To the Editor,
The overall best step-up therapy for children inadequately controlled on low dose inhaled steroids (ICS) is the addition of a long acting beta agonist (LABA) as compared to increasing the ICS dose or adding a leukotriene receptor-antagonist (LTRA). However, this preference for LABA step-up may not be shared by all races or /ethnicities. We performed a post-hoc analysis of differential treatment responses in relation to baseline history of eczema and race in 163 children 6–17 years of age enrolled in the “Best Add-on Therapy Giving Effective Response (BADGER)” trial. All participants in this study had uncontrolled asthma on fluticasone 100 mcg twice daily at base-line and subsequently received three step-up therapies in random order for 16 weeks each: fluticasone 250 µg twice daily (ICS step-up), fluticasone 100 µg + salmeterol 50 µg twice daily (LABA step-up), and fluticasone 100 µg twice daily + montelukast 5 or 10 mg daily (LTRA step-up) in a blinded, randomized, triple cross-over design. Rank-ordered logistic regression modeling was used to examine which patient characteristics were predictive of different patterns of treatment rankings in a similar analysis as used in the original BADGER report 1.
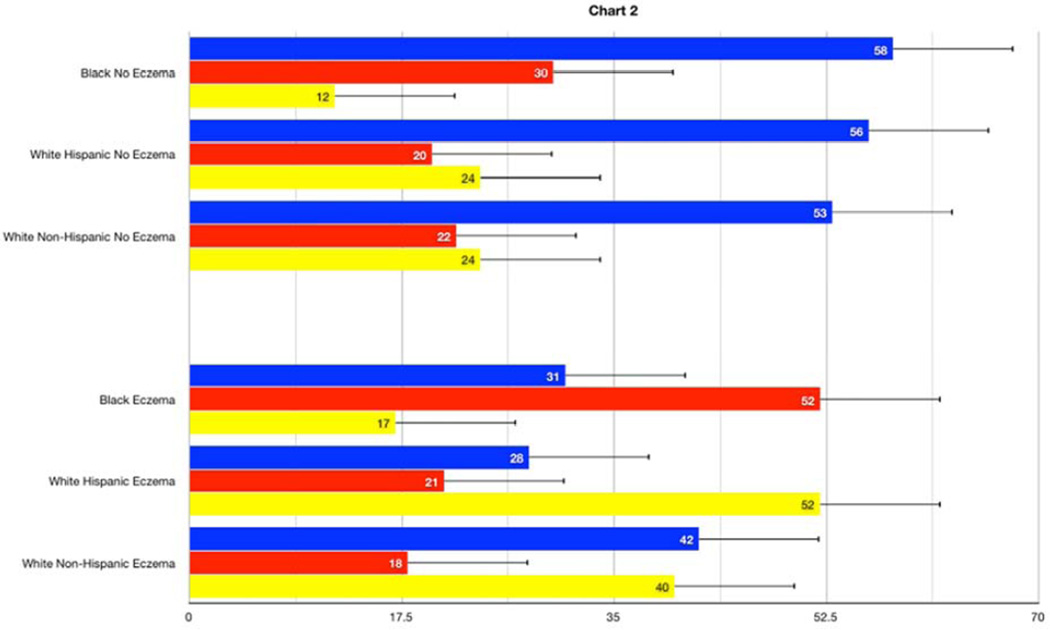
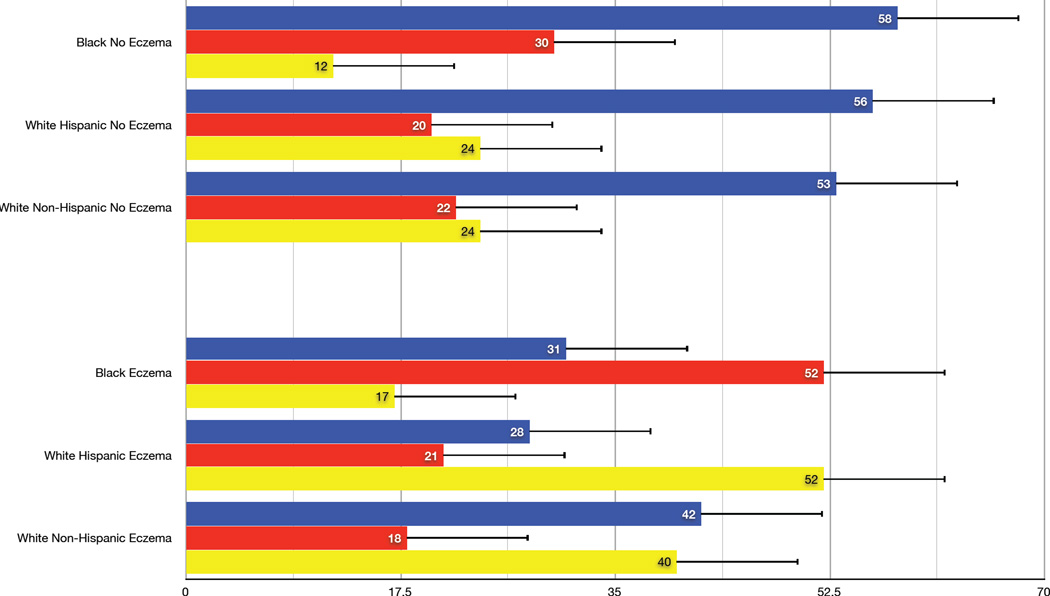
In the BADGER trial, 98% of the participants with asthma uncontrolled on low dose ICS had a differential response to the three step-up treatments. The best response for the entire study population was LABA step-up as this option was 1.5 times more likely to provide the best response compared to ICS or LTRA step-up. In the present post-hoc analysis of the BADGER data, this observation of a better LABA response was shown to be related substantially to eczema status. There was a strong pattern of best responses to LABA step-up in children without a history of eczema regardless of race or ethnicity status. In children with a past history of eczema, however, preferred responses to therapy depended on race/ethnicity (p=0.0002). In the eczema subgroup, Black participants (N = 29) responded best to ICS step-up, White-Hispanics (N=19) to LTRA step-up, and White non-Hispanics (N=31) demonstrated equivalent responses to step-up LABA or LTRA therapy (Figure 1).
Figure 1.
Overall probability of best response to step-up therapies with inhaled corticosteroid (ICS, red bars), long-acting beta agonist (LABA, blue bars, or a leukotriene-receptor antagonist (LTRA, orange bars) according to race/ethnicity by presence (Panel A) and absence (Panel B) of a history of eczema (active or past).
Based on this analysis, we speculate that children without a history of eczema respond best to step-up LABA therapy because their disease is less likely to be related to persistent airway inflammation. This hypothesis is supported by observations that the group with a history of eczema had more signs of atopic inflammation than the non-eczema group, including higher circulating eosinophils (p<0.015), a greater number of positive perennial allergen skin tests (p=0.05), and trend toward higher serum IgE levels (p = 0.11) (Table 1). A recently published post-hoc analysis of biomarker predictors in the BADGER study demonstrated that differential responses favoring LABA were related to a phenotype characterized by lower levels of inflammation (measured by lower levels of urinary leukotriene E4) and greater peripheral airway disease measured by impulse oscillometry 2. These studies are not directly comparable since the previous study reported changes in FEV1 as the main outcome instead of the present composite outcome, yet one could argue that the broad patterns differentiating LABA responders from non-LABA responders are similar.
Table 1.
Baseline characteristics of 163 patients with and without a history of eczema enrolled in the BADGER study
| Characteristics | Eczema 85 |
No-Eczema 78 |
P |
|---|---|---|---|
| Age (years) | 10.6±3.0 | 11.1±3.0 | 0.34 |
| Age at onset of eczema (years) | 2.6 ±2.3 | 3.4±3.1 | 0.06 |
| Height (cm) | 143.2±16.1 | 145.3±18.8 | 0.4 |
| Weight (kg) | 43.7±18.5 | 46.6±20.2 | 0.35 |
| Male Gender, n (%) | 50 (59%) | 56 (72%) | 0.10++ |
| Body mass index, (kg/m2) | 20.5±4.9 | 21.1±5.2 | 0.4 |
| Race or ethnic groups, n (%) | 0.3++ | ||
| Black | 29 (34%) | 16 (21%) | |
| White Hispanic or Latino | 19 (22%) | 20 (26%) | |
| White Non-Hispanic | 31 (37%) | 36 (46%) | |
| Other | 6 (7%) | 6 (7%) | |
| Number of (+) aeroallergen skin tests | 3.2±2.3 | 2.8±2.2 | 0.26 |
| Number of (+) perennial skin tests | 1.8±1.4 | 1.4±1.3 | 0.05 |
| Circulating eosinophil count (per ml) , median (25th, 75th quartile) | 347.1 (199.2, 532.6) | 256.0 (150.8, 408.0) | 0.015+ |
| Total serum IgE (kU/L) , median (25th, 75th quartile) | 347.0 (142.0,716.3) | 251.6 (65.5, 624.0) | 0.11+ |
| Exhaled Nitric Oxide (FeNO) (ppb), median (25th, 75th quartile) | 10.3 (7.0, 20.7) | 9.9 (6.6, 18.9) | 0.8+ |
| LTE4 (pg per mg creatinine)/FeNO (parts per billion) , median (25th, 75th quartile) | 5.2 (2.5, 10.4) | 5.0 (2.9, 9.7) | 0.9+ |
| LTE4 (pg per mg creatinine) , median (25th, 75th quartile) | 60.7 (40.6, 82.3) | 59.0 (44.0, 82.8) | 0.9+ |
| Pre-bronchodilator FEV1 (% predicted) | 98.8±13.3 | 97.3±14.2 | 0.5 |
| Maximum bronchodilator response*, % | 11.6±10.2 | 11.8±11.6 | 0.9 |
| Methacholine FEV1 PC20, (mg/ml) median (25th, 75th quartile) | 1.5 (0.6, 4.2) | 1.7 (0.6, 4.3) | 0.8+ |
| Asthma Control Test (ACT) | 20.8±2.8 | 20.1±4.1 | 0.2 |
p-value determined using Kruskal-Wallis test.
p-value determined using chi-square test.
Maximum bronchodilator response:
While response to LABA was primarily related to eczema status, race appeared to differentiate ICS responders (better in Black children) from LTRA responders (better in White children). A number of studies have reported a differential response to asthma therapy based on race 3,4,5,6, concluding that Black asthmatics demonstrate a relatively poor response to ICS therapy compared to White asthmatics. These studies perhaps infer that Blacks are less likely to respond fully to low doses of ICS therapy and are therefore more likely to exhibit uncontrolled disease due to partially treated atopic airway inflammation, while uncontrolled asthma in White children might be more likely to reflect non-eosinophilic inflammation or abnormal airway tone. This may partially explain why Black children with eczema respond preferentially to an increase in ICS therapy compared to adding LABA or LTRA to low-dose ICS.
In contrast, Black children had poor responses to LTRA therapy whether they had eczema or not. Inhibition of leukotriene activity by synthesis inhibitors or receptor antagonists has been established as an alternative for the treatment of patients with asthma. However, there are substantial variations in the treatment response possibly related to genetic variability. Drazen et al.7 defined a family of DNA sequence variants in the core promoter of the gene responsible for the 5-lipooxygenase pathway (ALOX5), leading to decreased transcription a relatively poor clinical response to anti-leukotriene therapy. Expression of some of these ALOX5 gene variants is higher in African-Americans, perhaps explaining the poor response to step-up LTRA therapy in BADGER 8. Further studies examining the interaction between genetic variation, race and response to LTRAs might be considered to test this hypothesis.
In conclusion, LABA step-up therapy is the preferred step-up therapy in asthmatic children without eczema. However, the preferred step-up therapy for those with eczema varies by race with Black children more likely to respond to increased ICS therapy, White Hispanics responding better to step-up LTRAs and White non- Hispanics to step-up LABA or LTRA therapy. Given the limitations of this relatively small and exploratory analysis, these findings will require replication in a larger prospective study. If validated, these novel findings might provide a personalized approach in targeting interventions for children with uncontrolled asthma based on specific and easily obtainable clinical features such as a history of eczema and race/ethnicity.
Clinical Summary.
In children with asthma without eczema, who are inadequately controlled on low-dose inhaled corticosteroids, addition of long-acting LABA step-up is the preferred treatment. However, children with asthma who also have eczema may respond better to increased ICS or LTRA-step-up depending on their race/ethnicity.
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute (HL064307, HL064288, HL064295, HL064287, HL064305, and HL064313), the National Institute of Allergy and Infectious Diseases (T32AI007635), and the Clinical Translational Science Award program of the National Center for Research Resources (UL1-RR025011 [Wisconsin] and UL1-RR024992 [St. Louis]). This study was performed in part by the General Clinical Research Centers at Washington University School of Medicine (M01-RR00036), and the University of Wisconsin (M01-RR03186). This study was also supported by Colorado CTSA Grant UL1 RR025780 from NCRR/NIH and UL1 TR000154 from NIH/NCATS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lemanske R, Mauger DT, Sorkness CA, Jackson DJ, Boehmer SJ, Martinez FD, et al. for the Childhood Asthma Research and Education Network of the National Heart, Lung and Blood Institute. Step-up therapy for children with uncontrolled asthma while receiving inhaled corticosteroids. N Engl J Med. 2010;362:975–985. doi: 10.1056/NEJMoa1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabinovitch N, Mauger DT, Reisdorph N, Covar R, Malka J, Lemanske RF, Jr, Morgan WJ, Guilbert TW, Zeiger RS, Bacharier LB, Szefler SJ. Predictors of Asthma Control and Lung Function Responsiveness to STEP-3 Therapy in Children with Uncontrolled Asthma. JACI. 2013 doi: 10.1016/j.jaci.2013.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi M, Thyne S, Choudhry S, Tsai HJ, Navarro D, Castro RA, et al. Ethnic-specific differences in bronchodilator responsiveness among African Americans, Puerto Ricans, and Mexicans with asthma. J Asthma. 2007;44:639–648. doi: 10.1080/02770900701554441. [DOI] [PubMed] [Google Scholar]
- 4.Naqvi M, Tcheurekdijan H, DeBoard JA, Williams LK, Navarro D, Castro RA, et al. Inhaled corticosteroids and augmented bronchodilator responsiveness in Latino and African American asthmatic patients. Ann Allergy Asthma Immunol. 2008;100:551–557. doi: 10.1016/S1081-1206(10)60055-5. [DOI] [PubMed] [Google Scholar]
- 5.Federico M, Covar R, Brown E, Leung DY, Spahn JD. Racial differences in T-lymphocyte Response to Glucocorticoids. Chest. 2005;127:571–578. doi: 10.1378/chest.127.2.571. [DOI] [PubMed] [Google Scholar]
- 6.Chan MTS, Leung DY, Szefler SJ, Spahn JD. Difficult-to-control asthma: Clinical characteristics of steroid-insensitive asthma. J Allergy Clin Immunol. 1998;101:594–601. doi: 10.1016/S0091-6749(98)70165-4. [DOI] [PubMed] [Google Scholar]
- 7.Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206. doi: 10.1056/NEJM199901213400306. [DOI] [PubMed] [Google Scholar]
- 8.Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, Wang J, Sylvester J, Holbrook J, Wise R, Weiss ST, Barnes K. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173:379–385. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]