Oxidative stress, the presence of reactive oxygen species (ROS) in excess of the antioxidant capacity in the heart induces myocardial damage, accumulation of which leads to ischemic heart disease and heart failure. NADPH oxidase (Nox) 2 and 4 are the major sources of O2− and H2O2 in the heart and play a crucial role in the regulation of growth and death in cardiomyocytes. Both Nox2 and Nox4 are upregulated in response to ischemia-reperfusion (I/R), thereby contributing to ROS production and consequent myocardial injury. Suppression of either one of them can reduce ROS and I/R injury in the heart. Importantly, however, a minimum level of ROS production by either Nox2 or Nox4 is essential for the activation of HIF-1α and inhibition of PPARα during I/R, such that combined suppression of both Nox2 and Nox4 exacerbates myocardial I/R injury. Thus, either excessive activation or suppression of Noxs below physiological levels can induce cardiac injury. Here we discuss both detrimental and salutary functions of Nox isoforms during myocardial I/R.
Noxs are the only known enzymes whose sole biological function is to purposefully produce O2− or H2O2, and they are major sources of reactive oxygen species (ROS) in cardiovascular cell types (Maejima, 2011). Thus far, seven members of the Nox family of proteins (Nox1-5 and Duox1,2) have been identified. Nox2 and Nox4 are abundantly expressed in cardiomyocytes and play a crucial role in the development of cardiac injury and remodeling (Maejima 2011). Nox2 is predominantly localized to the plasma membrane and its activity is regulated by cytosolic factors, including p47phox, p67phox, p40phox and Rac. Nox4 is predominantly localized on the intracellular membranes of organelles, including the nucleus, mitochondria, and the endoplasmic reticulum (ER). Nox4 is believed to be constitutively active and its activity is controlled primarily by its expression levels. We have shown recently that hypertrophic stimuli induce upregulation of Nox4, thereby leading to increased production of ROS, mitochondrial dysfunction and apoptosis in cardiomyocytes (Ago, 2010). Cardiac hypertrophy and dysfunction in response to pressure overload was significantly attenuated in cardiac-specific Nox4 knockout (KO) mice, and this was accompanied by preservation of mitochondrial function (Kuroda, 2010). Although Nox2 is involved in cardiac remodeling, including cardiomyocyte hypertrophy, apoptosis and interstitial fibrosis, after permanent coronary ligation (Looi, 2008), Nox2 is not essential for the development of cardiac hypertrophy after pressure overload (Grieve, 2006). In contrast, Nox1 is expressed mainly in the endothelial cells in the heart and is involved in endotoxin-induced cardiomyocyte apoptosis (Matsuno, 2012). These findings indicate that Noxs have isoform-specific functions in the heart during cardiac remodeling.
Less is known regarding the isoform specific functions of Noxs in myocardial ischemia-reperfusion (I/R) injury. A study using p47phox KO mice suggested that Nox2 does not contribute to myocardial injury after I/R (Hoffmeyer, 2000). However, studies directly examining the role of each Nox isoform in regulating myocardial I/R injury using isoform-specific KO mice were not reported until recently. We investigated the function of Nox2 and Nox4 in the heart using systemic Nox4 KO (sNox4 KO) and systemic Nox2 KO (sNox2KO) mice. Both types of mice exhibited similar levels of reduction in ROS production and attenuation of the infarct size after I/R, indicating that the ROS produced by Nox2 and Nox4 at distinct subcellular localizations contribute equally to the overall increase in ROS and myocardial injury in response to I/R (Matsushima, 2013a). I/R injury was also reduced in cardiac-specific Nox4 KO mice to a similar extent as in sNox4 KO mice, suggesting that the Nox4 in cardiomyocytes, rather than in non-myocytes, plays a dominant role in mediating I/R injury. Interestingly, however, transgenic mice with cardiac-specific overexpression of dominant negative (DN)-Nox, which broadly suppresses all Nox isoforms, and Nox2 and Nox4 double KO (DKO) mice exhibited larger infarcts after I/R, despite the fact that the level of oxidative stress was lower in DN-Nox and DKO mice than in single KO mice. Why was the I/R injury greater in DN-Nox and DKO mice despite the lower level of ROS? Hypoxia-inducible factor-1α (HIF-1α) is a master regulator of hypoxia-regulated gene expression mediating adaptation to low oxygen levels and/or oxidative stress. Upregulation of glycolytic genes such as phosphofructokinase by HIF-1α is believed to be a metabolic adaptation to hypoxia that maintains ATP production and prevents further ROS production by shunting glucose metabolites from mitochondria to glycolysis (Kim, 2006). Interestingly, although the level of HIF-1α was preserved after I/R in single KO mouse hearts, HIF-1α was downregulated in Tg-DN-Nox mouse hearts at baseline and after I/R. Both downregulation of HIF-1α and the increases in I/R injury were normalized when Tg-DN-Nox mice were crossed with mice deficient in proline hydroxylase 2 (PHD2), an enzyme that induces hydroxylation and degradation of HIF-1α and whose activity is inhibited by either hypoxia or ROS. H2O2 reduces Fe (II) levels through the Fenton reaction and inhibits PHD2 enzymatic activity (Gerald, 2004). These results suggest that ROS derived from either Nox2 or Nox4 are essential for suppression of PHD2 and stabilization of HIF-1α, which, in turn, mediates adaptive responses to I/R in the heart.
HIF-1α not only stimulates glycolysis but also inhibits fatty acid oxidation (FAO) through suppression of peroxisome proliferator-activated receptor-α (PPARα) in the heart. Whereas GLUT4 and hexokinase, key regulators of glucose metabolism, were downregulated, PPARα was upregulated in Tg-DN-Nox and DKO mouse hearts. Thus, ROS derived from either Nox2 or Nox4 may be involved in downregulation of PPARα through HIF-1α activation. In fact, downregulation of PPARα by crossing Tg-DN-Nox mice with PPARα KO mice reduced both infarct size and deposition of triglycerides after I/R compared to Tg-DN-Nox alone. Taken together, these data suggest that ROS derived from either Nox2 or Nox4 prevent I/R injury through activation of HIF-1α and consequent suppression of PPARα.
A recent report suggests that abolishing Nox activity through overexpression of DN-Nox, which broadly suppresses Nox isoforms, leads to a ‘markedly reduced state’ in the heart, which is characterized by low NAD(P)+/NAD(P)H and high GSH/GSSG ratios (Yu, 2014). This most likely occurs through decreased consumption of NAD(P)H due to the suppression of Noxs. Since Nox4 is localized on mitochondrial membranes and consumes NADH(Ago 2010), suppression of Nox4 could induce accumulation of NADH in mitochondria. Interestingly, when NADH accumulates in the presence of slow flow in the mitochondrial electron transport chain (ETC) during ischemia, direct transfer of electrons to oxygen takes place, resulting in excessive production of superoxide and severe irreversible damage of the heart (Yu 2014). Thus, Noxs regulate the redox state of cardiomyocytes not only through direct production of ROS but also through consumption of NAD(P)+/NAD(P)H. Although suppression of Nox can reduce production of O2−/H2O2 in mitochondria through the former action, it can paradoxically increase O2− production through the latter mechanism, especially when the ETC is damaged. How the balance between these counteracting mechanisms is regulated during I/R remains to be elucidated in vivo.
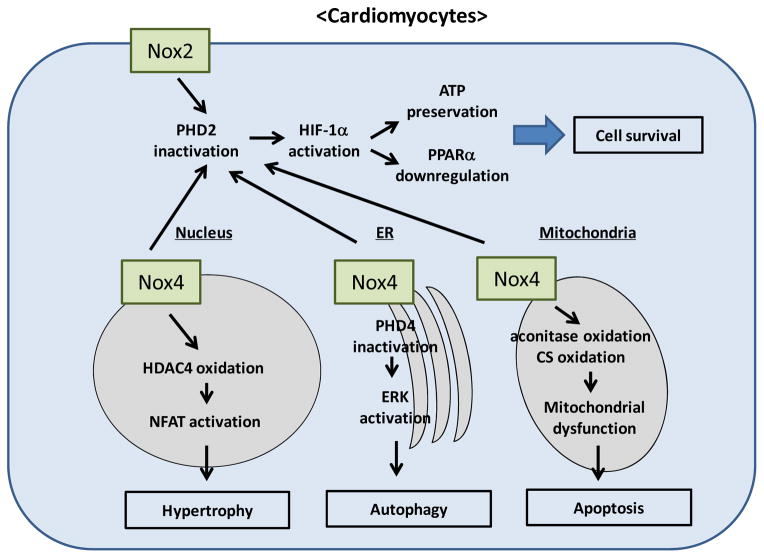
In addition to the level of ROS, the localization of ROS is also an important determinant of cell growth and death. Nox4 is localized in intracellular organelles, which allows ROS derived from Nox4 to act as signaling molecules to modulate the function of downstream targets in subcellular compartments. For example, nuclear ROS derived from Nox4 oxidize two critical cysteines of histone deacetylase 4 (HADC4), inducing nuclear export of HDAC4 and consequent activation of nuclear factor of activated T cells (NFAT), a master regulator of cardiac hypertrophy (Matsushima, 2013b). Mitochondrial ROS derived from Nox4 oxidize the cysteines of aconitase-2 and citrate synthase, leading to mitochondrial dysfunction and apoptosis in cardiomyocytes during aging and heart failure (Ago 2010). In the ER, Nox4 mediates autophagy during glucose deprivation in cardiomyocytes (Sciarretta, 2013). Nox4, which rapidly accumulates in the ER in response to energy stress, promotes autophagy through activation of the protein kinase RNA-activated-like ER kinase (PERK) pathway by suppressing ER-specific prolyl hydroxylase 4 (PHD4), which in turn activates PERK and autophagy. In this scenario, Nox2 is not involved in the induction of autophagy. Why, then, do Nox2 and Nox4 equally contribute to myocardial injury after I/R, despite their different subcellular localizations? I/R-induced upregulation of Nox2 and Nox4 induces O2− production in both plasma and intracellular membranes. O2− is rapidly dismutated to H2O2, which is diffusible and membrane permeable. Since PHD2, one of the critical targets of ROS mediating metabolic adaptation during I/R, is localized primarily in the cytosol, both Nox2 and Nox4 can contribute equally to the inactivation of PHD2 and stabilization of HIF-1α (Figure). On the other hand, PERK and the signaling complex regulating autophagy are localized in the ER. Given the selective upregulation of Nox4 in the ER during the early phase of glucose deprivation, Nox4, but not Nox2, appears to regulate autophagy.
Figure A.
schematic representation of the signaling pathways controlling growth and death of cardiomyocytes that are regulated by Nox isoforms. Nox2 and Nox4 are localized in different subcellular compartments and regulate signaling mechanisms locally or globally to affect metabolism, hypertrophy, autophagy and apoptosis.
Another important issue to consider when discussing the function of Noxs in the heart is that the function of Nox appears to be cell type-dependent. For example, cardiac-specific deletion of Nox4 inhibits pressure overload-induced cardiac hypertrophy and left ventricular dysfunction (Kuroda 2010). In contrast, systemic deletion of Nox4 exacerbates cardiac remodeling after pressure overload (Zhang, 2010). A side-by-side comparison, using mice derived from the same flox mice to generate cardiac and systemic KO mice, showed that cardiac and systemic Nox4 KO mice exhibited distinct cardiac phenotypes (Matsushima 2013b). Cardiac hypertrophy in response to pressure overload was significantly attenuated in cardiac-specific Nox4 KO mice but not in systemic Nox4 KO mice. Furthermore, interstitial fibrosis and diastolic dysfunction were exacerbated in systemic Nox4 KO mice (Matsushima 2013b). We speculate that Nox4 in cardiomyocytes promotes cardiac dysfunction and consequent hypertrophy, whereas Nox4 in non-myocytes protects the heart from fibrosis and diastolic dysfunction. Interestingly, Nox4 exhibits salutary effects against pathologically relevant stresses in some cell types. For example, Nox4 in endothelial cells is protective against angiotensin II-induced endothelial dysfunction, in contrast to Nox1 and Nox2 (Schroder, 2012). The function of Nox4 in non-cardiomyocytes, including cardiac fibroblasts and inflammatory cells, during pressure overload and I/R remains to be elucidated.
Although Nox may be an ideal target for clinical therapy to prevent oxidative stress, it must be taken into consideration that Noxs are also involved in physiological and adaptive cellular functions. Unconditional administration of antioxidants such as Vitamin E as a treatment for cardiovascular disease has not been successful, possibly due to the broad suppression of ROS. If Noxs are to be modulated in clinical settings, careful evaluation of how suppression of Nox would affect both the pathological and physiological functions of Noxs is required. Furthermore, if possible, modulation of Noxs should be conducted in an isoform-specific and cell type-specific manner. Unfortunately, none of the chemical inhibitors of Noxs developed thus far have achieved sufficiently high levels of specificity or isoform-selectivity. For example, apocynin (4-hydroxy-3-methoxyacetophenone) has been used widely as a Nox2 inhibitor. Although apocynin blocks translocation of p47phox and p67phox from the cytosolic fraction to the membrane fraction (Stolk, 1994), thereby inhibiting ROS production by Nox2, it also acts as an antioxidant, scavenging hydrogen peroxide and hydroxyl radicals (Heumuller, 2008), and has a paradoxical stimulatory effect on ROS production in non-leukocytes (Vejrazka, 2005). Diphenyleneiodonium (DPI), another commonly used NADPH oxidase inhibitor, also inhibits other flavin-containing enzymes, including nitric oxide synthase, xanthine oxidase, P-450 NADPH reductase and mitochondrial respiratory chain complex I (Tazzeo, 2009). Although more specific Nox inhibitors have been reported recently, these do not appear to be isoform specific either. VAS2870 (3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine) was identified originally as an inhibitor of Nox2, but it also inhibits Nox4 (Kleinschnitz, 2010), Nox1 (Sancho, 2011), and Duox (Niethammer, 2009), and GKT137831 (2-(2-chlorophenyl)-4-[3-(dimethylamino)phenyl]-5-methyl-1H-pyrazolo[4,3-c] pyridine-3,6 (2H,5H)-dione) inhibits not only Nox4 but also Nox1 and Nox2 (Jiang, 2012). Thus, despite its potential for treatment of I/R injury and heart failure, to our knowledge, a Nox4-specific inhibitor remains to be developed. Although the activity of Nox2 is modulated by cytosolic factors, the activity of Nox4 is regulated primarily by modulation of its expression level, such as through NF-κB-mediated transcription (Matsushima 2013b). Recently, Poldip2 was reported to be a novel positive regulator of Nox4 through its binding with Nox4 and p22phox, thereby affecting cell migration in vascular smooth muscle cells (Lyle, 2009). Thus, modulation of Poldip2 or Poldip2-Nox4/p22phox interaction may indirectly modulate the activity of Nox4. Elucidating the mechanism of posttranslational modification of Nox4 may allow identification of a specific approach to regulate the activity of Nox4.
In conclusion, Nox isoforms play an important role in mediating oxidative stress and myocardial injury after pressure overload and I/R. Suppressing Nox isoforms can effectively prevent excessive activation of ROS in a stimulus-specific manner. In the context of I/R, however, a certain amount of ROS derived from either Nox2 or Nox4 is required to protect the heart against I/R via powerful metabolic adaptations triggered by HIF-1α-PPARα-dependent mechanisms. Thus, although either Nox2 or Nox4 can be targeted for the treatment of I/R, maintenance of a minimum level of Nox activity is needed to preserve the physiological function of Nox. A better understanding of isoform-specific functions, cell type-specific functions and subcellular localization-specific functions of Noxs is needed before modulation of Nox isoforms can be considered as treatment for cardiac diseases, including heart failure and I/R.
Acknowledgments
The authors wish to thank Daniela Zablocki for critical reading of the manuscript and suggestions. This work was supported in part by U.S. Public Health Service Grants HL102738, HL67724, HL69020, HL91469, AG23039 and AG27211. This work was also supported by the Fondation Leducq Transatlantic Networks of Excellence. Dr. Matsushima has been supported by a Postdoctoral Fellowship from the Founders Affiliate, American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ago T, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circulation research. 2010;106:1253–64. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald D, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–94. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Grieve DJ, et al. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. Journal of the American College of Cardiology. 2006;47:817–26. doi: 10.1016/j.jacc.2005.09.051. [DOI] [PubMed] [Google Scholar]
- Heumuller S, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–7. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer MR, et al. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circulation research. 2000;87:812–7. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- Jiang JX, et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free radical biology & medicine. 2012;53:289–96. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15565–70. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looi YH, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–25. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- Lyle AN, et al. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation research. 2009;105:249–59. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima Y, et al. Regulation of myocardial growth and death by NADPH oxidase. Journal of molecular and cellular cardiology. 2011;50:408–16. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, et al. NOX1/NADPH oxidase is involved in endotoxin-induced cardiomyocyte apoptosis. Free radical biology & medicine. 2012;53:1718–28. doi: 10.1016/j.freeradbiomed.2012.08.590. [DOI] [PubMed] [Google Scholar]
- Matsushima S, et al. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1alpha and upregulation of peroxisome proliferator-activated receptor-alpha. Circulation research. 2013a;112:1135–49. doi: 10.1161/CIRCRESAHA.111.300171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima S, et al. Increased oxidative stress in the nucleus caused by Nox4 mediates oxidation of HDAC4 and cardiac hypertrophy. Circulation research. 2013b;112:651–63. doi: 10.1161/CIRCRESAHA.112.279760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer P, et al. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–9. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho P, Fabregat I. The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-beta-induced apoptosis of liver tumor cells. Biochemical pharmacology. 2011;81:917–24. doi: 10.1016/j.bcp.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Schroder K, et al. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circulation research. 2012;110:1217–25. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- Sciarretta S, et al. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circulation research. 2013;113:1253–64. doi: 10.1161/CIRCRESAHA.113.301787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk J, et al. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. American journal of respiratory cell and molecular biology. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Tazzeo T, et al. The NADPH oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal Ca(2+) pump. British journal of pharmacology. 2009;158:790–6. doi: 10.1111/j.1476-5381.2009.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejrazka M, et al. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochimica et biophysica acta. 2005;1722:143–7. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Yu Q, et al. Elimination of NADPH oxidase activity promotes reductive stress and sensitizes the heart to ischemic injury. J Am Heart Assoc. 2014;3:e000555. doi: 10.1161/JAHA.113.000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–6. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]