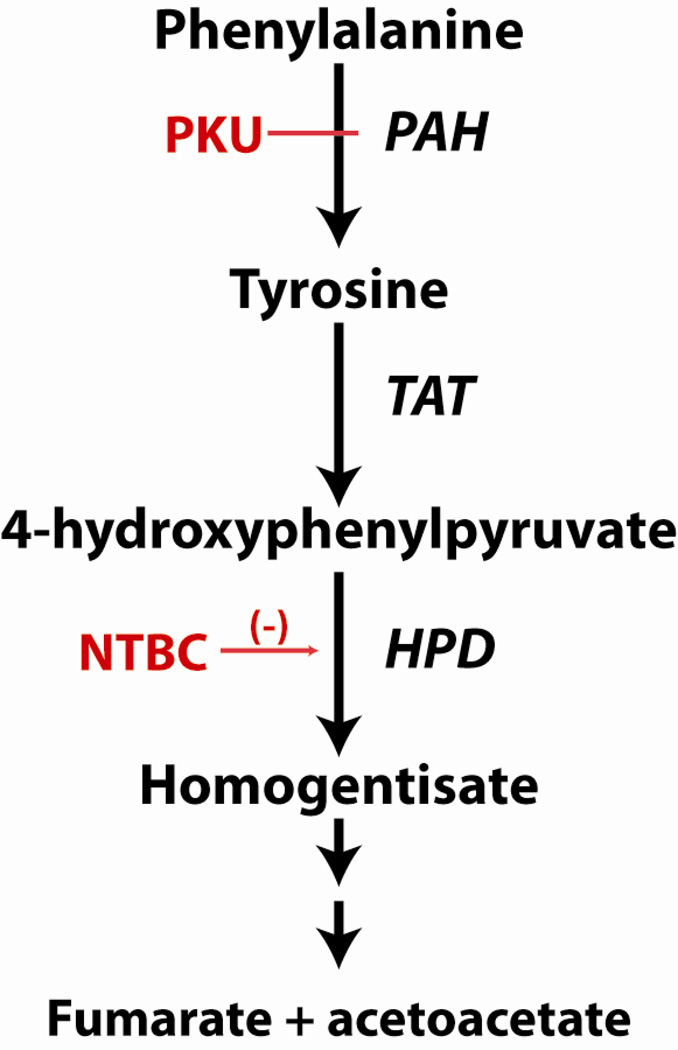
Figure 1. Phenylalanine Hydroxylation and the Tyrosine Degradation Pathway.
L-Phenylalanine is hydroxylated to L-tyrosine, primarily in liver, via the activity of phenylalanine hydroxylase (PAH, EC 1.14.16.1). Phenylketonuria (PKU) is caused by recessively inherited mutations in the Pah gene associated with reduced or absent PAH activity. Hyperphenylalaninemia is the result. L-Tyrosine is degraded to fumarate and acetoacetate beginning with deamination through the activity of tyrosine aminotransferase (TAT, EC 2.6.1.5). 4-hydroxyphenylpyruvate dioxygenase (HPD, EC 1.13.11.27) catalyzes the next step in L-tyrosine degradation, namely the oxidation of 4-hydroxyphenylpyruvate to homogentisate. 2-[2-nitro-4- (trifluoromethyl)benzoyl]cyclohexane-1,3-dione (NTBC, also known as nitisinone) is a reversible inhibitor of HPD activity. Oral administration of NTBC blocks the production of homogentisate and downstream metabolites; NTBC is therefore used in the treatment of the human inborn errors of metabolism alkaptonuria and tyrosinemia type 1. Blood tyrosine concentration is increased in mice and humans treated with NTBC.
Several different molecular mechanisms likely underlay the central nervous system (CNS) dysfunction associated with chronic hyperphenylalaninemia (Feillet et al. 2010), but abundant evidence implicates dysfunction of the dopaminergic and serotonergic neuronal systems, particularly of the prefrontal cortex, as having major roles in the symptoms of anxiety, depression and impaired executive functioning associated with poorly treated PKU (Christ et al. 2010; de Groot et al. 2010; Feillet et al. 2010). Two major theories have been proposed as the causes of brain dopamine and serotonin deficiency in PKU: relative deficiency in brain of L-tyrosine (Tyr) and L-tryptophan (Trp), the substrates for dopamine and serotonin synthesis respectively, and Phe-mediated competitive inhibition of tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) activities, the rate limiting steps in dopamine and serotonin synthesis within the brain (Figure 2). In the former, increased blood Phe inhibits the transport of Tyr and Trp (and of other large neutral amino acids (LNAA)) across the blood-brain barrier via the LAT neutral amino acid transporter system (Christensen et al. 1948; Choi and Pardridge 1986). Details of LNAA transport and the effects of hyperphenylalaninemia upon brain content of other LNAA have recently been presented (Vogel et al. 2013).
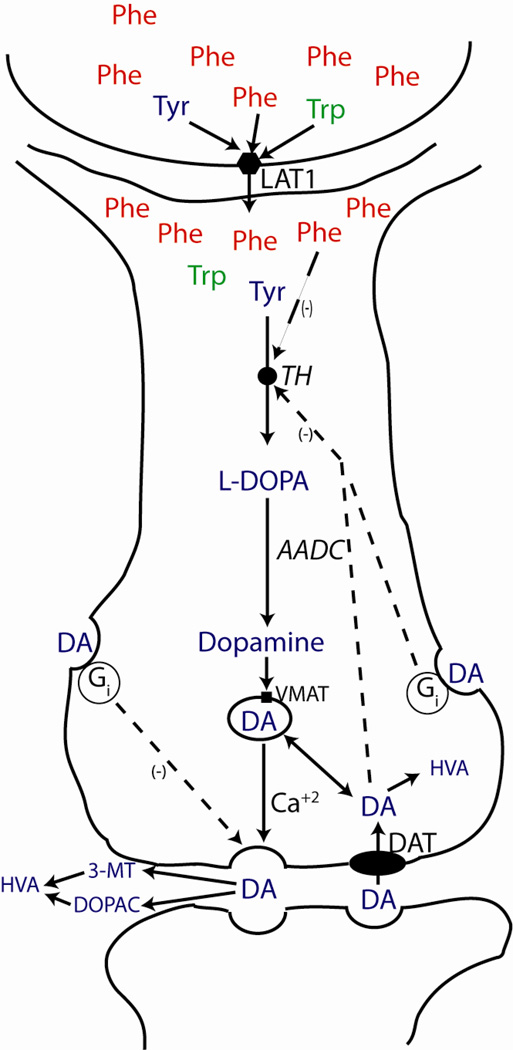
Figure 2. Monoamine neurotransmitter synthesis and regulation in neurons.
L-tyrosine ( ) and L-tryptophan (
) and L-tryptophan ( ) are the substrates for dopamine (
) are the substrates for dopamine ( ) and serotonin synthesis respectively. Both cross the blood brain barrier and enter neurons via diffusion facilitated by the LAT1 neutral amino acid transporter in competition with L-phenylalanine (
) and serotonin synthesis respectively. Both cross the blood brain barrier and enter neurons via diffusion facilitated by the LAT1 neutral amino acid transporter in competition with L-phenylalanine ( ) and other large neutral amino acids. Tyr is converted to L-DOPA by tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis. TH activity is stimulated by Tyr, but inhibited through Phe-mediated competition, DA or L-DOPA-mediated feedback inhibition, and via a DA-activated, G-protein coupled, synthesis-modulating autoreceptor. TH activity is also regulated through reversible phosphorylation triggered by a variety of stimuli (not shown). DA is synthesized from L-DOPA by aromatic amino acid decarboxylase (AADC), taken up into secretory vesicles via a monoamine specific transporter (VMAT) and secreted into the synapse following Ca+2 influx in response to a nerve stimulus. Neurotransmission terminates through DA reuptake via a specific transporter (DAT) or through degradation of dopamine to homovanillic acid (HVA). Extracellular DA can also act via another G protein-coupled autoreceptor to suppress further synaptic dopamine release. The production of HVA from DA requires both oxidation via the activity of monoamine oxidase (MAO) and methylation by catechol-O-methyl-transferase (COMT), but these sequential reactions can occur in either order. DA oxidation occurring first produces 3,4- dihydroxyphenylacetic acid (DOPAC) followed by methylation to HVA; initial DA methylation yields 3-methoxytyramine (3-MT) that is subsequently oxidized to HVA. The brain content of DOPAC and 3-MT are indicators of neuronal and synaptic DA turnover. Analogous mechanisms regulate the synthesis and secretion of serotonin from Trp followed by degradation to 5-hydroxyindoleacetic acid (5-HIAA) (not shown).
) and other large neutral amino acids. Tyr is converted to L-DOPA by tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis. TH activity is stimulated by Tyr, but inhibited through Phe-mediated competition, DA or L-DOPA-mediated feedback inhibition, and via a DA-activated, G-protein coupled, synthesis-modulating autoreceptor. TH activity is also regulated through reversible phosphorylation triggered by a variety of stimuli (not shown). DA is synthesized from L-DOPA by aromatic amino acid decarboxylase (AADC), taken up into secretory vesicles via a monoamine specific transporter (VMAT) and secreted into the synapse following Ca+2 influx in response to a nerve stimulus. Neurotransmission terminates through DA reuptake via a specific transporter (DAT) or through degradation of dopamine to homovanillic acid (HVA). Extracellular DA can also act via another G protein-coupled autoreceptor to suppress further synaptic dopamine release. The production of HVA from DA requires both oxidation via the activity of monoamine oxidase (MAO) and methylation by catechol-O-methyl-transferase (COMT), but these sequential reactions can occur in either order. DA oxidation occurring first produces 3,4- dihydroxyphenylacetic acid (DOPAC) followed by methylation to HVA; initial DA methylation yields 3-methoxytyramine (3-MT) that is subsequently oxidized to HVA. The brain content of DOPAC and 3-MT are indicators of neuronal and synaptic DA turnover. Analogous mechanisms regulate the synthesis and secretion of serotonin from Trp followed by degradation to 5-hydroxyindoleacetic acid (5-HIAA) (not shown).
Obviously, the most direct way to resolve dopamine and serotonin deficiency regardless of the specific underlying mechanism is to lower the blood Phe. As mentioned above however, many individuals struggle to maintain dietary adherence especially if ongoing problems with executive function or mood interfere with the patients’ ability to manage the diet. Oral Tyr (Lou et al. 1987; Lykkelund et al. 1988) or combination LNAA (Pietz et al. 1999) supplementation has been commonly prescribed to PKU patients, but the poor solubility of Tyr severely limits the amount of orally administered Tyr that can be absorbed. As an alternative, we propose a pharmacologic approach to increasing blood and brain Tyr: oral administration of 2-[2-nitro-4-(trifluoromethyl)benzoyl]cyclohexane-1,3-dione (NTBC, also known as nitisinone or Orfadin™), a reversible inhibitor of 4-hydroxyphenylpyruvate dioxygenase (HPD, EC 1.13.11.27), an intermediate step in the Tyr degradation pathway (Figure 1). NTBC is an FDA-approved drug for the safe and effective treatment of human tyrosinemia type 1. Our hypothesis is that chronic oral administration of NTBC to hyperphenylalaninemic individuals will lead to increased blood and brain Tyr concentrations, lower brain Phe, and will restore brain dopamine content. Here, we report the biochemical effects of chronic oral NTBC treatment in Pahenu2/enu2 mice, a model of human PAH deficiency.
Materials and methods
Animal husbandry
Animal care and experimentation were performed in accordance with the guidelines of the Dept. of Comparative Medicine, Oregon Health & Science University. C57Bl/6-Pahenu2/enu2 mice (henceforth designated Pah−/− mice) are homozygous for a missense mutation in exon 7 of the murine Pah gene, are completely deficient in liver PAH activity, are consequently hyperphenylalaninemic on an unrestricted diet, and are a representative animal model of human PKU (McDonald et al. 1990). Control animals included wild type C57Bl/6 mice (Pah+/+) bred and reared in our colony at OHSU. All mice were fed standard mouse chow (Lab Diet 5001, St. Louis, MO) ad libitum providing approximately 23% of energy as protein and housed under a standard 12 hours on, 12 hours off light cycle. Genotyping for the presence of the Pahenu2 mutation was performed by PCR-based analysis (Harding et al. 1998) or Taqman quantitative PCR assay. All surgical procedures were carried out with inhaled isoflurane general anesthesia to minimize pain and discomfort.
NTBC treatment trial
Two-month-old male and female Pah−/− mice in the treatment arm of the study received 2-[2-nitro-4-(trifluoromethyl)benzoyl]cyclohexane-1,3-dione (NTBC) mixed in drinking water at a concentration of 4 mg/ml while a control group of age matched Pah−/− mice consumed only normal drinking water. The trial was continued for 8 weeks when all animals were euthanized for tissue harvest.
Tissue harvest
All mice were fasted for four hours prior to tissue harvest to eliminate prandial effects on amino acid concentrations. Animals were sedated using inhaled isoflurane anesthesia. Blood was collected by cardiac puncture, and the mice were then euthanized by exsanguination. The animals were perfused with 20 ml normal saline via the left cardiac ventricle to clear blood from the cerebral circulation. Whole brain was rapidly excised from the cranium, split sagitally, and immediately submerged in liquid nitrogen. Half brains were stored at −80°C until pr ocessing for amino acid or neurotransmitter analysis.
Amino acid analysis
Amino acid concentrations in sera or brain tissue were measured either by high performance ion exchange liquid chromatography with post column ninhydrin derivitization (Slocum and Cummings 1991) or by precolumn derivitization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC, Waters AccQ Tag™derivitization system) followed by separation with ultra high performance liquid chromatography (Waters Acquity™ UPLC, Milford, MA). Results from the two different methods are tightly correlated (data not shown). For analysis of amino acid concentrations in brain, half-brains were mechanically homogenized in 5 volumes 10% trichloroacetic acid. The homogenates were clarified by centrifugation at 500 X g and supernatants stored at −80°C until analysis. For br ain amino acid analysis using the Waters MassTrak amino acid system (http://www.waters.com/waters/en_US/Amino-Acid-Analysis/nav.htm?cid=514511), norvaline (250 µM final concentration) was added to the 10% trichloroacetic acid prior to tissue homogenization as an internal recovery standard. 100 µl clarified brain homogenate was neutralized by adding 100 µl 0.7 M NaOH; 20 µl of neutralized clarified brain homogenate was derivitized with AQC according to the instructions of the Waters MassTrak amino acid analysis kit (final volume of the derivitization reaction = 100 µl). 1 µl of this final derivitized solution was injected onto the UPLC for analysis. Measured brain homogenate amino acid concentrations were corrected for wet weight of the initial tissue sample and reported as nmol/g brain tissue.
Brain monoamine neurotransmitter analysis
Monoamine neurotransmitter concentrations (dopamine, HVA, DOPAC, 3-MT, serotonin, and 5-HIAA) were measured in brain tissue by HPLC and electrochemical detection according to a published method (Ogburn et al. 2006). Mouse half-brains were mechanically homogenized in ice cold 10% perchloric acid. Measured brain homogenate monoamine neurotransmitter concentrations were corrected for wet weight of the initial tissue sample and reported as nmol/g brain tissue.
Statistical analysis
All data are presented as mean ± SEM. Blood and brain amino acids and brain neurotransmitter concentrations measured in Pah+/+, Pah−/− and NTBC-treated Pah−/− mice were compared using one-way ANOVA calculated using Prism 5.0 software. Intergroup p values were calculated using Bonferonni’s Multiple Comparison Test. In some cases, p values between two groups were calculated using a one-tailed Student’s t test.
Results
NTBC treatment reverses hypopigmentation in Pahenu2/enu2 mice
Phe competitively inhibits the enzyme tyrosinase, which is the rate-limiting step in the production of melanin (Miyamoto and Fitzpatrick 1957), and hyperphenylalaninemia is associated with hypopigmentation in humans with PKU and in Pah−/− mice. To test whether NTBC treatment could functionally overcome this pigmentation defect, adult Pah−/− mice were treated with NTBC in drinking water. By eight weeks of therapy, NTBC-treated mice developed fully pigmented black hair, compared to untreated controls (Figure 3). This finding suggests that NTBC was able to positively affect the imbalance between Tyr and Phe in this pathway.
Figure 3. NTBC treatment reverses PKU-associated hypopigmentation.
Oral NTBC treatment for eight weeks yielded reversal of hypopigmentation in treated Pahenu2/enu2 mice (left) in comparison to untreated littermates (right). PKU-associated hypopigmentation is due to Phe-mediated competitive inhibition of tyrosinase enzyme, the rate-limiting step in melanin synthesis; increased Tyr and lower Phe due to NTBC treatment reverses tyrosinase inhibition.
NTBC treatment lowers blood and brain phenylalanine in Pahenu2/enu2 mice
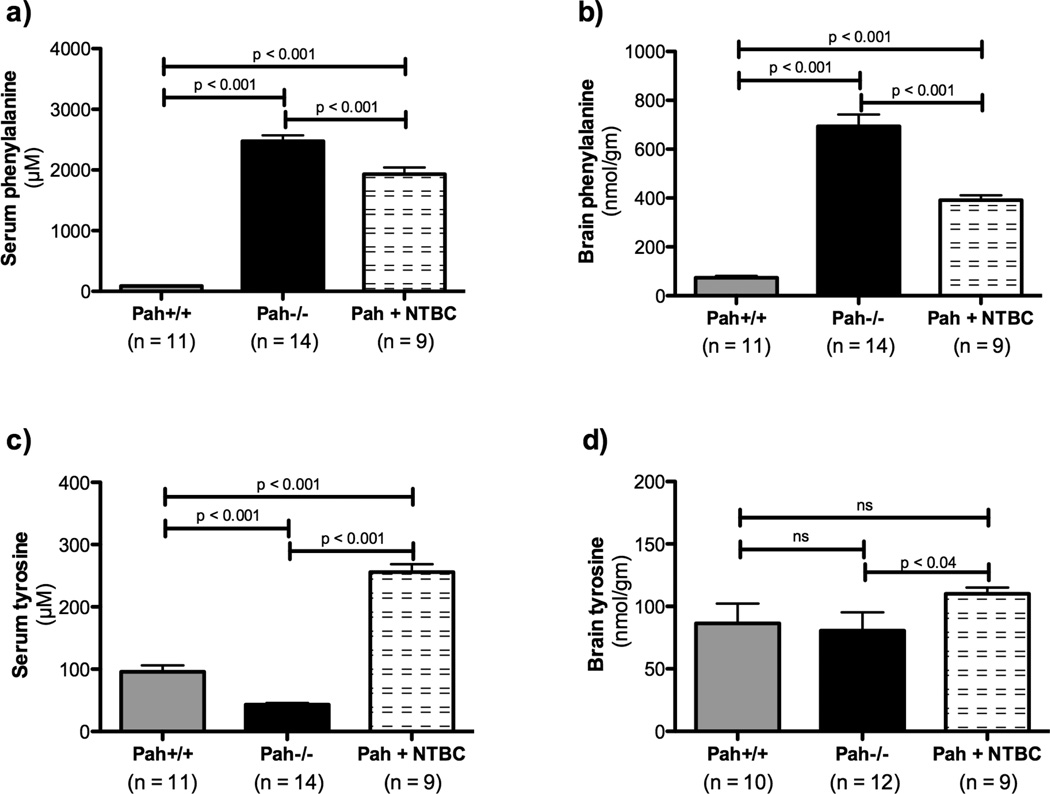
We determined the effect of NTBC treatment upon blood and brain Phe and Tyr content, and these results in wild type, untreated Pah−/−, and NTBC-treated Pah−/−, mice are presented in Table 1 and depicted in Figure 4. As expected in PAH deficiency, serum Phe concentration was extremely elevated in Pah−/− mice (2474 ± 99 µM) in comparison to wild type C57Bl6-Pah+/+ mice (89 ± 7 µM)(Figure 4a), while serum Tyr is significantly reduced (Pah+/+ Tyr = 96 ± 11 µM, Pah−/− Tyr = 43 ± 3 µM, Figure 4c). Likewise, brain Phe content is elevated in Pah−/− mice (693 ± 49 nmol/g) in comparison to Pah+/+ mice (74 ± 8 nmol/g)(Figure 4b). Brain tyrosine content was however not significantly different between Pah−/− mice (81 ± 15 nmol/g) and Pah+/+ mice (86 ± 16 nmol/g)(Figure 4d). NTBC treatment, as expected, led to an almost six fold increase in serum Tyr (256 ± 13 µM)(Figure 4c), and a slight but significant increase in brain Tyr (110 ± 5 nmol/g)(Figure 4d). Both serum Phe (1933 ± 109 µM) and most importantly brain Phe (391 ± 20 nmol/g) were significantly reduced by NTBC treatment, although not to concentrations measured in Pah+/+ mice (Figures 4a and 4b).
Table 1.
Effect of NTBC treatment upon serum and brain amino acids in Pahenu2/enu2 mice.
| Serum (µM) |
Brain (nmol/g) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Amino acid | Pah −/− |
Pah−/− +NTBC |
Mean % change |
p value |
Pah− /− |
Pah−/− +NTBC |
Mean % change |
p value |
| Phenylalanine | 2474 ± 99 | 1933 ± 109 | −21.9 | 0.0009 | 693 ± 49 | 391 ± 20 | −43.6 | < 0.0001 |
| Tyrosine | 43 ± 3 | 256 ± 13 | +595 | < 0.0001 | 81 ± 15 | 110 ± 5 | +35.8 | 0.0385 |
| Tryptophan | 63 ± 3 | 48 ± 4 | −23.8 | 0.0045 | 20 ± 1 | 12 ± 1 | −40.0 | 0.0004 |
| Valine | 252 ± 10 | 242 ± 11 | −4.0 | 0.515 8 | 164 ± 9 | 111 ± 5 | −32.3 | 0.0002 |
| Leucine | 176 ± 8 | 159 ± 11 | −9.7 | 0.215 8 | 284 ± 16 | 193 ± 10 | −32.0 | 0.0002 |
| Isoleucine | 116 ± 5 | 109 ± 9 | −6.0 | 0.528 7 | 105 ± 6 | 80 ± 3 | −23.8 | 0.0028 |
| Methionine | 61 ± 5 | 99 ± 18 | +62.3 | 0.078 3 | 62 ± 4 | 51 ± 3 | −17.7 | 0.0452 |
| Histidine | 69 ± 3 | 69 ± 3 | 0 | 0.922 9 | 114 ± 6 | 75 ± 4 | −34.2 | < 0.0001 |
| Threonine | 150 ± 9 | 171 ± 13 | +14.0 | 0.096 6 | 261 ± 14 | 201 ± 8 | −23.0 | 0.0022 |
| Lysine | 259 ± 32 | 252 ± 20 | −2.7 | 0.419 9 | 312 ± 17 | 265 ± 12 | −15.1 | 0.0210 |
| Aspartic acid¶ | 13 ± 2 | 13 ± 12 | 0 | 0.4717 | 2073 ± 126 | 1895 ± 113 | −8.6 | 0.1552 |
Serum and brain amino acid concentrations expressed as mean ± SE. p values calculated using one-tailed Students’ t test.
Data for which the difference between the means of NTBC-treated and untreated Pahenu2/enu2 mice was significant (p < 0.05) are displayed in blue  .
.
Aspartic acid is displayed here as a reference amino acid that is not appreciably transported by the LAT-1 transporter.
Figure 4. Effect of NTBC treatment upon phenylalanine and tyrosine concentrations in blood and brain of Pahenu2/enu2 mice.
Serum tyrosine concentration in Pahenu2/enu2 mice (Pah−/−) is significantly lower than in wild type (Pah+/+) mice (c) while serum (a) and brain (b) phenylalanine are significantly elevated as expected. There was a trend toward lower brain tyrosine in Pahenu2/enu2 mice in comparison to controls (d) but this difference did not reach statistical significance. NTBC treatment of Pahenu2/enu2 mice led to significantly increased serum (c) and brain tyrosine (d) while decreasing both serum (a) and brain (b) phenylalanine. Data are presented as mean ± SEM. Statistical analysis utilized one-way ANOVA with intergroup p values calculated using Bonferonni’s Multiple Comparison Test.
NTBC treatment increases the content of dopamine and its metabolites in Pahenu2/enu2 mouse brain
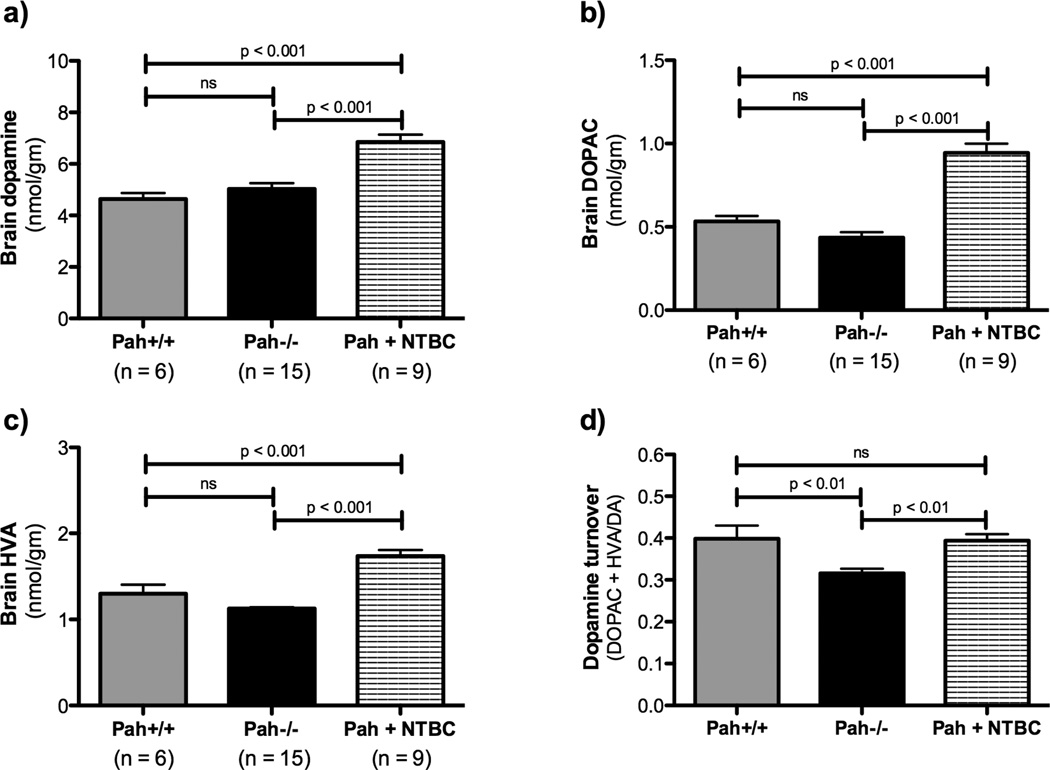
Although dopamine deficiency in specific brain regions has been previously reported in the Pahenu2/enu2 mouse model (albeit on the BTBR genetic background)(Puglisi-Allegra et al. 2000), we did not detect a significant difference between dopamine content of C57Bl/6-Pahenu2/enu2 mouse half brain homogenates (5.0 ± 0.2 nmol/gm) and that of wild type C57Bl/6 mice (4.6 ± 0.2 nmol/gm) (Figure 5). The ratio of (DOPAC + HVA)/DA, a marker of dopamine turnover, was significantly depressed in Pah−/− mice in comparison to wild type animals (Pah−/−, 0.32 ± 0.01 vs. Pah+/+, 0.40 ± 0.03, p = 0.003 by one-tailed Student’s t test). NTBC treatment yielded a significant increase in half-brain dopamine content (6.8 ± 0.3 nmol/gm brain tissue) in comparison to untreated Pah−/− mice (5.0 ± 0.2 nmol/gm, p < 0.0001), significantly increased HVA and DOPAC, and complete restoration of dopamine turnover ((DOPAC + HVA)/DA = 0.39 ± 0.02, p = 0.0002 in comparison to untreated Pah−/− mice).
Figure 5. Effect of NTBC treatment upon brain dopamine and its metabolites in Pahenu2/enu2 mice.
NTBC treatment was associated with significant increases in the brain content of dopamine and its degradation products. The deficit in dopamine turnover ((DOPAC + HVA)/DA) exhibited by Pah−/− mice was corrected by NTBC treatment. Data are presented as mean ± SEM. Statistical analysis utilized one-way ANOVA with intergroup p values calculated using Bonferonni’s Multiple Comparison Test.
Combined hyperphenylalaninemia and hypertyrosinemia further impairs large neutral amino acid uptake into brain of NTBC-treated Pahenu2/enu2 mice
Movement of amino acids across the blood-brain barrier is mediated by a system of several amino acid transporters (Vogel et al. 2013) with overlapping amino acid specificities. Facilitated diffusion of Phe, Tyr and other LNAA (tryptophan, valine, leucine, isoleucine, methionine, threonine and histidine) from capillary circulation into brain parenchyma is mediated predominantly but not exclusively by the LAT1 transporter (also known as SLC7A5)(Kanai et al. 1998; Mastroberardino et al. 1998) Affinity of the LAT1 transporter for LNAA is high relative to blood concentrations of the amino acids guaranteeing that at normal physiologic blood amino acid levels the LAT1 transporter is already saturated (Choi and Pardridge 1986). Additionally, LAT1 has the highest affinity to Phe followed by Tyr, among all LNAA. Therefore, both Phe and Tyr are effective inhibitors of the trans-BBB transport of other LNAA. In this experiment, even though blood Phe concentration had decreased on NTBC therapy, the combination of hyperphenylalaninemia and hypertyrosinemia in NTBC-treated Pah−/− mice led to significant reduction in the brain content of other select large neutral amino acids (Table 1) including tryptophan (Trp), the precursor to serotonin. The brain Trp content of NTBC-treated mice decreased to 12.4 ± 0.78 nmol/gm from 20.1 ± 1.4 nmol/gm in untreated mice (p < 0.0001).
CNS deficiency of LNAA other than Tyr and Trp could have additional adverse effects upon the brain. Impaired protein synthesis in brain due to LNAA deficiency has been proposed as a possible contributor to the PKU phenotype (Feillet et al. 2010). Methionine deficiency might also impair critical methylation reactions in brain. We, however, have not further investigated the possible consequences of CNS LNAA deficiency in Pahenu2/enu2 mice.
Pahenu2/enu2 mice exhibit brain serotonin deficiency
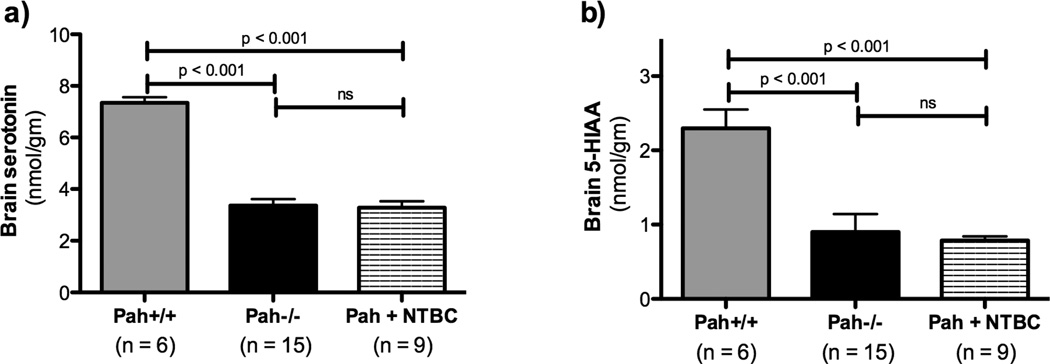
The serotonin content of Pah−/− mouse brain was severely depressed in comparison to wild type mice (Pah−/− = 3.4 ± 0.2 nmol/gm, Pah+/+ = 7.3 ± 0.2 nmol/gm, p < 0.0001)(Figure 6). NTBC treatment did not significantly alter brain serotonin content (3.3 ± 0.3 nmol/gm). Similarly, the brain content of 5-HIAA, a serotonin metabolite, was depressed in both untreated and NTBC-treated Pah−/− mice in comparison to Pah+/+ animals. There was a trend toward lower 5-HIAA content in the NTBC treated group in comparison to untreated Pah−/− mice, but this difference did not reach statistical significance. There was no change in the ratio of 5-HIAA/serotonin, a marker of serotonin turnover, with NTBC treatment (data not shown).
Figure 6. Effect of NTBC treatment upon brain serotonin and its metabolites in Pahenu2/enu2 mice.
Pahenu2/enu2 mice exhibit severe brain serotonin deficiency relative to wild type mice. NTBC treatment was associated with a trend toward lower brain serotonin content probably because of decreased availability of tryptophan, the substrate for serotonin synthesis, but this difference did not reach statistical significance. Statistical analysis utilized one-way ANOVA with intergroup p values calculated using Bonferonni’s Multiple Comparison Test.
Discussion
We reasoned that pharmacologic inhibition of the tyrosine degradation pathway would effectively increase blood Tyr and consequently decrease brain Phe in Pahenu2/enu2 mice. NTBC is an FDA-approved oral drug for the treatment of human tyrosinemia type 1 (HT1). In HT1 patients, the blood tyrosine is further increased by NTBC treatment, but inhibition of the tyrosine degradation pathway decreases the production of the tyrosine metabolite fumarylacetoacetate, the substance responsible for liver and kidney dysfunction in HT1 (Lindstedt et al. 1992). Elevated blood Tyr at the concentrations achieved in our NTBC-treated mice has not been associated with any adverse effects in humans. NTBC is also used to decrease production of homogentisic acid in alkaptonuria (Introne et al. 2011). Importantly, this study showed that doses of NTBC much lower than those used for the treatment of HT1 (0.03 vs. 1 mg/kg/day) are fully effective in raising blood Tyr concentration. Our results reported here clearly demonstrate that NTBC treatment was associated with an almost six fold increase in serum Tyr concentration and a modest increase in brain Tyr in Pah−/− mice; consequently, brain Phe decreased 44% but not down to concentrations found in Pah+/+ mice. Perhaps a higher dose of NTBC could have an even larger effect upon brain Phe. NTBC treatment of Pah−/− mice also caused an approximately 20% reduction in blood Phe concentration. Our clinical experience in humans with HT1 has revealed similar anecdotal evidence that NTBC treatment is associated with decreased blood Phe. In this animal experiment, NTBC treatment was also associated with decreased serum Trp. The mechanism for this effect of NTBC upon aromatic amino acids in blood has not been elucidated. One possible mechanism is an increase in the fractional excretion of Phe or Trp in urine; increased Tyr in the renal glomerular filtrate could compete with Phe or Trp for reuptake in the proximal renal tubule leading to excretion of a greater fraction of filtered amino acid. In this experiment however, we were unable to measure a consistent effect of NTBC upon the fractional excretion of Phe (data not shown). Oral administration of LNAA mixtures including Tyr has been associated with lower blood Phe in human trials, presumably due to competition for intestinal amino acid transport into enterocytes from the gut lumen (Matalon et al. 2006) but it is unclear how pharmacologically-induced hypertyrosinemia could alter the competition for intestinal absorption of other LNAA at the luminal side of the enterocyte. Elucidation of the mechanism behind decreased blood Phe and Trp in response to NTBC therapy will require further detailed investigation.
NTBC treatment was associated with a 36% increase in brain dopamine content in Pahenu2/enu2 mice and similar increases in markers of brain dopamine turnover. This increase in dopamine occurred despite minimal change in brain Tyr content but with a significant decrease in brain Phe. This result implies that Phe-mediated inhibition of TH activity is primarily responsible for PKU-associated dopamine deficiency. Phe at high concentration can itself be hydroxylated to Tyr through TH activity, potentially partially mitigating brain Tyr deficiency; local Tyr synthesis from Phe in brain may be the reason that the dopamine content of Pah−/− mouse brain was similar to that of Pah+/+ brain in our experiment. The measurement of normal or only mildly decreased brain dopamine content in Pahenu2/enu2 half brain in this experiment does not however guarantee that dopamine content in specific brain regions, intracellular dopamine trafficking, synaptic release, or turnover are not effected by elevated brain Phe concentration. In fact, our data suggest that dopamine turnover was impaired in Pah−/− mice despite near normal brain dopamine content. Also, increased brain dopamine content in NTBC-treated mice would not necessarily guarantee improved dopaminergic neuronal signaling; direct effects upon neural transmission from dopaminergic neurons remain to be demonstrated. Experiments similar to previously published work (Puglisi-Allegra et al. 2000; Pascucci et al. 2002) employing microdialysis in prefrontal cortex, a highly dopaminergic brain region, are planned to directly evaluate the effect of NTBC treatment upon synaptic monoamine neurotransmitters in Pahenu2/enu2 mice. Also, any potential behavioral effects of NTBC treatment secondary to improved brain dopamine status remain to be demonstrated.
NTBC treatment did not correct brain serotonin deficiency in Pahenu2/enu2 mice. Brain Trp content decreased following NTBC treatment suggesting that the combination of hyperphenylalaninemia and hypertyrosinemia led to further impairment of Trp transport into brain of NTBC-treated Pahenu2/enu2 mice. Similar effects of NTBC treatment upon the brain content of other LNAA were also demonstrated. However, brain serotonin content did not further decrease in NTBC-treated mice. This result suggests again that Phe-mediated inhibition of TPH activity likely plays a role in deficient serotonin synthesis in PKU brain; reduction of brain Phe may have partially restored TPH activity and maintained serotonin synthesis despite decreased availability of Trp, the TPH substrate. This conclusion is consistent with previously published data (Pascucci et al. 2002) showing that the brain content of 5-hydroxytryptophan, the product of the TPH reaction, is severely reduced in Pahenu2/enu2 mice despite only slightly decreased brain Trp content. Clearly however, and in contrast to dopamine, serotonin synthesis is still severely limited in NTBC-treated Pahenu2/enu2 mouse brain.
Parenthetically, Tyr supplementation as sole therapy for infants with PKU without reduction of blood Phe does not prevent mental retardation (Batshaw et al. 1981) so the effect of chronically elevated Phe upon developing brain must involve additional pathophysiologic mechanisms beyond brain Tyr deficiency or TH inhibition. CNS Tyr and Trp deficiencies and attendant dopamine and serotonin deficiencies can explain only a portion of the PKU phenotype and is likely more relevant in adolescents and adults who have lost dietary adherence. Also, the pathogenesis of neuropsychiatric dysfunction in poorly controlled adults with PKU is likely multifactorial (Feillet et al. 2010); correction of CNS dopamine status alone may not fully ameliorate the neuropsychiatric phenotype associated with hyperphenylalaninemia.
Our results suggest that the most likely cause of brain dopamine and serotonin deficiency in PKU is Phe-mediated inhibition of brain TH and TPH activities rather than decreased substrate availability. If this observation is true, then reduction in brain Phe by whatever available treatment or alternatively stimulation of TH and TPH activities, perhaps by supplementation with tetrahydrobiopterin (BH4) cofactor (Koshimura et al. 1990), likely would have greater impact upon monoamine neurotransmitter synthesis than simply increasing brain Tyr or Trp supply.
Conclusion
This experiment demonstrated significant effects upon brain Phe, Tyr, and dopamine content in Pahenu2/enu2 mice treated with oral NTBC. A controlled direct comparison of NTBC therapy vs. oral Tyr supplementation alone or an evaluation of combined NTBC/Tyr therapy needs yet to be completed. Additionally, because NTBC-induced hypertyrosinemia was associated with decrease brain concentrations of other LNAA including Trp, combination therapy with NTBC and LNAA including Trp or L-hydroxytryptophan (Pascucci et al. 2009) to treat CNS serotonin deficiency would likely be necessary to fully address monoamine neurotransmitter and cerebral LNAA deficiency in PKU. We propose that oral NTBC therapy could be an adjunct treatment for CNS dopamine deficiency in humans with chronic hyperphenylalaninemia due to PKU.
Take home message.
Oral nitisinone (NTBC) therapy yielded increased blood and brain L-tyrosine, lower blood and brain L-phenylalanine, and consequently increased brain dopamine synthesis in a murine model of phenylketonuria (PKU).
Acknowledgements
This work was supported in part by NIH Grants R01 DK059371 and R01 NS080866 and a grant from the National PKU Alliance (NPKUA, www.npkua.org). We thank Gloria Baca, Baoyu Lin, Lindsey Stetson, and Katie Cobb for able technical assistance and Dr. Melanie Gillingham for critical review of the manuscript.
Abbreviations
- PKU
phenylketonuria
- PAH
phenylalanine hydroxylase
- NTBC
2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
- Phe
L-phenylalanine
- Tyr
L-tyrosine,
- Trp
L-tryptophan
- LNAA
large neutral amino acids
- L-DOPA
L-3,4-dihydroxyphenylalanine
- DA
dopamine
- DOPAC
L-3,4-dihydroxyphenylacetic acid
- 3-MT
3-methoxytyramine
- HVA
homovanillic acid
- 5-HTP
L-5-hydroxytryptophan
- 5-HIAA
5-hydroxyindoleacetic acid
Footnotes
Compliance with Ethics Guidelines
Cary O. Harding, Shelley Winn, Markus Grompe, Mike Gibson, Erland Arning, and Teodoro Bottiglieri declare that they have no conflict of interest for this manuscript.
All institutional and national guidelines for the care and use of laboratory animals were followed.
Authors contributions:
Cary Harding was the principal investigator on this project. He designed the experiments, oversaw their execution, performed the data analysis and wrote the manuscript.
Shelley Winn executed the animal experiments described and thoroughly reviewed the manuscript.
Mike Gibson assisted with experimental design and also performed some of the amino acid analyses on tissue samples. He also reviewed the manuscript.
Erland Arning and Teodoro Bottiglieri performed the brain neurotransmitter analyses and reviewed the manuscript.
Markus Grompe assisted with experimental design, data analysis, provided NTBC for the animal experiment, and reviewed the manuscript.
Contributor Information
Cary O. Harding, Department of Molecular and Medical Genetics, Oregon Health & Science University, Portland, OR, USA
Shelley R. Winn, Department of Molecular and Medical Genetics, Oregon Health & Science University, Portland, OR, USA
K. Michael Gibson, Section of Clinical Pharmacology, College of Pharmacy, Washington State University, Spokane, WA, USA.
Erland Arning, Institute of Metabolic Disease, Baylor Research Institute, Dallas, TX, USA.
Teodoro Bottiglieri, Institute of Metabolic Disease, Baylor Research Institute, Dallas, TX, USA.
Markus Grompe, Department of Pediatrics, Oregon Health & Science University, Portland, OR, USA.
REFERENCES
- Azen CG, Koch R, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Amer. J. Dis. Child. 1991;145(1):35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Valle D, et al. Unsuccessful treatment of phenylketonuria with tyrosine. J Pediatr. 1981;99(1):159–160. doi: 10.1016/s0022-3476(81)80985-7. [DOI] [PubMed] [Google Scholar]
- Choi TB, Pardridge WM. Phenylalanine transport at the human blood-brain barrier. Studies with isolated human brain capillaries. J Biol Chem. 1986;261(14):6536–6541. [PubMed] [Google Scholar]
- Christ S, Huijbregts S, et al. Executive function in early-treated phenylketonuria: Profile and underlying mechanisms. Mol Genet Metab. 2010;99(Suppl 1):S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Christensen HN, Streicher JA, et al. Effects of feeding individual amino acids upon the distribution of other amino acids between cells and extracellular fluid. J Biol Chem. 1948;172(2):515–524. [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, et al. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Feillet F, van Spronsen FJ, et al. Challenges and pitfalls in the management of phenylketonuria. Pediatrics. 2010;126(2):333–341. doi: 10.1542/peds.2009-3584. [DOI] [PubMed] [Google Scholar]
- Harding C. Progress toward cell-directed therapy for phenylketonuria. Clin Genet. 2008;74(2):97–104. doi: 10.1111/j.1399-0004.2008.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CO, Wild K, et al. Metabolic engineering as therapy for inborn errors of metabolism - development of mice with phenylalanine hydroxylase expression in muscle. Gene Therapy. 1998;5(5):677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introne WJ, Perry MB, et al. A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab. 2011;103(4):307–314. doi: 10.1016/j.ymgme.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, et al. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273(37):23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Koshimura K, Miwa S, et al. Enhancement of dopamine release in vivo from the rat striatum by dialytic perfusion of 6R-L-erythro-5,6,7,8-tetrahydrobiopterin. J Neurochem. 1990;54(4):1391–1397. doi: 10.1111/j.1471-4159.1990.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Lindstedt S, Holme E, et al. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340(8823):813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- Lou HC, Lykkelund C, et al. Increased vigilance and dopamine synthesis by large doses of tyrosine or phenylalanine restriction in phenylketonuria. Acta Paediatr Scand. 1987;76(4):560–565. doi: 10.1111/j.1651-2227.1987.tb10521.x. [DOI] [PubMed] [Google Scholar]
- Lykkelund C, Nielsen JB, et al. Increased neurotransmitter biosynthesis in phenylketonuria induced by phenylalanine restriction or by supplementation of unrestricted diet with large amounts of tyrosine. Eur J Pediatr. 1988;148(3):238–245. doi: 10.1007/BF00441411. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395(6699):288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Matalon R, Michals-Matalon K, et al. Large neutral amino acids in the treatment of phenylketonuria (PKU) J Inher Metab Dis. 2006;29(6):732–738. doi: 10.1007/s10545-006-0395-8. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Bode VC, et al. Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc Natl Acad Sci U S A. 1990;87(5):1965–1967. doi: 10.1073/pnas.87.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Fitzpatrick T. Competitive inhibition of mammalian tyrosinase by phenylalanine and its relationship to hair pigmentation in phenylketonuria. Nature. 1957;179(4552):199–200. doi: 10.1038/179199b0. [DOI] [PubMed] [Google Scholar]
- Ogburn KD, Bottiglieri T, et al. Prostaglandin J2 reduces catechol-O-methyltransferase activity and enhances dopamine toxicity in neuronal cells. Neurobiol Dis. 2006;22(2):294–301. doi: 10.1016/j.nbd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Andolina D, et al. 5-Hydroxytryptophan rescues serotonin response to stress in prefrontal cortex of hyperphenylalaninaemic mice. Int J Neuropsychopharmacol. 2009;12(8):1067–1079. doi: 10.1017/S1461145709990381. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, et al. Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport. 2002;13(18):2561–2564. doi: 10.1097/00001756-200212200-00036. [DOI] [PubMed] [Google Scholar]
- Pietz J, Kreis R, et al. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J Clin Invest. 1999;103(8):1169–1178. doi: 10.1172/JCI5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi-Allegra S, Cabib S, et al. Dramatic brain aminergic deficit in a genetic mouse model of phenylketonuria. Neuroreport. 2000;11(6):1361–1364. doi: 10.1097/00001756-200004270-00042. [DOI] [PubMed] [Google Scholar]
- Slocum RH, Cummings JG. Amino acid analysis of physiological samples. In: Hommes FA, editor. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. New York: Wiley-Liss; 1991. pp. 87–126. [Google Scholar]
- Thompson AJ, Smith I, et al. Neurological deterioration in young adults with phenylketonuria. Lancet. 1990;336:602–605. doi: 10.1016/0140-6736(90)93401-a. [DOI] [PubMed] [Google Scholar]
- VanZutphen KH, Packman W, et al. Executive functioning in children and adolescents with phenylketonuria. Clin Genet. 2007;72(1):13–18. doi: 10.1111/j.1399-0004.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- Vogel KR, Arning E, et al. Non-physiological amino acid (NPAA) therapy targeting brain phenylalanine reduction: pilot studies in PAHENU2 mice. J Inherit Metab Dis. 2013;36(3):513–523. doi: 10.1007/s10545-012-9524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health. 2004;16(1):41–45. doi: 10.1515/ijamh.2004.16.1.41. [DOI] [PubMed] [Google Scholar]