Abstract
Dementia and gait impairments often coexist in older adults and patients with neurodegenerative disease. Both conditions represent independent risk factors for falls. The relationship between cognitive function and gait has recently received increasing attention. Gait is no longer considered merely automated motor activity but rather an activity that requires executive function and attention as well as judgment of external and internal cues. In this review, we intend to: (1) summarize and synthesize the experimental, neuropsychological, and neuroimaging evidence that supports the role played by cognition in the control of gait; and (2) briefly discuss the implications deriving from the interplay between cognition and gait.
In recent years, the dual task paradigm has been widely used as an experimental method to explore the interplay between gait and cognition. Several neuropsychological investigations have also demonstrated that walking relies on the use of several cognitive domains, including executive-attentional function, visuospatial abilities, and even memory resources. A number of morphological and functional neuroimaging studies have offered additional evidence supporting the relationship between gait and cognitive resources. Based on the findings from 3 lines of studies, it appears that a growing body of evidence indicates a pivotal role of cognition in gait control and fall prevention. The interplay between higher-order neural function and gait has a number of clinical implications, ranging from integrated assessment tools to possible innovative lines of interventions, including cognitive therapy for falls prevention on one hand and walking program for reducing dementia risk on the other.
Keywords: gait, cognitive function, aging, falls, neuroimaging, executive function
Gait disorders are common in older adults and represent a core feature of most neurodegenerative diseases. In a large, population-based study, the prevalence of any gait abnormality among people older than 70 years of age was 35% and increased further with age, reaching 46% in subjects older than 85 years.1 Gait impairments have been associated with an increased risk for falls and immobility, which in turn, contributes to greater disability, to institutionalization with consequent increases in health care costs, and ultimately, to death.1–3
Cognitive decline is another independent risk factor for falls.4,5 Gait disorders and falls are more prevalent in demented patients compared with nondemented subjects, and there is a direct relationship between cognitive impairment severity and increased gait abnormalities.6,7 Given these associations and the impact of cognitive impairment and gait abnormalities on functional independence, it is not surprising that the relationship between cognitive function and gait performance has received increasing attention in the last decade as the population of older adults rapidly expands. Gait is no longer considered merely automated motor activity, but rather an activity that requires executive function and attention as well as motivation and judgment of external and internal cues.8 Dysfunction in specific gait features has been associated with increased risk of cognitive decline and Alzheimer’s disease (AD)9; and gait abnormality, as measured by quantitative gait assessment, was reported to be frequent in older adults diagnosed with mild cognitive impairment (MCI) in a large, community-based cohort.10 Finally, elderly fallers have been found to display specific executive and attention dysfunctions,11 and a recent systematic review and meta-analysis of 27 prospective studies conducted in healthy older adults with at least 1 year of follow-up reported a significant relationship between executive dysfunction and increased risk of falling.12 These findings highlight the multiple links between gait, cognitive function, and falls.
The purpose of the present review is 2-fold. We provide an overview of the available evidence that can help to better elucidate the pathophysiological mechanisms underlying the relationship between specific cognitive domains/brain areas and different gait components by focusing on 3 separate lines of evidence. Second, we assess the possible “translational” relevance of these theoretical links and models. Toward this end, the topics of the present review include the following:
A summary of the experimental, neuropsychological, and neuroimaging evidence that supports the role played by cognition in gait control among several study populations with varying degrees of motor and cognitive impairments, including older adults, subjects with MCI, patients affected by dementia, and subjects with Parkinson’s disease (PD). This review is meant to be illustrative, but not exhaustive.
A discussion of the clinical implications derived from the interplay between cognition and gait.
Gait and Cognition: The Dual-Task Paradigm
The cognitive contribution to gait control is supported by experimental evidence offered by the dual-task (DT) paradigm, including both the “absolute” performance under DT and the interference that expresses the change on walking between DT and single-task performance. When subjects are asked to walk while performing another task, eg, serial subtracting or verbal fluency testing, the change in performance in either or both tasks indicates the extent of the cognitive demand.13 The simultaneous performance of 2 tasks not only leads to a competition for attention resources, but it also forces the brain to decide which task to unconsciously prioritize when no specific instructions are given about task prioritization. Although single-tasking and fast walking may also rely on attention, the degree of the cognitive challenge is likely to be less. This explains why the DT methodology has been widely used in recent years to explore the interplay between gait and cognition in both normal aging and several neurological diseases. Here, we briefly review key studies on these issues.
The DT Paradigm in Healthy Subjects
Most reports14–19 show that DT conditions exert a detrimental effect on gait variables in healthy adults of all ages, with stronger effects reported in older adults.15,17 In particular, a DT might interfere with gait by reducing walking speed and increasing variability (eg, stride-length variability, stride-time variability), which are measures of stability impairment.20–22 However, when evaluating the effects of prioritization, healthy young adults and, less so, older adults tended to give priority to motor over cognitive tasks.14,23 This result would support a “posture first” strategy that represents the unconscious strategy to avoid hazards in order to prevent falls. Nevertheless, even healthy young adults may not always prioritize gait,24,25 suggesting that a more complex model would be needed to account for prioritization strategies.26 Table 1 summarizes key findings on DT effects in healthy subjects.
TABLE 1.
Effect of DT on gait in healthy subjects: summary of main findings
| Study | Population | Gait assessment | DT | Main results |
|---|---|---|---|---|
| Lindenberger et al.15 (2000) | Young adults (n = 47); middle-aged adults (n = 45); older adults (n = 48) | Time taken to walk on a determined track | Memorization | ↓ Walking speed during DT and poorer memorization performance were associated with increasing age. |
| Bloem et al.23 (2001) | Young adults (n = 50); older adults (n = 13) | Time taken to complete each task | Set of tasks of increasing difficulty while walking | ↑ Motor errors amount was in relation with DT complexity. In complex tasks, young adults gave priority to motor tasks over cognitive tasks. |
| Beauchet et al.16 (2005) | Young adults (n = 49) | Quantitative gait assessment | Counting back from 50 | ↑ Gait variability and ↓ walking speed during DT. |
| Verghese et al.14 (2007) | Older adults (n = 189) | Quantitative gait assessment | Recitation of alternate letters of the alphabet | ↓ Walking speed during talking task prioritization as compared to walking speed during talking-walking task (attention to both talking and walking). Verbal output was not different on the 2 conditions. |
| Hollman et al.17 (2007) | Older adults (n = 20); middle-aged adults (n = 20); young adults (n = 20) | Quantitative gait assessment | Spelling 5-letter words in reverse | ↑ Gait variability and ↓ walking speed in older versus middle-aged and young adults during DT. DT performance was poorer in older versus middle-aged and young adults. |
| Hausdorff et al.19 (2008) | Older adults (n = 228) | Quantitative gait assessment | Serial 3 and 7 subtracting, phonememonitoring | ↓ Gait speed and swing time and ↑ gait variability during DT. Gait variability but not gait speed under DT was correlated with executive function. |
Criteria for papers selection: sizable sample, novelty of content, English language.
DT, dual task.
The DT Paradigm in MCI and AD
The DT effect is larger in subjects with cognitive impairment than in controls,18,27–30 and an increase in the DT complexity further worsens the gait measures.29,30 Furthermore, when comparing different grades of cognitive impairment (eg, MCI subjects and AD patients), more severe cognitive impairment (ie, AD) is associated with more detrimental DT effects on gait.30 Table 2 summarizes findings on the effects of DT in MCI and AD.
TABLE 2.
Effect of dual task on gait in MCI, AD, and PD subjects: summary of main findings
| Study | Population | Gait assessment | DT | Main results |
|---|---|---|---|---|
| Camicioli et al.27 (1997) | Older adults (n = 43); AD patients (n = 15) | Time and steps number taken to walk 30 feet | Verbal fluency task | ↓ Walking speed in AD patients versus older adults during DT. |
| Sheridan et al.28 (2003) | AD (n = 28) | Quantitative gait assessment | Forward digit span | ↑ Gait variability and ↓ walking speed during DT. EF performance scores were associated with ↑ gait variability during DT. |
| Montero-Odasso et al.29 (2012) | MCI (n = 43); CS (control subjects) (n = 25) | Quantitative gait assessment | Serial subtracting and semantic fluency task | ↑ Gait variability and ↓ walking speed in MCI versus CS during DT in relation with increasing DT complexity. |
| Muir et al.30 (2012) | AD (n = 23); MCI (n = 29); CS (n = 22); PD (n = 15) | Quantitative gait assessment | Semantic fluency task; serial subtracting (by 1s and 7s) | ↑ Gait variability and ↓ walking speed in AD and MCI versus CS during DT in relation with DT complexity. |
| Yogev et al.34 (2007) | Elderly fallers (n = 15); CS (n = 11) | Quantitative gait assessment | Serial subtracting | ↑ Gait asymmetry during DT in PD and in elderly fallers versus CS. |
| Bloem et al.32 (2001) | Young adults (n = 50); older adults (n = 20); PD (n = 20) | Time taken to complete each task | Set of tasks of increasing difficulty while walking | In addition to slow performance, PD patients gave priority to cognitive task rather then gait. |
| Hausdorff et al.33 (2003) | PD (n = 10) | Quantitative gait assessment | Serial subtracting | During normal walking, gait variability was correlated with fall risk, disease duration and severity, motor and cognitive function. When walking during DT, gait variability ↑. |
| Plotnik et al.35 (2009) | PD (n = 21); CS (n = 13) | Quantitative gait assessment | Serial subtracting | ↓ Bilateral coordination and ↑ gait variability in PD versus CS during DT |
| Plotnik et al.38 (2011) | PD (n = 30) | Quantitative gait assessment | Serial subtracting | Stride length, gait speed and variability, bilateral coordination and gait asymmetry were affected by DT. Set-shifting performance accounted for such effect. |
| Plotnik et al.21 (2011) | PD (n = 30, fallers and nonfallers) | Quantitative gait assessment | Serial subtracting | ↑ Gait variability, ↓ walking speed and ↓ bilateral coordination in fallers versus nonfallers. |
| Yogev-Seligmann et al.36 (2012) | PD (n = 20); CS (n = 20) | Quantitative gait assessment | Verbal fluency task with:(1) no prioritization; (2)gait prioritization; (3) task prioritization | ↓ Walking speed in both groups under DT without prioritization. ↑ Gait variability during all 3 DT in both groups. Task prioritization abilities were similar in PD and CS. |
| Yogev-Seligmann et al.37 (2013) | Young adults (n = 21); older adults (n = 15); PD (n = 18) | Quantitative gait assessment | Verbal fluency task during: (1) standing; (2) cycling; and (3) walking | DT effects were largest for walking, intermediate for cycling and smallest for standing in all groups but with maximum cost in PD, an intermediate cost in older and a minimum cost in young adults. |
| Fuller et al.42 (2013) | PD (n = 154) | Time taken to walk 50 feet | Verbal fluency task | During DT, PD patients showed ↓ verbal fluency and ↓ gait speed. Decrement on verbal fluency and not on walking speed during DT resulted associated with greater disability. |
Criteria for papers selection: sizable sample, novelty of content, English language. For a complete review on the DT effect in PD see also Kelly et al.43 (2012).
MCI, mild cognitive impairment; AD, Alzheimer’s disease; PD, Parkinson’s disease; DT, dual task; EF, executive function; CS, control subjects.
The DT Paradigm in PD
The primary effects of PD are seen in motor control, but cognitive function is also diminished, even in the early stages of disease.31 Thus, it is not altogether surprising that DT also affects the gait of patients with PD.22,32–37 In particular, PD patients display greater gait asymmetry,34 reduced bilateral coordination, and increased gait variability, compared to age-matched controls during DT conditions.22,33,35,36,38 In addition to reduced speed, these latter 2 variables have been associated with an increased risk for falling.22 Several reports have also described the concept of “wrong prioritization” (first task and then posture) that increases the risk of falling in PD patients,32,39 but PD patients who were cognitively intact have shown task prioritization abilities similar to those of healthy older adults.36 The magnitude of the DT impact on gait in PD therefore appears to be directly related to the underlying cognitive dysfunction.22,38,40,41 Evaluation of the relationship between DT interference and disability in PD revealed that the decrement in verbal fluency was associated with greater disability, whereas the change in gait speed was not.42 Furthermore, Yogev-Seligmann et al.37 have shown that the DT effect was largest for walking, intermediate for cycling, and smallest for standing, in young adults, older adults, and in cognitively intact patients with PD. Interestingly, the costs were highest for the PD patients and lowest for the young adults. Those results support the idea that walking represents a complex motor task requiring both bilateral coordination and dynamic postural control that are continuously monitored by cognitive resources. Table 2 summarizes the main findings on the effects of DT on the gait of PD patients.43
In conclusion, gait changes while performing another task, especially highly demanding ones that require simultaneous involvement of multiple cognitive skills,23,32,44 strongly support the hypothesis that shared higher-order neural networks are involved in the simultaneous performance of 2 tasks. Although the effects of DTs on gait are now well-described, it is not yet clear if the interference is a reflection of limited processing capacity or if it is a more specific deficit in the ability to perform walking and another complex task simultaneously. Be that as it may, walking is clearly altered when the concurrent task competes for common neural resources,45 and the degree of the alteration has a direct relationship with the extent of the frontal cognitive reserve that depends on both aging and disease-related pathological changes. It is, however, not yet clear if there are distinctions, in magnitude or quality, between the effects of aging and pathology on the DT gait interference.
A recent meta-analysis reported that changes in gait performance during DT were associated with increased risk for falling among healthy older adults and, to a considerable extent, among frail older adults.46 This idea, however, was not confirmed in a recent study on PD patients, possibly due to the mild stage and/or the normal cognitive status of the cohort.47 In the latter study,47 certain gait variability measures were unexpectedly unaffected during DT conditions. This may be due to the use of an auditory Stroop task, which provides a beneficial cueing effect. Indeed, external cues may influence gait through several possible mechanisms, eg, compensatory cerebellar-parieto-premotor pathways, or by facilitating task prioritization (executive function) and/or attentional control.48
Neuropsychological Evidence Linking Cognition and Gait
Many studies have demonstrated an association between poorer executive/attention domain and gait dysfunction in older adults,4,5,19,24,49–52 in patients with MCI and those with AD,28,53,54 and in patients with PD.40,55–59 Most studies that assessed the relationship between impairment of specific cognitive domains and poorer gait components consistently showed that poorer executive/attention domain performance was associated with increased gait variability measures, especially during tasks that demanded cognitive skills in older adults,19,24,51,60 in AD patients,28 and in PD patients.55 Furthermore, poorer executive function and increased gait variability measures during DT predicted future falls in older adults.4,5 In addition to gait variability measures, executive dysfunction has also been associated with reduced gait speed during highly demanding DTs in older adults,50 in patients with MCI,53,54 in AD patients53 and in PD patients (the association was weak in the latter group).40 In addition, freezing of gait (FOG) has been associated with executive dysfunction57,59,61 and with a worse progression of cognitive decline58 in PD patients.
Several more recent studies have demonstrated a visuospatial contribution to gait in older adults52 and in PD patients.56,61–63 In particular, gait stability, as evaluated by either quantitative gait assessment52,63 or Unified Parkinson’s Disease Rating Scale (UPDRS) subitems,56,62 was consistently associated with visuospatial abilities. Visual sampling (saccadic frequency) may explain this relationship in PD.64 Furthermore, different aspects of FOG in PD were correlated with visuospatial deficits in combination with executive dysfunction.61
Finally, some studies have shown the less intuitive contribution of memory to gait control in older adults.9,10,60,65 In those studies, memory was associated with cadence.9,10,60 Furthermore, 1 longitudinal study showed that memory impairment, in combination with executive dysfunction, was associated with greater gait speed decline.65 Table 3 summarizes the main findings on the relationship between neuropsychological findings and gait.
TABLE 3.
Relationship between cognitive domains and gait variables: summary of main neuropsychological findings
| Study | Population | Cognitive domains evaluated | Gait assessment | Main findings |
|---|---|---|---|---|
| Hausdorff et al.49 (2005) | Older adults (n = 43) | Executive/memory. In addition, tapping and catching abilities were evaluated. | Quantitative gait assessment measures | Better catching but not tapping performance was associated with walking (↑ gait speed and ↓ gait variability). ↓ Gait variability was associated with better executive function |
| Springer et al.24 (2006) | Young adults (n = 19); older adults including fallers and nonfallers (n = 41) | Executive/attention, memory | Quantitative gait assessment | ↑ Gait variability measures were increased during DT only in the fallers. ↑ Gait variability measures correlated with worse executive function. |
| Coppin et al.50 (2006) | Older adults (n = 737) | Executive/attention | Gait velocity assessment | Poor executive functions were associated with ↓ gait speed during complex walking tasks. |
| Hausdorff et al.19 (2008) | Older adults (n = 228) | Executive/attention, memory, visuospatial | Quantitative gait assessment | ↑ Gait variability but not ↓ gait speed during DT correlated with worse executive/attention performance. |
| van Iersel et al.51 (2008) | Older adults (n = 100) | Executive/attention, memory | Quantitative gait assessment | Executive functions were associated with ↑ gait variability measures during the most demanding DT (animal naming). |
| Herman et al.5 (2010) | Older adults (n = 262) followed for 2 y | Executive/attention, memory, visuospatial, global intelligence | Quantitative gait assessment | Baseline poorer executive function and ↑ gait variability during DT predicted future falls over the 2-year follow-up period. |
| Mirelman et al.4 (2012) | Older adults (n = 256) followed for 5 y | Executive/attention, memory, visuospatial | Quantitative gait assessment, falls report | Baseline executive/attention performance and gait variability under DT predicted future falls. |
| Sheridan et al.28 (2003) | AD (n = 28) | Executive/attention | Quantitative gait assessment | ↑ Gait variability during DT correlated with worse executive/attention performance. |
| Persad et al.53 (2008) | MCI (n = 26); AD (n = 15); CS (n = 12) | Executive/attention, memory, visuospatial | Gait velocity assessment on walkways of increasing cognitive demand | AD and MCI subjects with executive dysfunction were slower than CS and MCI without executive dysfunction; executive functioning was associated with ↓ gait speed during the most demanding walkway. |
| Montero-Odasso et al.54 (2009) | MCI (n = 55) | Executive/attention | Gait velocity test | Working memory was associated with ↓ gait speed especially during DT. |
| Yogev et al.55 (2005) | PD (n = 30); CS (n = 28) | Executive/attention, memory | Quantitative gait assessment | Executive functions were associated with ↑ gait variability measures during DT. |
| Rochester et al.40 (2008) | PD (n = 130) | Executive | Quantitative gait assessment | ↓ Gait speed during DT was weakly associated with executive functioning. |
| Amboni et al.57 (2008) | PD with FOG (n = 13); PD without FOG (n = 15) | Executive function | FOG-Q | PD with FOG versus PD without FOG performed worse on executive testing with a direct relation between FOG severity and executive impairment. |
| Amboni et al.58 (2010) | PD with FOG (n = 13); PD without FOG (n = 15) followed for 2 y | Executive function | FOG-Q | PD with FOG versus PD without FOG displayed a worse progression of executive dysfunction over a 2-year follow-up period. |
| Naismith et al.59 (2010) | PD with different FOG severity (n = 31) | Executive function | FOG-Q | FOG symptoms were selectively associated with poorer performance on tasks of set-shifting. |
| Martin et al.52 (2013) | Older adults (n = 422) | Executive/attention, memory, visuospatial | Quantitative gait assessment | Poorer executive functions were associated with worse performance on most gait measures and ↑ gait variability measures; visuospatial abilities were associated with ↑ double support phase variability. |
| Uc et al.56 (2005) | PD (n = 76); older adults (n = 161) | Executive/attention, memory, visuospatial | Subitems stability and gait UPDRS III | Poorer visuospatial abilities and executive function were associated with worse gait and postural stability. |
| Domellof et al.62 (2011) | Newly diagnosed PD (n5103) | Executive/attention, memory, visuospatial, language | Subitems UPDRS III | Axial signs (gait and stability) were associated with worse visuospatial memory and visuospatial functioning. |
| Amboni et al.63 (2012)a | PD (n = 43) | Executive/attention, memory, visuospatial | Quantitative gait assessment | The stability factor was found to be strongly and directly correlated with visuospatial domain performance. |
| Nantel et al.61 (2012) | PD (n = 30) | Executive/attention, visuospatial | FOG-Q; alternating SIP | Visuospatial deficits correlated with all SIP freeze metrics, FOG-Q total score; executive function deficits correlated with FOG-Q, SIP arrhythmicity and duration of FOG episodes. |
| Verghese et al.9 (2007)a | Older adults (n = 427) followed for 5 y | Executive/attention, memory, general cognition | Quantitative gait assessment | Baseline rhythm factor was associated with memory decline; baseline pace factor was associated with decline on executive function; both baseline rhythm and variability factor were associated with later dementia. |
| Verghese et al.10 (2008)a | Amnestic MCI (n = 54); nonamnestic MCI (n = 62); CS (n = 295) | Executive/attention, memory, language | Quantitative gait assessment | Subjects with amnestic MCI had worse rhythm and variability scores than those with nonamnestic MCI and controls. Subjects with nonamnestic MCI had worse performance on the pace domain than the other 2 groups. |
| Watson et al.65 (2010) | Older adults (n = 909) followed for 5 y | Executive/attention, memory, global cognition | Gait velocity assessment | Baseline poorer performance in global function, memory and executive function were associated with greater gait speed decline per year. |
| Holtzer et al.60 (2012) | Nondemented older adults (n = 671) | Executive/attention, verbal IQ, memory | Quantitative gait assessment | Executive/attention and memory predicted speed during DT; memory predicted cadence during both ST and DT; executive/ attention was predictor for gait variability during both ST and DT. |
Criteria for paper selection: novelty of content, evaluation of both cognition and gait, English language.
DT, dual task; AD, Alzheimer’s disease; MCI, mild cognitive impairment; CS, control subjects; PD, Parkinson’s disease; FOG: freezing of gait; FOG-Q, FOG questionnaire; UPDRS III, Unified Parkinson’s Disease Rating Scale–Motor Function, Part III; SIP, stepping in place; IQ, intelligence quotient; ST, single task.
Factor analysis approach.
Neuroimaging Findings Supporting the Relationship Between Cognition and Gait
A number of neuroimaging findings offer additional support to the relationship between gait and cognitive resources. Several magnetic resonance imaging (MRI) studies have evaluated the contribution of white-matter hyperintensities (WMHs) and/or the role of focal brain atrophy on both gait and cognitive dysfunction in older adults.66–73 WMHs were independently associated with gait disturbances and cognitive impairment either irrespective of66 or together with brain atrophy.71 Furthermore, periventricular leukoaraiosis was associated with poorer mobility and poorer executive performance in subjects with MCI, even after adjusting for ventriculomegaly.68 Conversely, ventricular dilatation,69 a marker of subcortical atrophy, or corpus callosum atrophy67 was independently linked to both reduced gait speed and cognitive impairment in subjects with WMHs. In addition, subjects with high-level gait disorders (defined as cautious gait with reduced mobility, significant fear of falling, abnormal postural responses, mild extrapyramidal signs, and frontal release signs73) displayed WM changes, as revealed by reduced fractional anisotropy and increased displacement values, in regions related to the motor system, as well as in cognitive and affective-related areas.73
Reduced volumes of both sensorimotor and frontoparietal regions, the former being a motor domain and the latter being a cognitive domain involved in visuospatial and executive abilities, have been associated with impaired quantitative gait variables; namely, reduced stride length and increased support time.70 Of note, a smaller volume in the prefrontal area was linked to both slower gait and reduced processing speed, suggesting a shared anatomic basis for both cognitive and motor function.72
In addition to these morphological findings, a few studies have assessed neurochemical and functional changes associated with gait and cognitive impairment in older adults.74–76 In 1 study, smaller hippocampal volume was associated with reduced stride length in a general elderly population.74 In contrast, decreased hippocampal metabolism was related to both reduced stride length and increased stride length variability (dynamic stability measure) in subjects with memory impairment, thus indicating a possible relationship among memory, gait and instability.74 A more recent MRI and MR spectroscopy study that evaluated the relationship between primary motor cortex and gait variables in subjects with MCI75 showed that the neurochemistry and volume of the primary motor cortex were associated with gait performance. In particular, stride-time variability was sensitive to neuronal function, whereas gait velocity was more affected by inflammatory damage and volumetric changes. Somewhat different findings were described in a pilot functional MRI (fMRI) study designed to identify the neural correlates of executive functioning in fallers.76 In that study, fallers showed reduced activation of the posterior lobe of the right cerebellum compared to nonfallers. Interestingly, certain regions of the cerebellum have been related to executive function in another study.77
A very recent study found significant reduction in the volumes of gray matter (GM) in several areas involved in motor and mental functions in PD patients with the postural instability gait difficulty (PIGD) PD subtype compared with patients with the tremor dominant (TD) PD subtype.78 In addition, 2 studies79,80 investigated the relationship between FOG, cognition, and GM atrophy. Despite some possible differences in local GM loss in the 2 studies that might have been due to the significantly different disease stage and/or disease duration of the 2 populations, FOG was associated with both GM atrophy in the posterior cortical regions and executive dysfunction. Another study designed to evaluate brain connectivity by means of resting-state fMRI reported that connectivity disruption of “executive-attention” and visual neural networks may be associated with FOG in PD.81 Taken together, these findings suggest that the association of frontal cognitive impairment and deficits in visuoperceptual areas might represent the anatomofunctional basis for FOG in PD.
Two positron emission tomography (PET) studies investigated the role of cholinergic system and β-amyloid on instability and gait disturbances in PD.82,83 Cholinergic system dysfunction was particularly associated with a history of falls,82 whereas neocortical β-amyloid deposition was linked with increased PIGD features,83 further supporting the key contribution of cognitive function to gait and falls. Consistent with this idea, a recent study that used the short-latency afferent inhibition method to reflect cholinergic activity demonstrated an association between gait dysfunction, cholinergic deficiency, and attention impairment in PD.84 The main findings on the relationships between gait, cognition, and neuroimaging results are summarized in Table 4.
TABLE 4.
Relationship among gait, cognition, and neuroimaging results: summary of main findings
| Study | Population | Technique | Gait assessment | Cognitive assessment | Main findings |
|---|---|---|---|---|---|
| Gouw et al.66 (2006) | Older adults (n = 574) | MRI | SPPB | MMSE | Subjects with disturbances of gait and balance (SPPB ≤ 10) had ↑ WMH than subjects with normal physical performance. Subjects with mild cognitive deficits (MMSE ≤ 25) had ↑ WMH than subjects with normal cognition. |
| Ryberg et al.67 (2007) | Older adults (n = 569) | MRI | Gait speed; history of falls; SPPB | MMSE; subjective memory complaints | Corpus callosum atrophy was independently associated with impaired global cognitive and motor function in subjects with age-related white-matter changes. |
| Onen et al.68 (2008) | MCI (n = 61) | MRI | Mobility assessment | Memory and attention tests | Performance on the TUG test was the only clinical predictor of periventricular leukoaraiosis; there was a greater presence of poor TUG test performances in patients with lower executive scores than in those with lower memory scores. |
| Rosano et al.70 (2008) | Older adults (n = 220) | MRI | Quantitative gait assessment | Digit Symbol Substitution Test; modified MMSE | ↓ Sensorimotor regions and ↓ frontoparietal regions were associated with ↓ stride length and ↑ support times. |
| Palm et al.69 (2009) | Older adults (n = 858) | MRI | Gait speed | Neuropsychological battery | Ventricular dilatation was found to be associated with both ↓ gait speed and cognitive dysfunction. |
| Rosano et al.71 (2010) | Older adults (n = 795) | MRI/MTR | Gait speed | Executive tests | ↓ Gait speed was associated with ↓ MTR, ↑ WMH, and brain atrophy in women, and with ↑ WMH and brain atrophy in men. Executive test scores substantially attenuated these associations. |
| Rosano et al.72 (2012) | Older adults (n = 214) | MRI | Gait speed | Neuropsychological battery | ↓ Prefrontal area volume was associated with ↓ gait speed and ↓ information processing speed. |
| Kafri et al.73 (2013) | HLGD (n = 13); elderly CS (n = 9); middle-age CS (n = 13) | MRI, DTI; Q-space imaging | Clinical assessment | Clinical assessment | HLGD patients had ↓ fractional anisotropy and ↑ displacement values in regions related to the motor system, as well as in cognitive and affective-related areas. |
| Liu-Ambrose et al.76 (2008) | Older adults (n = 83) | fMRI | History of falls | Frontal task (attention and response inhibition) during fMRI scanning | As compared with nonfallers, fallers displayed ↓ activation of the posterior lobe of the right cerebellum. |
| Zimmerman et al.74 (2009) | Older adults (n = 48) | MRI/MRS | Quantitative gait assessment | Clinical Dementia Rating Scale, memory test | ↓ Hippocampal volume was associated with ↓ stride length; ↓ hippocampal metabolism was associated with ↑ stride length variability; ↓ hippocampal metabolism was related to both ↓ stride length and ↑stride length variability in subjects with memory deficits. |
| Annweiler et al.75 (2013) | MCI (n = 20) | MRI/MRS | Quantitative gait assessment | Neuropsychological battery | The neurochemistry and volume of the primary motor cortex were associated with gait performance. Stride time variability was sensitive to neuronal function, whereas gait velocity was more affected by inflammatory damage and volumetric changes. |
| Rosenberg-Katz et al.78 (2013) | p-PIGD PD (n = 30); p-TD PD (n = 29) | MRI/resting state fMRI | UPDRS subitems | Computerized cognitive battery | GM was reduced in the p-PIGD versus p-TD in several areas involving motor as well as cognitive functions. ↑ GM volumes in the pre-SMA were associated with stronger connectivity between pre-SMA and the putamen in p-PIGD. |
| Tessitore et al.79 (2012) | PD with FOG (n = 12); PD without FOG (n = 12); CS (n = 12) | MRI | FOG-Q | Neuropsychological battery | Voxel-based morphometry analysis in PD with FOG versus PD without FOG and CS showed ↓ GM volume in the left posterior cortical areas. Patients with FOG scored lower on tests exploring both executive function and spatial programming. |
| Kostic et al.80 (2012) | PD with FOG (n = 17); PD without FOG (n = 20); CS (n = 34) | MRI | FOG-Q | Neuropsychological battery focused on executive function | PD with FOG versus PD without FOG and CS showed ↓ GM volume in the left frontal and parietal cortical areas. FOG severity correlated with frontal executive deficits. |
| Tessitore et al.81 (2012) | PD with FOG (n = 16); PD without FOG (n = 13); CS (n = 15) | Resting state fMRI | FOG-Q | Neuropsychological battery | PD with FOG exhibited ↓ functional connectivity within both executive-attention and visual networks. FOG severity was correlated with ↓ connectivity within the two networks. PD with FOG scored lower on frontal testing. |
| Bohnen et al.82 (2009) | PD without dementia (n = 44); CS (n = 15) | AChE and VMAT2 PET imaging | History of falls | MMSE | ↓ Cortical AChE activity in PD fallers as compared to PD nonfallers and CS. There was no difference in nigrostriatal dopaminergic activity between PD fallers and nonfallers. |
| Müller et al.83 (2013) | PD (n = 44) | PIB and VMAT2 PET imaging | UPDRS | Dementia Rating Scale | ↑ PIGD features severity was associated with ↑ β-amyloid cortical deposition independently of nigrostriatal dopaminergic activity changes. |
Criteria for papers selection: novelty of content, evaluation of both cognition and gait in the context of neuroimaging studies, English language.
MRI, magnetic resonance imaging; SPPB, short physical performance battery; MMSE, Mini–Mental State Examination; WMH, white-matter hyperintensities; MCI, mild cognitive impairment; TUG, timed up-and-go test; MTR, magnetization transfer ratio; HLGD, high-level gait disorders; CS, control subjects; DTI, diffusion tensor imaging; fMRI, functional magnetic resonance imaging; MRS, magnetic resonance spectroscopy; p-PIGD, predominantly postural instability gait difficulty; PD, Parkinson’s disease; p-TD, predominantly tremor dominant; UPDRS, Unified Parkinson’s Disease Rating Scale; GM, gray matter; pre-SMA, pre-supplementary motor area; FOG, freezing of gait; FOG-Q, FOG questionnaire; VMAT2, vesicular monoamine transporter type 2; AchE, acetylcholinesterase; PIB, Pittsburg Compound B.
Summary of Evidence From Experimental, Neuropsychological, and Neuroimaging Studies
The 3 different lines of evidence herein reported consistently support the interaction between cognitive function and gait performance. In particular, it is possible to point to 3 levels of associations: (1) unspecific association; (2) an association between more specific findings and specific gait measures; and (3) a specific association among gait dysfunction, cognitive impairment, and reduced cholinergic activity.
Unspecific Association
Broad neuroimaging findings (eg, generalized brain atrophy/WMH) have been related to gait speed, an overall measure of gait, suggesting that the association between gait and cognition has a nonspecific nature.
An Association Between More Specific Findings and Specific Gait Measures
Experimental and neuropsychological findings support the important role played by attention and executive function in gait control and the regulation of gait speed and variability. These neuropsychological data suggest a more specific role of cognitive function. In particular, gait may be modulated by frontal cognitive networks that use and manipulate sensory information via cortical sensory association areas, namely the parietal and occipital cortices.85 The frontal-visuospatial network may be one of the neural substrates that regulates both velocity and gait variability, a measure of dynamic stability. This network is involved in the flexible adaptation of motor behavior, especially whenever the environmental context changes. Interestingly, neuroimaging studies further support these relationships by showing associations between frontoparietal brain atrophy and/or reduced neural activation, on one hand, and gait variability, instability or FOG, on the other hand. Of note, a few neuropsychological findings and neuroimaging data such as reduced hippocampal metabolism also support the contribution of memory to gait control. Because the hippocampus has been associated with keeping rhythmic locomotion within a learned motor plan,74 it could be speculated that either memory plays a direct role in rhythm maintenance, or memory and rhythm might share common neural networks; alternatively, the association between memory and gait might be unspecific.
Partially in agreement with this model, Lord et al.86 found a direct correlation between attention and gait speed using a factor analysis approach in a sample of older adults. Nevertheless, they did not find a relationship between executive function and gait variability measures or a correlation between memory and rhythm. This could be an artifact of the factor analysis approach and/or related to the high-functioning cognitive status of the population. Future studies should more fully address this issue.
A Specific Association Among Gait Dysfunction, Cognitive Impairment, and Reduced Cholinergic Activity
In this scenario, the cholinergic system may play a pivotal role as a unifying factor. In fact, the nucleus basalis of Meynert supplies the majority of the cholinergic input to the cerebral cortex, modulating hippocampus activity and the cognitive frontoparietal networks.87 Moreover, the cholinergic pedunculopontine nucleus (PPN) may exert a key role on both attention88 and locomotion.89 The recent neuroimaging82 and electrophysiological findings84 reported above (in the section Neuroimaging findings supporting the relationship between cognition and gait) further corroborate the intriguing cholinergic hypothesis.90
Implications
The main findings summarized herein show that cognitive impairment and gait abnormalities, as well as dementia and increased risk for falls, often coexist. Furthermore, this growing body of data has demonstrated that gait abnormalities predict the future development of dementia, and cognitive impairment increases the risk of falls (see Fig. 1). Extending this innovative idea,91 we suggest that integrated cognitive/motor assessment tools are needed for both dementia and fall risk estimation. In other words, any tool aimed at assessing gait impairment and fall risk should ideally take into account cognitive resources92 and, in turn, assessment tools for identifying subjects at risk of developing dementia should include gait assessment, perhaps speed, gait variability, and other measures.
FIG. 1.
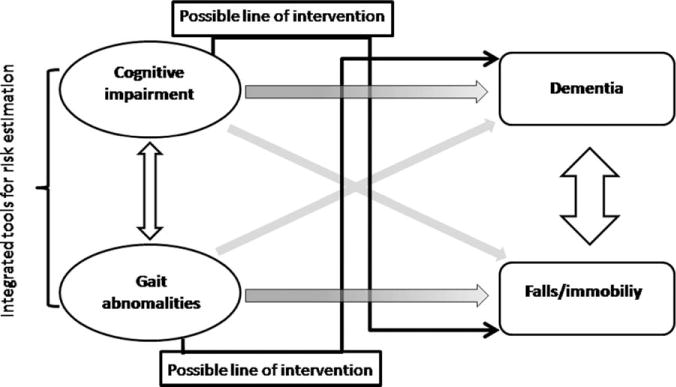
Traditionally, cognitive impairment predicted dementia, whereas gait abnormalities increased the risk for falling (shaded arrows). A more complete understanding suggests that cognitive impairment and gait abnormalities, as well as dementia and falls, are associated with each other (white arrows). According to recent evidence, gait abnormalities predict dementia and cognitive impairment increases falls risk (gray arrows); based on the close relationship between cognition and gait, on one hand, integrated tools for risk estimation are needed (bracket), on the other hand, possible lines of intervention (black arrows) could rely on enhancing cognition for falls prevention and on walking training for lessening dementia risk. Adapted from Montero-Odasso et al.91
Consistent with this idea, very recently Verghese et al.93 introduced a new construct that they refer to as motor cognitive risk (MCR) syndrome. This syndrome integrates motor and cognitive deficits and aims at enhancing the early detection of subjects who have an increased risk of developing dementia. Operationally, MCR identifies a condition of MCI plus reduced speed gait. In a prospective study, the MCR construct predicted dementia better then MCI alone or gait speed alone.93 Although these intriguing findings can be interpreted in several distinct ways, they further support the idea that gait and cognition are related to each other.94
Another implication deriving from the model regards possible lines of intervention (see Fig. 1). These include: (1) cognitive therapy for falls prevention; and (2) walking programs for reducing dementia risk, and are briefly reviewed in the next 2 sections.
Cognitive Therapy for Falls Prevention
A recent systematic review95 concluded that factors such as the degree of cognitive impairment could affect the success of interventions for falls prevention; cognitive training interventions might represent an option to bridge this gap. Further, as the body of evidence herein reported shows, cognitive abilities are apparently critical to safe ambulation. A number of studies have evaluated the effectiveness of cognitive training interventions, mainly based on DT training, in improving gait and balance, and in reducing falling risks in older adults and demented or parkinsonian patients. Overall, the effects of these preliminary therapies on gait measures are encouraging. Nevertheless, their effectiveness in reducing fall risk per se has not been evaluated (2 recent reviews: Montero-Odasso et al.91 and Segev-Jacubovski et al.96).
A further possible line of intervention to enhance cognitive function to prevent falls is the use of cognitive pharmacological therapy. Several cognitive enhancers including methylphenidate, cholinesterase inhibitors, and memantine have been used in a number of pilot studies to assess their effect on gait in older adults or PD patients. Preliminary results are generally promising, but larger clinical trials are needed. Furthermore, it is worth noting that, apart from indirect effects via cognition, these drugs might affect gait by modifying the cholinergic, noradrenergic, dopaminergic, and glutamatergic networks involved in gait (2 recent reviews: Montero-Odasso et al.91 and Segev-Jacubovski et al.96).
Walking Programs for Reducing Dementia Risk
In the last 2 decades, several studies demonstrated the effectiveness of physical activity for preventing cognitive decline. A recent large meta-analysis concluded that physical activity is inversely associated with dementia risk.97 Nevertheless, few studies have specifically assessed and conclusively shown the effectiveness of walking programs for preventing cognitive decline.98–101 In the light of the close relationship between cognition and gait, one explanation might be that walking requires the coactivation and the interfacing of multiple cognitive domains and might, therefore, act as a training ground for cognitive skills. It may, perhaps, be more effective than other types of physical activity in enhancing cognitive function. Future trials specifically designed to verify this hypothesis are needed.
Future Directions
Based on the wide amount of emerging data, cognition and gait appear to be closely related in a complex fashion. The temporal relationship between cognitive decline and gait dysfunction is still not fully understood. Previously, Verghese et al.9 showed that quantitative gait measures might predict future risk of cognitive decline and dementia in initially nondemented older adults. More recently, a longitudinal population-based study concluded that slow gait speed precedes cognitive decline, whereas baseline cognition is not associated with later changes in gait speed. Taken together, these findings support the idea that gait impairment antedates cognitive dysfunction and may even represent a reliable risk factor for cognitive decline.
Nevertheless, in agreement with the opposite notion that cognitive decline precedes later gait impairment,65,103 Holtzer et al.104 reported that the protective effect of executive function and episodic memory against speed decline is greater in subjects with higher cognitive reserve. This might suggest that cognitive reserve plays a pivotal role in regulating later changes in both age-related and disease-related gait impairment. Untangling the temporal and the likely causative relationships between cognitive and gait impairments (eg, first gait changes or cognitive dysfunction?) could aid in the early identification of subjects who have an increased risk for falls or dementia. To better elucidate this issue, longitudinal studies are needed. Finally, future studies should also extend the present understandings to other issues (eg, motor recklessness, cautious gait, fear of falling, and depression) that may also be related to gait-cognition interactions.
In conclusion, a growing body of experimental, neuropsychological, and neuroimaging evidence supports the pivotal role of cognition in gait control and the prevention of falls. The close interplay between higher-order neural function and gait points to a number of clinical implications ranging from integrated assessment tools to possible innovative lines of interventions and future directions.
Footnotes
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 4.Mirelman A, Herman T, Brozgol M, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012;7:e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65:1086–1092. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Iersel MB, Hoefsloot W, Munneke M, Bloem BR, Olde Rikkert MG. Systematic review of quantitative clinical gait analysis in patients with dementia. Z Gerontol Geriatr. 2004;37:27–32. doi: 10.1007/s00391-004-0176-7. [DOI] [PubMed] [Google Scholar]
- 7.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 8.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc. 2008;56:1244–1251. doi: 10.1111/j.1532-5415.2008.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausdorff JM, Doniger GM, Springer S, Yogev G, Simon ES, Giladi N. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing. 2012;41:299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
- 13.Abernethy B. Dual-task methodology and motor skills research: some applications and methodological constraints. J Hum Mov Stud. 1988;14:101–132. [Google Scholar]
- 14.Verghese J, Kuslansky G, Holtzer R, et al. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88:50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- 16.Beauchet O, Dubost V, Herrmann FR, Kressig RW. Stride-to-stride variability while backward counting among healthy young adults. J Neuroeng Rehabil. 2005;2:26. doi: 10.1186/1743-0003-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture. 2007;26:113–119. doi: 10.1016/j.gaitpost.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Theill N, Martin M, Schumacher V, Bridenbaugh SA, Kressig RW. Simultaneously measuring gait and cognitive performance in cognitively healthy and cognitively impaired older adults: the Basel motor-cognition dual-task paradigm. J Am Geriatr Soc. 2011;59:1012–1018. doi: 10.1111/j.1532-5415.2011.03429.x. [DOI] [PubMed] [Google Scholar]
- 19.Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1335–1343. doi: 10.1093/gerona/63.12.1335. [dup of 51] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hausdorff JM, Rios D, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 21.Hausdorff JM. Gait dynamics in Parkinson’s disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos. 2009;19:026113. doi: 10.1063/1.3147408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotnik M, Giladi N, Dagan Y, Hausdorff JM. Postural instability and fall risk in Parkinson’s disease. Impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp Brain Res. 2011;210:529–538. doi: 10.1007/s00221-011-2551-0. [DOI] [PubMed] [Google Scholar]
- 23.Bloem BR, Valkenburg VV, Slabbekoorn M, Willemsen MD. The Multiple Tasks Test: development and normal strategies. Gait Posture. 2001;14:191–202. doi: 10.1016/s0966-6362(01)00141-2. [DOI] [PubMed] [Google Scholar]
- 24.Springer S, Giladi N, Peretz C, Yogev G, Simon ES, Hausdorff JM. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 25.Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, Dickstein R, Giladi N, Hausdorff JM. How does explicit prioritization alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Phys Ther. 2010;90:177–186. doi: 10.2522/ptj.20090043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27:765–770. doi: 10.1002/mds.24963. [DOI] [PubMed] [Google Scholar]
- 27.Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: the effect of a dual task in aging and Alzheimer’s disease. Neurology. 1997;48:955–958. doi: 10.1212/wnl.48.4.955. [DOI] [PubMed] [Google Scholar]
- 28.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 29.Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil. 2012;93:293–299. doi: 10.1016/j.apmr.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35:96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 31.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 32.Bloem BR, Valkenburg VV, Slabbekoorn M, van Dijk JG. The multiple tasks test. Strategies in Parkinson’s disease. Exp Brain Res. 2001;137(3–4):478–486. doi: 10.1007/s002210000672. [DOI] [PubMed] [Google Scholar]
- 33.Hausdorff JM, Balash J, Giladi N. Effects of cognitive challenge on gait variability in patients with Parkinson’s disease. J Geriatr Psychiatry Neurol. 2003;16:53–58. doi: 10.1177/0891988702250580. [DOI] [PubMed] [Google Scholar]
- 34.Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res. 2007;177:336–346. doi: 10.1007/s00221-006-0676-3. [DOI] [PubMed] [Google Scholar]
- 35.Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of gait and Parkinson’s disease: the effects of dual tasking. J Neurol Neurosurg Psychiatry. 2009;80:347–350. doi: 10.1136/jnnp.2008.157362. [DOI] [PubMed] [Google Scholar]
- 36.Yogev-Seligmann G, Rotem-Galili Y, Dickstein R, Giladi N, Hausdorff JM. Effects of explicit prioritization on dual task walking in patients with Parkinson’s disease. Gait Posture. 2012;35:641–646. doi: 10.1016/j.gaitpost.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Yogev-Seligmann G, Giladi N, Gruendlinger L, Hausdorff JM. The contribution of postural control and bilateral coordination to the impact of dual tasking on gait. Exp Brain Res. 2013;226:81–93. doi: 10.1007/s00221-013-3412-9. [DOI] [PubMed] [Google Scholar]
- 38.Plotnik M, Dagan Y, Gurevich T, Giladi N, Hausdorff JM. Effects of cognitive function on gait and dual tasking abilities in patients with Parkinson’s disease suffering from motor response fluctuations. Exp Brain Res. 2011;208:169–179. doi: 10.1007/s00221-010-2469-y. [DOI] [PubMed] [Google Scholar]
- 39.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Rochester L, Nieuwboer A, Baker C, et al. Walking speed during single and dual tasks in Parkinson’s disease: which characteristics are important? Mov Disord. 2008;23:2312–2318. doi: 10.1002/mds.22219. [DOI] [PubMed] [Google Scholar]
- 41.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35:715–728. doi: 10.1016/j.neubiorev.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 42.Fuller RL, Van Winkle EP, Anderson KE, et al. Dual task performance in Parkinson’s disease: a sensitive predictor of impairment and disability. Parkinsonism Relat Disord. 2013;19:325–328. doi: 10.1016/j.parkreldis.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dualtask walking deficits in people with Parkinson’s disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719. doi: 10.1155/2012/918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srygley JM, Mirelman A, Herman T, Giladi N, Hausdorff JM. When does walking alter thinking? Age and task associated findings. Brain Res. 2009;1253:92–99. doi: 10.1016/j.brainres.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klingberg T. Limitations in information processing in the human brain: neuroimaging of dual task performance and working memory tasks. Prog Brain Res. 2000;126:95–102. doi: 10.1016/S0079-6123(00)26009-3. [DOI] [PubMed] [Google Scholar]
- 46.Beauchet O, Annweiler C, Dubost V, et al. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16:786–795. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 47.Smulders K, Esselink RA, Weiss A, Kessels RP, Geurts AC, Bloem BR. Assessment of dual tasking has no clinical value for fall prediction in Parkinson’s disease. J Neurol. 2012;259:1840–1847. doi: 10.1007/s00415-012-6419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rochester L, Burn DJ, Woods G, Godwin J, Nieuwboer A. Does auditory rhythmical cueing improve gait in people with Parkinson’s disease and cognitive impairment? A feasibility study Mov Disord. 2009;24:839–845. doi: 10.1002/mds.22400. [DOI] [PubMed] [Google Scholar]
- 49.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 50.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Olde Rikkert MG. Executive functions are associated with gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- 52.Martin KL, Blizzard L, Wood AG, et al. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:726–732. doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- 53.Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. J Gerontol A Biol Sci Med Sci. 2008;63:1350–1355. doi: 10.1093/gerona/63.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9:41. doi: 10.1186/1471-2318-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22:1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 56.Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- 57.Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- 58.Amboni M, Barone P, Picillo M, et al. A two-year follow-up study of executive dysfunctions in parkinsonian patients with freezing of gait at on-state. Mov Disord. 2010;25:800–802. doi: 10.1002/mds.23033. [DOI] [PubMed] [Google Scholar]
- 59.Naismith SL, Shine JM, Lewis SJ. The specific contributions of set-shifting to freezing of gait in Parkinson’s disease. Mov Disord. 2010;25:1000–1004. doi: 10.1002/mds.23005. [DOI] [PubMed] [Google Scholar]
- 60.Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control. 2012;16:64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nantel J, McDonald JC, Tan S, Bronte-Stewart H. Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson’s disease. Neuroscience. 2012;221:151–156. doi: 10.1016/j.neuroscience.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Domellöf ME, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson’s disease. Mov Disord. 2011;26:2183–2189. doi: 10.1002/mds.23814. [DOI] [PubMed] [Google Scholar]
- 63.Amboni M, Barone P, Iuppariello L, et al. Gait patterns in Parkinsonian patients with or without mild cognitive impairment. Mov Disord. 2012;27:1536–1543. doi: 10.1002/mds.25165. [DOI] [PubMed] [Google Scholar]
- 64.Galna B, Lord S, Daud D, Archibald N, Burn D, Rochester L. Visual sampling during walking in people with Parkinson’s disease and the influence of environment and dual-task. Brain Res. 2012;1473:35–43. doi: 10.1016/j.brainres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65:1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gouw AA, Van der Flier WM, van Straaten EC, et al. Simple versus complex assessment of white matter hyperintensities in relation to physical performance and cognition: the LADIS study. J Neurol. 2006;253:1189–1196. doi: 10.1007/s00415-006-0193-5. [DOI] [PubMed] [Google Scholar]
- 67.Ryberg C, Rostrup E, Stegmann MB, et al. Clinical significance of corpus callosum atrophy in a mixed elderly population. Neurobiol Aging. 2007;28:955–963. doi: 10.1016/j.neurobiolaging.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 68.Onen F, Henry-Feugeas MC, Roy C, Baron G, Ravaud P. Mobility decline of unknown origin in mild cognitive impairment: an MRI-based clinical study of the pathogenesis. Brain Res. 2008;1222:79–86. doi: 10.1016/j.brainres.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 69.Palm WM, Saczynski JS, van der Grond J, et al. Ventricular dilation: association with gait and cognition. Ann Neurol. 2009;66:485–493. doi: 10.1002/ana.21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosano C, Sigurdsson S, Siggeirsdottir K, et al. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2010;31:1197–1204. doi: 10.1016/j.neurobiolaging.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kafri M, Sasson E, Assaf Y, et al. High-level gait disorder: associations with specific white matter changes observed on advanced diffusion imaging. J Neuroimaging. 2013;23:39–46. doi: 10.1111/j.1552-6569.2012.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Annweiler C, Beauchet O, Bartha R, et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain. 2013;136(Pt 3):859–871. doi: 10.1093/brain/aws373. [DOI] [PubMed] [Google Scholar]
- 76.Liu-Ambrose TY, Nagamatsu LS, Handy TC, Leghari A. Does impaired cerebellar function contribute to risk of falls in seniors? A pilot study using functional magnetic resonance imaging. J Am Geriatr Soc. 2008;56:2153–2155. doi: 10.1111/j.1532-5415.2008.01984.x. [DOI] [PubMed] [Google Scholar]
- 77.Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 78.Rosenberg-Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology. 2013;80:1476–1484. doi: 10.1212/WNL.0b013e31828cfaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tessitore A, Amboni M, Cirillo G, et al. Regional graymatter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am J Neuroradiol. 2012;33:1804–1809. doi: 10.3174/ajnr.A3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kostic VS, Agosta F, Pievani M, et al. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- 81.Tessitore A, Amboni M, Esposito F, et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Parkinsonism Relat Disord. 2012;18:781–787. doi: 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 82.Bohnen NI, Müller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Müller ML, Frey KA, Petrou M, et al. β-amyloid and postural instability and gait difficulty in Parkinson’s disease at risk for dementia. Mov Disord. 2013;28:296–301. doi: 10.1002/mds.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rochester L, Yarnall AJ, Baker MR, et al. Cholinergic dysfunction contributes to gait disturbance in early Parkinson’s disease. Brain. 2012;135(Pt 9):2779–2788. doi: 10.1093/brain/aws207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chow TW, Cummings J. Frontal-subcortical circuits. In: Miller BL, Cummings J, editors. The Human Frontal Lobes: Functions and Disorders. New York: The Guilford Press; 2007. pp. 25–43. (Science and Practice of Neuropsychology Series). [Google Scholar]
- 86.Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci. 2013;68:820–827. doi: 10.1093/gerona/gls255. [DOI] [PubMed] [Google Scholar]
- 87.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 88.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 89.Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson’s disease. Brain. 2000;123:1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- 90.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26:2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 91.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Snijders AH, Verstappen CC, Munneke M, Bloem BR. Assessing the interplay between cognition and gait in the clinical setting. J Neural Transm. 2007;114:1315–1321. doi: 10.1007/s00702-007-0781-x. [DOI] [PubMed] [Google Scholar]
- 93.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68:412–418. doi: 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hausdorff JM, Buchman AS. What links gait speed and MCI with dementia? A fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci. 2013;68:409–411. doi: 10.1093/gerona/glt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oliver D, Connelly JB, Victor CR, et al. Strategies to prevent falls and fractures in hospitals and care homes and effect of cognitive impairment: systematic review and meta-analyses. BMJ. 2007;334:82. doi: 10.1136/bmj.39049.706493.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Segev-Jacubovski O, Herman T, Yogev-Seligmann G, Mirelman A, Giladi N, Hausdorff JM. The interplay between gait, falls and cognition: can cognitive therapy reduce fall risk? Expert Rev Neurother. 2011;11:1057–1075. doi: 10.1586/ern.11.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 98.van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med. 2008;42:344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- 99.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 100.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maki Y, Ura C, Yamaguchi T, et al. Effects of intervention using a community-based walking program for prevention of mental decline: a randomized controlled trial. J Am Geriatr Soc. 2012;60:505–510. doi: 10.1111/j.1532-5415.2011.03838.x. [DOI] [PubMed] [Google Scholar]
- 102.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–937. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buchman AS, Boyle PA, Leurgans SE, Barnes LL, Bennett DA. Cognitive function is associated with the development of mobility impairments in community-dwelling elders. Am J Geriatr Psychiatry. 2011;19:571–580. doi: 10.1097/JGP.0b013e3181ef7a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holtzer R, Wang C, Lipton R, Verghese J. The protective effects of executive functions and episodic memory on gait speed decline in aging defined in the context of cognitive reserve. J Am Geriatr Soc. 2012;60:2093–2098. doi: 10.1111/j.1532-5415.2012.04193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


