Abstract
To investigate neural mechanisms that support semantic functions in aging, we recorded scalp EEG during an object retrieval task in 22 younger and 22 older adults. The task required determining if a particular object could be retrieved when two visual words representing object features were presented. Both age groups had comparable accuracy although response times were longer in older adults. In both groups a left fronto-temporal negative potential occurred at around 750 msec during object retrieval, consistent with previous findings (Brier et al., 2008). Only in older adults a later positive frontal potential was found peaking between 800 and 1000 msec during no retrieval. These findings suggest younger and older adults employ comparable neural mechanisms when features clearly facilitate retrieval of an object memory, but when features yield no retrieval, older adults use additional neural resources to engage in a more effortful and exhaustive search prior to making a decision.
Keywords: semantic, object memory, memory retrieval, feature, ERP, aging
1. Introduction
Many aspects of semantic memory are separable from the neural systems and mechanisms that underpin episodic and procedural memory (Binder & Desai, 2011; Martin, 2007; Tulving, 1972). Several current models of semantic memory are supported by evidence indicating that an object is represented in multiple neural systems that are closely related to those systems activated originally upon experiencing the features of that object (Allport, 1985; Hart & Gordon, 1992; Martin, 2007; Martin & Chao, 2001). Features associated with objects, either living or non-living things, typically span multiple sensorimotor and cognitive domains and are an essential part of object representation (Goldberg, Perfetti, & Schneider, 2006; Hart, Anand, & Zoccoli et al., 2007; Kellenbach, Brett, & Patterson, 2001; Noppeney & Price, 2002). For example, a lion can be linked to more concrete features such as its roar and mane as well as to more abstract features such as being threatening (Kraut, Kremen, & Moo et al., 2002; Kraut, Moo, Segal, & Hart, 2002; Kraut, Pitcock, Calhoun, Freeman, & Hart, 2006).
Since features are represented in a distributed fashion in the brain (Binder & Desai, 2011; Hart et al., 2007; Martin & Chao, 2001), one would expect age-related functional and/or structural alterations, including changes in white matter that mediates synchronized connectivity between different brain regions (Grady, 2012; Hedden & Gabrieli, 2004), to affect the long-range communication demanded by memory retrieval from this distributed representation. However, semantic memory, unlike other cognitive functions such as episodic memory, attention, and executive function, does not seem to undergo as much change with age (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002; Li, Lindenberger, & Hommel et al., 2004). How then does the neural mechanism support semantic memory in normal older adults such that only minimal age-related changes are noticeable?
Our goal in this study is to examine how age affects semantic memory retrieval by studying younger and older adults using EEG, a non-invasive tool which provides a high temporal resolution measure of neural activity (Luck, 2005; Luck & Kappenman, 2013; Kiefer & Pulvermüller, 2012). We used the Semantic Object Retrieval Task (SORT), which examines how objects are retrieved via explicit evaluation of object-associated features (Kraut, Kremen et al., 2002; Kraut, Cherry, & Pitcock et al., 2006, 2007). For example, in the SORT paradigm, presenting the features “humps” and “desert” normally facilitates retrieval of “camel”. By eliciting the process of combining these features that are represented across the brain to retrieve an object memory, we could untangle the dynamic neural mechanisms that operate to integrate object features to form a coherent object representation. The SORT task has been applied as an effective measure to detect impairment in lexico-semantic memory retrieval in various clinical populations and thus serves a useful task to examine age-related changes in retrieval (Mild Cognitive Impairment, MCI, and Alzheimer’s disease, AD: Kraut, Cherry et al., 2006; Kraut et al., 2007; schizophrenia: Assaf, Rivkin, & Kuzu et al., 2006; Gulf War Illness: Calley, Kraut, & Spence et al., 2010; aging effects of concussion in former professional football athletes: Hart, Kraut, & Womack et al., 2013).
Successful performance in the SORT requires successful retrieval of semantic object memory. Data from previous activation, brain stimulation, and lesion studies suggest that the retrieval process of semantic memory involves the left inferior frontal gyrus (IFG) with its sub-regions subserving semantic selection and controlled retrieval (Badre & Wager, 2002; Thompson-Schill, Swick, Farah et al., 1997; Whitney, Kirk, O’Sullivan et al., 2011). Other areas that may also assist in semantic memory retrieval localize to the left angular gyrus (AG) and left poster middle temporal gryus (MTG) (Noonan, Jefferies, Visser, & Lambon Ralph, 2013; Whitney et al., 2011). In addition to these regions, activation studies on the SORT have shown object memory retrieval elicited increased BOLD signal in the pre-supplementary area (pre-SMA) of the medial frontal cortex, caudate, and thalamus (Assaf, Calhoun et al., 2006; Kraut, Kremen et al., 2002; Kraut, Moo et al., 2002; Kraut et al., 2003). Lesion studies using the SORT have shown relatively selective deficits in object memory retrieval compared to other semantic memory tasks (e.g., category, association) in patients with localized thalamic strokes (Segal, Williams, Kraut, & Hart, 2003; Pergola, Bellebaum, & Gehlhaar et al., 2013). Based on these findings, Hart et al. (2013) proposed a central role of the pre-Supplementary Motor Area (pre-SMA)-thalamus-caudate circuit in mediating the semantic memory retrieval process especially in tasks such as the SORT that involves feature integration, as part of the Neural Hybrid Model of semantic memory (Hart et al., 2007; Hart et al., 2013).
An ERP study of young adults using the SORT showed a left fronto-temporal component starting at around 750 msec post-stimulus that was more negative in retrieval than non-retrieval stimulus pairs (Brier, Maguire, Tillman, Hart, & Kraut, 2008). A power analysis of the EEG changes during the SORT showed earlier alpha desynchronization (8~12 HZ)/delta synchronization (~1 Hz) as well as later frontal beta synchronization (20–35 Hz, after one second post-stimulus), which were posited to represent semantic search and object retrieval, respectively (Ferree et al., 2009; Hart et al., 2013). Moreover, Slotnick et al. (2002) recorded intra-thalamic electrical activity while a patient was performing the SORT task and found beta synchronization at long latency in both thalamic hemispheres time-locked to the stimulus. This 750 msec ERP component appears later than the typical N400, a negative scalp evoked potential considered to be a marker of semantic processing that peaks at about 400 msec post-stimulus onset in response to semantic/contextual incongruity (Kutas & Federmeier, 2000; Kutas & Hillyard, 1980).
Majority of the previous ERP studies involving the N400 effect have used tasks based on semantic priming, contextual constraints, etc. (Kutas & Federmeier, 2000; Wlotko, Lee, & Federmeier, 2010). Most of these tasks do not mandate retrieval of a specific concept (e.g., objects) but are related to processing of meaning, inquiring about category or semantic relatedness between stimuli (probed as individual words/pictures or in the context of a sentence; Kiefer, 2001; Kutas & Hillyard, 1980). Tasks based on priming do not even require direct (explicit) evaluation of semantic information. For example, subjects are asked to judge if the second stimulus is a real word or not, while the relations between the first (prime) and second stimuli (target) are manipulated to examine priming effects due to semantic association, category, etc. (Holcomb & Anderson, 1993; Kiefer, 2005). The SORT task is characteristically different from these noted above in that participants are required to directly evaluate whether the features result in retrieval of an object memory or not and to indicate so by making a explicit response. In terms of the neural basis of semantically-based ERPs, N400 is most consistently associated with access to semantic memory storage represented in the temporal regions such as the left MTG, inferior temporal gyrus, and left anterior medial temporal lobe (Nobre & McCarthy, 1995; McCarthy, Nobre, Bentin, & Spencer, 1995; Lau et al., 2008). On the other hand, the semantic retrieval process is more strongly associated with longer latency waveform characteristics (“post-N400 positivity”, Van Patten & Luka, 2006), especially in the case of semantic selection and effortful retrieval, which may involve frontal areas such as the left IFG (van Petten & Luka, 2006; Lau et al., 2008). FMRI findings on the SORT (Assaf, Calhoun et al., 2006; Kraut, Kremen et al., 2002) also support this contention, finding that only object memory retrieval (during the SORT) elicited increased signal in bilateral inferior frontal gyri, pre-SMA, caudate, and thalamus, whereas both SORT and object association (i.e., semantic association judgment) elicited increased BOLD in left MTG, angular gyrus, and inferior temporal gyrus. These findings suggest that the ERP marker related to the SORT task involves frontal (pre-SMA, left IFG) and/or temporal (left MTG, inferior temporal) semantic systems that are engaged in semantic selection and integrative retrieval processes.
Other late ERP effects that occur after the N400 time window, such as P600, a positive deflection occurring at around 600 msec post-stimulus onset, have been suggested to be associated with other semantic related processes such as processing sentences with conflicting semantic structure (Bornkessel-Schlesewsky & Schlesewsky, 2008), with conflicting thematic structures (Kuperberg, Sitnikova, Caplan, & Holcomb, 2003; Kuperberg, 2007), and with incongruous syntactic structures (Van Petten & Luka, 2012). These late components have been dissociated from N400 in some experiments thus suggesting their functional roles different from N400 (Kuperberg, 2007). Also, these late effects are not exclusive to sentence-level processing or verbal content, and have also been observed when stimuli were single words (Hill, Strube, Roesch-Ely, & Weisbrod, 2002; Meyer & Federmeier, 2007; Swaab, Brown, & Hagoort, 1998) or pictures (Ganis & Kutas, 2003; McPherson & Holcomb, 1999; Mudrik, Lamy, & Deouell, 2010; Sitnikova, Holcomb, Kiyonaga, & Kuperberg, 2008).
ERP studies that have examined the effects of age on lexico-semantic tasks have shown varying age-related effects on several components. General findings include delayed N400 latency and/or decreased N400 amplitude as well as difference in lateralization due to age (Federmeier & Kutas, 2005; Gianquino, Ranghi, & Butler, 2007; Kutas & Iragui, 1998) with some exceptions (Grieder, Crinelli, & Koenig et al., 2012). In terms of later components, prior studies have observed attenuated amplitude (Meyer & Federmeier, 2010) and qualitative changes in scalp distribution (Galdo-Avarez, Lindin, & Díaz et al., 2009; Harbin, Marsh, & Harvey, 1984) in older adults. The reason for variable findings in age-related effects might reflect variations in task designs. It has been posited that older adults may recruit a decreased amount of neural resource when message-level or executive processing is required, but they can still show comparable activity at word-level processing compared to younger adults (Federmeier, van Petten, Schwartz, & Kutas, 2003; Wlotko et al., 2010). Therefore, in view of the word stimuli used in the SORT, we hypothesized that older adults would present differences between retrieval and non-retrieval stimulus pairs around 750 msec similar to young adults in Brier et al.’s study (2008). In order to test this hypothesis, we undertook conventional windowed analyses between 750 and 1000 msec post-stimulus as in Brier et al. (2008) to replicate the results in younger adults and to examine similarities between younger and older adults in the current study. To further examine qualitative differences in ERPs between younger and older adults that might not be easily detected by visual inspection and thus windowed ERP measures, we performed data-driven exploratory analyses that prove to be a useful tool for high-density ERPs (Dien & Frishkoff, 2005; Dien, Michelson, & Franklin, 2010; Spence, Brier, Hart, & Ferree, 2013). We expected the data-driven analysis to yield findings not only confirming results from our conventional windowed analyses, but also to provide further insights into age associated differences between groups for the SORT. Understanding typical age-related changes in semantic object memory retrieval will help develop a better functional marker of the breakdown in word/semantic memory retrieval abilities observed in individuals with MCI and AD; this could potentially be useful in evaluating and monitoring therapeutic effects at the neural level.
2. Methods
2.1 Participants
We studied 22 younger adults (15 women; Mage = 21.7 years, SD = 3.2) and 22 older adults (17 women; Mage= 63.9 years, SD = 6.4). All participants were native English speakers and right handed. Younger adults were undergraduate students from the University of Texas at Dallas who completed this study for research credits. Community dwelling older adults were recruited from an exercise training study of normal aging population, who did not have subjective memory complaints or cognitive impairments, as confirmed by a standard neuropsychological battery (Chapman, Aslan, & Spence et al., 2013). Only the pre-training data were used for the current study. We excluded participants with a history of neurological or psychiatric disorders, traumatic brain injury, learning disabilities and communication disorders, uncorrected visual and hearing impairments. Informed consent was obtained from all participants in accordance with the protocols approved by the Institutional Review Boards of the University of Texas at Dallas and of the University of Texas Southwestern Medical Center.
2.2 Experimental Paradigm and Procedures
We administered the ERP version of the Semantic Object Retrieval Test (SORT) (Brier et al., 2008) to the participants. The stimuli in this paradigm comprise 112 pairs of words. The two words represent features or attributes of objects (living or non-living). In retrieval word pairs (56 pairs), the two words represent features of a particular object. Non-retrieval word pairs (56 pairs) were created by randomly pairing the words. For example, the word pair ‘humps’ and ‘desert’ would facilitate memory retrieval of ‘camel’ (retrieval pair). In contrast, ‘humps’ and ‘monitor’ should not facilitate memory retrieval of any object (non-retrieval pair). The retrieval and non-retrieval word pairs have been validated in previous studies with independent groups of subjects from different age groups (Brier et al., 2008; Kraut, Kremen et al., 2002; Kraut, Cherry et al., 2007; Kraut et al., 2007).
Words were presented simultaneously, one pair at a time, with one word above the other, for three seconds. In between trials, a + sign was presented at the center for three seconds as a visual fixation target. Participants were instructed to respond ‘yes’ or ‘no’ depending on whether they could think of any particular object upon seeing each word pair. Responses were made by pressing a button under the right index finger for ‘yes’ and a button under the right middle finger for ‘no’. Responses had to be made within the 3 seconds during which the word pairs remained on the screen. Any responses made after three seconds would be recorded as incorrect. Reaction time (RT) and accuracy were recorded for each trial. Retrieval and non-retrieval trials were presented in a pseudo-randomized order. The entire task lasted about 11 minutes.
Word stimuli were presented on an LCD screen placed about 46 inches from the participant using Stim software (Compumedics Neuroscan, USA). The two words in each pair spanned about 5 degrees of both vertical and horizontal visual angel, with the horizontal measure varying slightly by word length. All the words were presented in black color, in lower case Times New Roman font, against a white background.
2.3 Behavioral analysis
We analyzed RT and accuracy separately using repeated-measures 2-way ANOVAs (between-subject factor: group [younger adults vs. older adults]; within-subject factor: condition [retrieval vs. non-retrieval]). All statistical analyses of behavioral data were performed using IBM SPSS Statistics 21 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.).
2.4 EEG data acquisition and processing
Continuous EEG was recorded from a 64-electrode elastic cap (Neuroscan Quickcap) while the participants were doing the task, through a Neuroscan SynAmps2 amplifier and using Scan 4.5 software (Compumedics Neuroscan, USA; sampling rate: 1kHz, DC-200Hz). Electrode impedances were typically below 10 kΩ. The reference electrode was located at midline between Cz and CPz and vertical electroocculogram (VEOG) was recorded at sites above and below left eye.
Data were processed off-line using Neuroscan Edit software (Compumedics Neuroscan). Poorly functioning electrodes were identified by visual inspection of the data and were excluded. Electrodes that had impedance over 20 kΩ were also excluded from analysis. No more than 5% of the total number of electrodes was rejected in any individual subject (following guidelines by Picton et al., 2000; average percentage of bad electrodes: 1.6% and 1.9% in younger and older adult groups, respectively). The continuous EEG data were high-pass filtered at 0.15Hz and corrected for eye blinks using the spatial filtering function in the Scan 4.5 software (Semlitsch, Anderer, Schuster, & Presslich, 1986; Compumedics Neuroscan, USA).
EEG data were then processed using an in-house processing schema based on EEGLAB functions (Delorme & Makeig, 2004) in MATLAB (the MathWorks Inc.). EEG data were first segmented into multiple trial-by-trial EEG epochs (−200 to 1800 msec). Only correct trials were included for analysis. Additionally, we applied a digital low-pass filtering with a cutoff value of 30 Hz (linear finite impulse response function) to minimize high frequency noise, such as muscle activity. Epochs with peak signal amplitude of more than 75 μV were rejected. Finally, outlier epochs were identified by rejection algorithms in EEGLAB (rejecting improbable and abnormally distributed data lying over 5 standard deviations from the mean, using pop_jointprob.m and pop_rejkurt.m EEGLAB algorithms, respectively) and excluded from analysis. The average retaining rates were 78.3% (38 trials) and 77% (39 trials) in the younger adult group, and 81.6% (40 trials) and 83.1% (42 trials) in the older adult group, for retrieval and non-retrieval trials, respectively. All subjects except for one in the older group retained more than 30 trials in both retrieval and non-retrieval trials to be included for ERP analysis.
An algorithm computing the average based on spherical splines fitted to the data was then applied to interpolate EEG data to the sites of the bad electrodes (Ferree et al., 2009). This was based on the assumption that the number of bad electrodes typically consisted of less than 5% of all 62 electrodes, and that the bad electrodes were not all contiguous (as previously applied in Brier et al., 2012; Maguire et al., 2010; Spence et al., 2013). After artifact/epoch rejection was done and the missing electrodes were replaced, the EEG epochs were then re-referenced (global average potential from all electrodes) and baseline corrected (average potential of the pre-stimulus period between −200 and 0 msec).
2.5 ERP analysis
2.5.1 Hypothesis-driven Windowed Measures
In order to examine if the current study replicated previous findings (Brier et al., 2008), we focused on the left fronto-temporal area (F7) during the time window between 750 and 1000 msec post-stimulus as observed previously. We measured the mean amplitude within the time window. We first examined the difference between retrieval and non-retrieval trials using paired-t tests in the younger adult group. Subsequently, we tested for group difference and interaction by running repeated-measures 2-way ANOVAs (between-subject factor: group [younger adults vs. older adults]; within-subject factor: condition [retrieval vs. non-retrieval]) on mean amplitude in IBM SPSS Statistics 21.
2.5.2 Data-driven STAT-PCA analysis
The pre-processed ERP data were analyzed by STAT-PCA (Ferree et al, 2009; Spence et al, 2013), a procedure that utilizes statistical inference at each temporal and spatial unit (STAT) followed by a principal component (factoring) analysis (PCA) to isolate the most salient temporal and spatial properties of the ERPs.
In the current study, the spatial units were electrodes and the temporal units were 50-msec time windows within an epoch (0–1800 msec post-stimulus). We thus had 62 × 36 = 2232 combinations of space and time (62 electrodes and 36 time windows in an epoch). For each one of the 2232 spatial/temporal units, we implemented a linear mixed model
to examine the effects of group (λi), condition (ϒk) and the interaction between group and condition (λϒ)ik, on the scalp potential Yijkl, where each subscript indexes group, subject, condition and trial, respectively. The statistical inference was performed in the SAS software 9.4 using Proc Mixed, and the variances associated with subject variability, bj(i), and trial variability, εijkl, were estimated by restricted maximum likelihood. To account for multiple tests, we derived an inferential threshold based on the false discovery rate (FDR, Benjamini & Hochberg, 1995) rather than individual p-values, setting the FDR equal to 0.05 for each set of 2232 tests corresponding to group, condition and their interaction. The average potential of each of these three effects of interest was retained if the corresponding test was below threshold; otherwise, they were set to zero.
We then applied the principal components analyses (PCA) to the 3 matrices of thresholded average potentials (Ferree et al, 2009; Spence et al, 2013), each 36 temporal units by 62 electrodes, and retained the main spatial components by parallel analysis (Horn, 1965.) For each retained spatial component, the temporal components were simply the resulting PCA scores. The retained spatial/temporal components can be visualized by plotting spatial PCA loadings in topographical distribution across scalp and temporal PCA scores as a time-series.
3. Results
3.1 Behavioral Data
Younger adults had mean accuracy of 85.7 % (SD = 5.7) and 92.3% (SD = 5.1) and mean RT of 1207 msec (SD = 195) and 1476 msec (SD= 303) for retrieval and non-retrieval trials, respectively. Older adults had mean accuracy of 87% (SD = 5.6) and 92.6% (SD = 5.1) and mean RT of 1394 msec (SD = 255) and 1703 msec (SD = 396) for retrieval and non-retrieval trials, respectively. While there was no significant difference in accuracy due to age (p > .1), the RTs were significantly longer in older adults (1548.5 msec) compared to younger adults (1341.7 msec), F(1, 42) = 6.3, p = .016. Overall, both groups took longer and were more accurate in responding to non-retrieval trials (mean RT = 1589.6 msec; mean accuracy = 92.4%) than to retrieval trials (mean RT = 1300.6 msec; mean accuracy = 86.3%); for RT, F(1, 42) = 67.7, p < .001, and accuracy, F(1, 42) = 31.9, p = < .001. There was no group by condition interaction in either RT or accuracy (p > .1).
3.2 ERP Data
3.2.1 Results on Hypothesis-driven Windowed Measures
The group average ERPs were plotted for visual inspection (Figure 1). The analysis showed that for F7, mean amplitude between 750 and 1000 msec post-stimulus was significantly more negative in retrieval trials than non-retrieval in young adults, t(21) = 3.28, p = .004 (Table 1; Figure 2). To examine whether this effect was different between the two age groups, the 2-way ANOVAs showed that regardless of group (age), the mean amplitude was more negative in retrieval trials (−.93 μV) than non-retrieval (.05 μV), F(1, 42) = 21.4, p < .001 (Table 1). No effects of group or group by condition interaction were significant (p > .1). Therefore, the results replicated previous findings in Brier et al. 2008.
Figure 1. Group average ERPs in retrieval and non-retrieval trials.
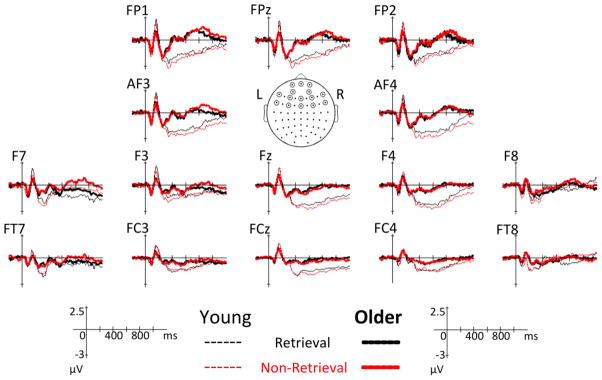
Group avarege ERPs are overlaid for young (thin line) and older adults (thick line) at select electrodes at both hemispheres. Black line: retrieval trials; red line: non-retrieval trials.
Table 1.
ERP mean amplitude at F7 (750–1000 msec)
| Group | Retrieval (μV) | Non-retrieval (μV) | Paired t tests (p values) |
|---|---|---|---|
| Young-F7 | −1.3 (2.1) | −.35 (2) | .004** |
| Older-F7 | −.59 (2) | .46 (1.6) | .01* |
Values: mean (standard error). P < .05 (*). P < .01(**).
Figure 2. Group average ERPs at F7.
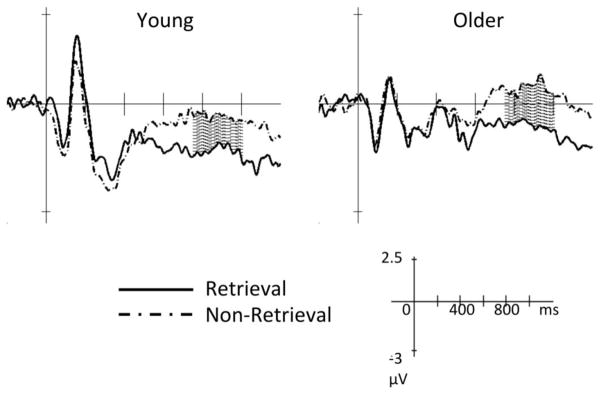
Groups average ERPs of young (left) and older adults (right) showing the condition effect (retrieval vs. non-retrieval) at the left fronto-temporal site (F7) between 750 amnd 1000 msec post-stimulus (as represented within the shadowed areas). Solid line: retrieval trials; dashed line: non-retrieval trials.
We performed further analyses to address the potential contribution of RT difference between retrieval and non-retrieval trials to the left fronto-temporal components, and found that RT difference did not contribute prominently to the ERP component of interest (See Supplementary Analysis 1).
3.2.2 Results on Data-driven STAT-PCA analysis
For effects of condition, group, and their interaction we found 303, 208, 24 spatial/temporal units (out of total 2322 units), respectively, that survived at FDR = 0.05.
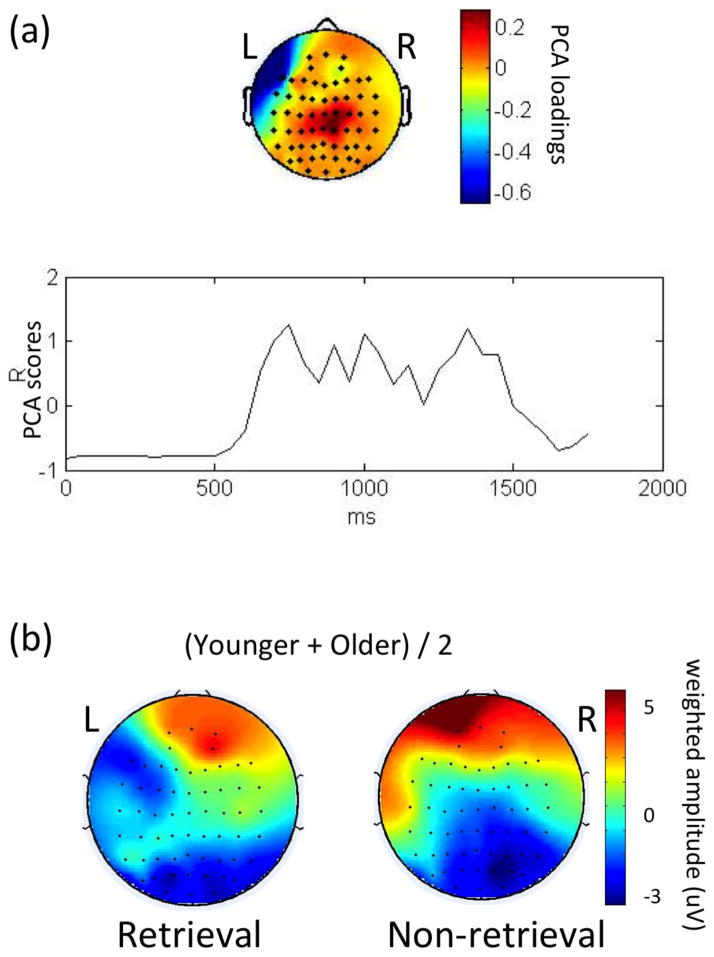
For condition differences (retrieval vs. non-retrieval), two spatial PCA factors were detected (48.6% and 20.9% of total variance, respectively). The first factor was found near the left fronto-temporal region (near F7, Figure 3a) starting at around 750 msec post-stimulus, and of smaller magnitude, near the centro-parietal region (near Cz, CPz, Figure 3a), which is consistent with the findings of Brier et al., (2008) study. In the left fronto-temporal region the retrieval condition contributed larger negative potentials relative to the non-retrieval condition, whereas in the centro-parietal region, negative potentials were larger in the non-retrieval trials relative to retrievals (Figure 3b). ERP waveforms (Figures 1&2) demonstrated that this effect was found at the left side (F7) compared to that on the right side (F8). This effect was again replicated here using STAT-PCA, consistent with our hypothesis-driven analysis in the previous section. The second spatial PCA factor was centered over the frontal region (near FP2, not shown) but much less consistent in time and across subjects compared to the first factor. Most importantly, it had no clear peak and started after 1400 msec post-stimulus onset, which is later than the average RT in the younger adults and the RT of most of the older adults. Since we primarily focused on neural processes that occurred before a motor response was made, we did not pursue this finding further.
Figure 3. PCA results of the condition differences.
PCA results of condition differences. The 1st spatial factor is depicted in (a) and the weighted ERP topography for each condition is shown in (b). In (a), the upper part of the figure illustrates a condition effect in the left fronto-temporal region (F7, FT7) accompanied by a weaker effect at the middle centro-parietal region (Cz, CPz); the lower part illustrates the spatial factor that peaked at around 750 msec post-stimulus and that lasted beyond 1400 msec. In (b), the ERPs weighted by the temporal PCA scores for each condition (averaged across groups) show that the left fronto-temporal effect was found with retrieval trials having more negative potentials than non-retrieval trials; the co-existing middle centro-parietal effect was found with non-retrieval having more negative potentials than retrieval trials.
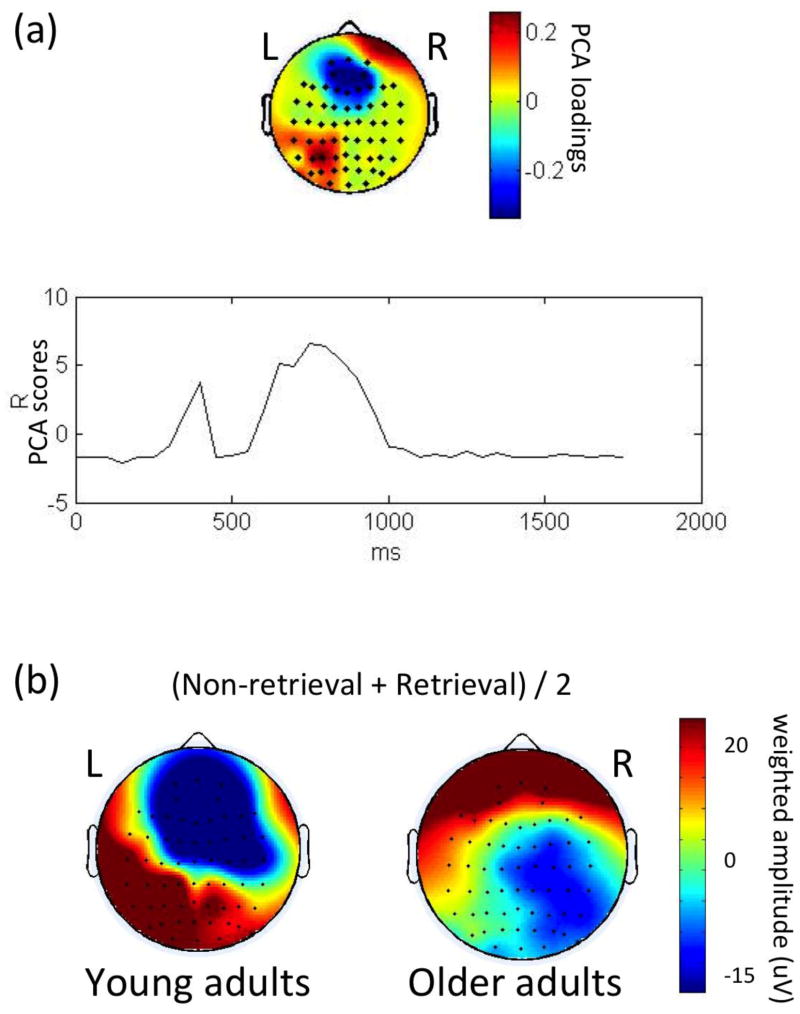
For group differences (young vs. old), one spatial PCA factor was found (71.5% of total variance), centered over the mid frontal regions (near Fz, FCz) and peaked both at 400 msec and at 750 msec post-stimulus (Figure 4a). While a more positive scalp potential was observed in the older group, during these time frames, a negative scalp potential was observed in the younger group (Figure 4b). ERP waveforms (Figure 1) demonstrated that this effect was found at the frontal midline electrodes (Fz, FCz).
Figure 4. PCA results of the group differences.
PCA results of the group differences. The 1st spatial factor is shown in (a) and the weighted ERP topography for each group is shown in (b). In (a), the upper part of the figure illustrates a group effect in the frontal region (Fz, FCz); the lower part illustrates that the spatial factor peaked at around both 400 and 750 msec post-stimulus. In (b), the ERPs weighted by the temporal PCA scores for each group (averaged across retrieval and non-retrieval trials) show that the frontal effect was contributed by negative potentials in the younger and positive potentials in the older adults.
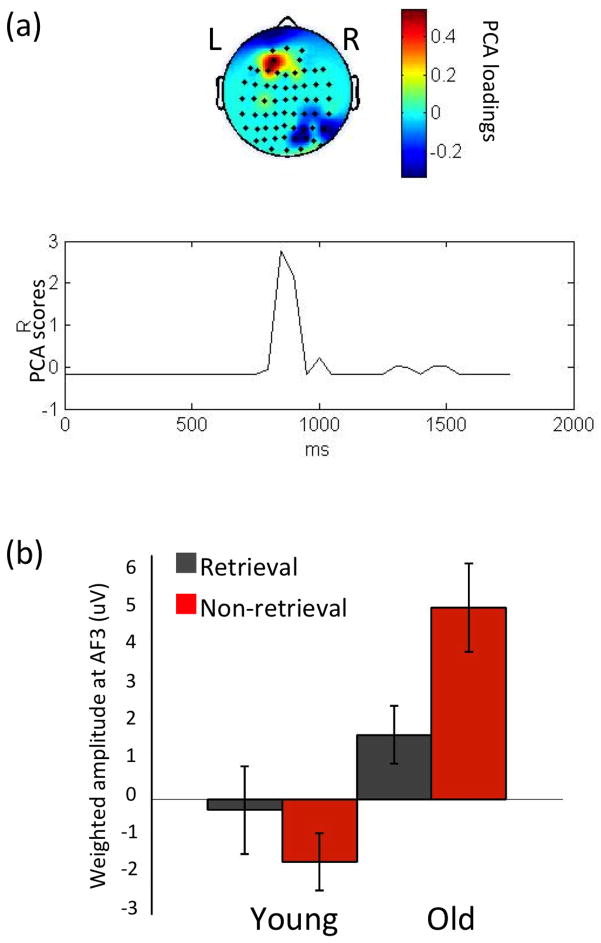
For the group by condition interaction, one spatial PCA factor was found (67.2% of total variance), focused in frontal electrodes (AF3, F3) that had the strongest effect between 800 and 1000 msec post-stimulus (Figure 5a). This interaction was mostly a result of condition effects in the older group, with a more positive frontal potential in non-retrieval than in retrieval trials (Figures 5b). The pattern seemed to be reversed in the younger group (Figures 5b), but in the younger group, this waveform started long before the 800–1000 msec window and was not nearly as large in magnitude. ERP waveforms (Figure 1) demonstrated that this effect was found in opposite directions for different age groups on the left (AF3) compared to the right (AF4). Finally, we conducted windowed measures analysis to confirm the PCA findings here could be replicated using a more conventional approach, with the caveat in mind, however, that this was a post-hoc approach and could not be ascertained temporally and spatially before the STAT-PCA was performed (see Supplementary Analysis 2).
Figure 5. PCA results of the group by condition interaction.
PCA results of the group by condition interaction. The 1st spatial factor is illustrated in (a) and weighted amplitudes at the electrode AF3 for each condition and group are shown in (b). In (a), the upper part of the figure shows a condition effect that differs by group in the frontal region (AF3, F3); the lower part illustrates the spatial factor peaked between 800 and 1000 msec post-stimulus. In (b), the ERPs weighted by the temporal PCA scores at AF3 show that the frontal interaction effect was contributed by non-retrieval having more positive potentials than retrieval trials in the older adults, while non-retrieval having slightly more negative potentials than retrieval trials in the younger adults; error bars represent standard errors.
4. Discussion
In this study, we used ERPs to investigate how age differences modulated semantic object memory retrieval during the SORT task. We observed differences in scalp potentials between retrieval and non-retrieval trials starting at around 750 msec in the left fronto-temporal regions, lasting longer than 1000 msec, in both younger and older adults. In addition, there was a frontal positive potential that was found to arise between 800 and 1000 msec in older adults. Behaviorally, we found that older adults performed as accurately as younger adults. Comparable accuracy in both groups indicates that the overall ability to bind features to retrieve object representations is relatively preserved with aging. Although RT differences between young and old were observed, these RT differences were not specific to either retrieval or non-retrieval trials, and hence may have been due to generalized age-related slowing (Lima, Hale, & Myerson, 1991; Salthouse, 1996).
The left fronto-temporal ERP component common to both groups started late (around 750 msec) and lasted until around 1400 msec post-stimulus. The same effect was also shown in Brier et al. (2008) in young adults. The left fronto-temporal ERP component in the current study occurred later than the typical N400 time frame and is likely to be functionally different from N400. Such late effects have been observed by others in studies that have examined semantic related processes at the sentence level (Bornkessel-Schlesewsky & Schlesewsky, 2008; Kuperberg et al., 2003; Kuperberg, 2007; Van Petten & Luka, 2012) as well as processing of single words (Hill et al., 2002; Meyer & Federmeier, 2007; Swaab et al., 1998) and pictures (Ganis & Kutas, 2003; McPherson & Holcomb, 1999; Mudrik et al., 2010; Sitnikova et al., 2008). Overall, these late effects have not been found to be affected predominantly by the perceptual properties of the stimuli or the semantic relationships between them, but instead are largely affected by how stimuli are interpreted and processed, as demonstrated when the instructions and experimental designs were manipulated at the conceptual level (Kuperberg, 2007). We postulate that the cognitive processes at this stage include the memory retrieval of an object facilitated by the probe words (such as humps and desert in the SORT; Hart et al., 2013). Object retrieval here requires integration of two concepts (features) to make an explicit, conscious connection. The effortful nature of the SORT was supported by the substantially longer RT for retrieval trials (> 1 second) than what is normally observed in lexical judgment and object categorization tasks that also use words as stimuli (Hill et al., 2002; Grieder et al., 2012). Late ERP effects have also been observed in studies on truth verification (Wiswede, Koranyi, Müller, Langner, & Rothermund, 2012), metaphor processing (Arzouan, Goldstein, & Faust, 2007; Coulson & Van Petten, 2002), and creative thinking (Kröger et al., 2013). Finally, as we tested in the results, this component was much less likely to be contributed by RT difference between retrieval and non-retrieval trials (Supplementary Analysis 1).
Semantic memory studies that have used word pairs similar to ours have observed N400 between 250 and 500 msec (Kutas & Federmeier, 2000). These studies have found that the amplitude of N400 is modulated by the degree of semantic relatedness/congruity between word pairs. Specifically, smaller N400 amplitudes have been observed in pairs where a target word is followed by a related (dog-cat) as compared to an unrelated (dog-car) word (Bentin, McCarthy, & Wood, 1985). In our task, the retrieval pairs (humps and deserts) had slightly more relatedness or congruity than non-retrieval pairs (humps and monitor), leading to the expectation of seeing a N400 effect, with larger amplitude during non-retrieval trials compared to retrieval trials. One possible reason for not observing the N400 effects in our study may be related to the fact that our task required participants to evaluate whether the word pairs yield retrieval of an object memory or not. It is likely that the focus on object retrieval in our task engages neural circuits beyond those that are essential for access to semantic storage (such as left MTG, superior temporal sulcus, and inferior temporal gyrus; see a comprehensive review by Lau et al., 2008) thus resulting in a late negative potential around 750 msec. It is plausible the pre-SMA-thalamus-caudate circuit in addition to the left IFG implicated in previous activation studies on the SORT are involved during the object retrieval process (Assaf, Calhoun et al., 2006; Kraut, Kremen et al., 2002). Furthermore, the SORT stimuli are feature pairs created based on whether they in combination would lead to retrieval of a particular object, rather than how closely related or congruous these two features may be. Therefore, the difference in semantic relatedness or congruity may not be large enough to elicit consistent N400 effects between retrieval and non-retrieval trials. Another possible reason for not observing N400 may be related to stimulus repetition. The N400 effect has been shown to be degraded after repetition (Besson, Kutas, & McCarthy, 1999; Kiefer, 2001). As a result, since the SORT the individual word stimuli were presented twice within the experiment to balance between retrieval and non-retrieval trials, the N400 effect might have been diminished. Nevertheless, the extent to which repetition of the stimuli once would eliminate the N400 effect is debatable (see Debruille & Renoult, 2009; Renoult, Wang, Mortimer, & Debruille, 2012).
While it appears that both younger and older adults used comparable neural mechanisms when features clearly facilitated retrieval of an object memory, we found a frontal positive ERP component between 800 and 1000 msec post-stimulus only in older adults. ERP showed that non-retrieval trials elicited a larger and more positive potential than retrieval trials, suggesting that this frontal component represents increased neural recruitment in response to non-retrieval trials. Maintaining an active search process for potential linkage to an object during non-retrieval trials appeared to be more taxing than in retrieval trials. The longer RT in the non-retrieval trials suggests the search was more exhaustive and effortful before the search was terminated prior to making a response. It is likely that older adults recruited additional neural resources to test and rule out possibilities prior to making a behavioral response on non-retrieval trials. Given that there was no difference in the accuracy of response between younger and older adults, it is possible that these additional neural resources in older adults serve as a compensatory mechanism for maintaining and/or terminating search when features result in no retrieval. The frontal ERP component could be mediated by age-related changes in the frontal lobes, which are known to occur earlier than age-related changes in other brain regions (Grady, 2012; Hedden & Gabrieli, 2004). However, ERP studies provide high spatial-temporal but relatively poor spatial resolution. Future investigation using fMRI (previously done only in young adults; Assaf, Calhoun et al., 2006; Kraut, Kremen et al., 2002;), which affords better spatial resolution than does EEG, will be needed to test this hypothesis in the aging population.
As we contended earlier, semantic relatedness and congruity could play a less important role in the SORT task and do not contribute significantly to the late ERP component we observed. Still, we acknowledge that a plausible account for the late ERP finding reported in the current study may be attributed to semantic relatedness or congruity effects that differ between retrieval and non-retrieval conditions. For example, the additional frontal component in older adults may reflect a delayed detection of congruity compared to young adults. Future studies should (1) include retrieval and non-retrieval trials contingent upon disparate degrees of semantic relatedness/congruity and (2) use non-repeated stimuli in order to examine these other possibilities.
The operation associated with non-retrieval trials has been observed to be dysfunctional in patients with Alzheimer’s disease and schizophrenia performing the SORT (Assaf et al., 2006; Kraut, Cherry et al., 2006). In these studies, a predominant type of error noted was false positive answers on non-retrieval trials or ‘overbinds’. For example, when asked for the name of the object in reading “humps” and “alarm”, an Alzheimer’s patient wrote “volcano” as the false positive error. Another example is “skywriter” for the word pair “propeller” and “writing”. Thus it should be emphasized that semantic memory retrieval dysfunction could manifest in both ways: failure to retrieve (false negative) and failure to suppress inappropriate memory representations when falsely combining features to either an unrelated or a nonexistent object (false positive). We posit that the latter dysfunction can result from an inability to maintain and terminate search processes effectively. Normal older adults appear to maintain and terminate the search successfully by utilizing neural mechanisms as measured by the late frontal ERP component. Therefore, deviations to this late frontal component could potentially be used as a neurophysiological marker for detecting semantic dysfunction in patient populations. However, further research will be required to replicate the results with a larger sample of subjects to test the validity of using this ERP component as a marker.
Although condition differences in older adults seemed to contribute the most to the interaction observed in the frontal electrodes, we also observed a pattern reversal when comparing younger and older adults; while we observed frontal negativity in younger adults, a frontal positivity was noted in older adults. The group difference peaked twice at 400 and 750 msec post-stimulus and was not specific to trial type. It is plausible that structural differences or changes in the brain between the groups could be contributing to this pattern reversal. At the neural level, it could be that in one age group, different cellular populations are being synaptically impinged relative to the other age group. Those different neural impingements could be happening within the same regions of brain, thus causing activation of distinct neural generators with different polarities. Alternatively, the activity could be in different brain regions altogether, with consequent opposite-polarity summations at the scalp. The neural bases for these ERP differences can not be ascertained at this point.
We acknowledge that the older adult participants were recruited through a physical fitness study (Chapman et al., 2013) that had strict inclusion and exclusion criteria. Those potential participants who had physical or cognitive issues and were not able to endure the study were excluded. Thus, the older adult subjects in our study were in general healthier than their peers in the overall population, which may have contributed to their behavioral performance being comparable to that exhibited by the cohort of young participants. However, this fact makes the ERP differences in the face of preserved performance even more striking, since many individual differences can create variability in the ERPs of general older adult populations (Meyer & Federmeier, 2010; Wlotko et al., 2010). Second, more studies will be needed to investigate the time-frequency or phase coherence information on the cortical oscillations associated with the object memory retrieval process associated with the SORT. Finally, in the current design subjects only use their right hand to respond to the stimuli, leaving the uncertainty as to whether unilateral responses contributed in an important way to the left fronto-temporal ERP component. A study design incorporating bilateral hand responses could plausibly help to address this issue.
In conclusion, our findings suggest that older adults employ additional neural resources during semantic processing that requires more extensive and exhaustive search within the semantic network. When the search ends with object memory retrieval, older adults recruit similar neural mechanisms as younger adults. Our results support the contention that the stability of this semantic memory function in older age relies not only on successfully retrieving an object memory but also on successfully terminating search before false-positive object memory retrieval occurs: this process has been found to be impaired in patient populations (Assaf et al., 2006; Kraut, Cherry et al., 2006). The semantic ERP markers observed in the current study may have potential applications in characterizing early semantic deterioration and distinguishing neurophysiological changes due to disease from those due to aging alone (Chiang et al., 2012).
Supplementary Material
Highlights.
We used ERPs to examine neural basis of semantic object retrieval in normal aging.
Behavioral performance in older adults was comparable to younger adults.
Comparable neural mechanisms used in both groups when features facilitated retrieval.
Older adults used additional neural resources when features yielded no retrieval.
Acknowledgments
This work was supported by a grant from the National Institute of Health (RC1-AG035954), the RGK foundation, and the Berman Research Initiative at the Center for BrainHealth. The authors thank Dr. Elizabeth K. Bartz, Rajen Patel, Monique Salinas, Erin Venza, Audette Rackley, and Claire Gardner for their invaluable assistance in data collection. A special thanks to Dr. Francesca Filbey for her insightful review on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allport DA. Distributed memory, modular subsystems and dysphasia. In: Newman SK, Epstein R, editors. Current Perspectives in Dysphasia. Edinburgh: Churchill Livingstone; 1985. pp. 207–244. [Google Scholar]
- Arzouan Y, Goldstein A, Faust M. Brainwaves are stethoscopes: ERP correlates of novel metaphor comprehension. Brain Research. 2007;1160:69–81. doi: 10.1016/j.brainres.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart JJ, Pearlson GD. Neural correlates of the object-recall process in semantic memory. Psychiatry Research: Neuroimaging. 2006;147(2–3):115–126. doi: 10.1016/j.pscychresns.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Assaf M, Rivkin PR, Kuzu CH, Calhoun VD, Kraut MA, Groth KM, et al. Abnormal object recall and anterior cingulate overactivation correlate with formal thought disorder in schizophrenia. Biological Psychiatry. 2006;59(5):452–459. doi: 10.1016/j.biopsych.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Semantic retrieval, mnemonic control, and prefrontal cortex. Behavioral and Cognitive Neuroscience Reviews. 2002;1(3):206–218. doi: 10.1177/1534582302001003002. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Statistics in Society) 1995;57:289–300. [Google Scholar]
- Bentin S, McCarthy G, Wood CC. Event-related potentials, lexical decision and semantic priming. Electroencephalography and Clinical Neurophysiology. 1985;60(4):343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- Besson M, Kutas M, Van Petten C. An event-related potential (ERP) analysis of semantic congruity and repetition effects in sentences. Journal of Cognitive Neuroscience. 1992;4(2):132–149. doi: 10.1162/jocn.1992.4.2.132. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornkessel-Schlesewsky I, Schlesewsky M. An alternative perspective on ‘semantic P600’ effects in language comprehension. Brain Research Reviews. 2008;59(1):55–73. doi: 10.1016/j.brainresrev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Brier MR, Maguire MJ, Tillman GD, Hart J, Jr, Kraut MA. Event-related potentials in semantic memory retrieval. Journal of the International Neuropsychological Society: JINS. 2008;14(5):815–822. doi: 10.1017/S135561770808096X. [DOI] [PubMed] [Google Scholar]
- Calley CS, Kraut MA, Spence JS, Briggs RW, Haley RW, Hart J., Jr The neuroanatomic correlates of semantic memory deficits in patients with gulf war illnesses: A pilot study. Brain Imaging and Behavior. 2010;4(3–4):248–255. doi: 10.1007/s11682-010-9103-2. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Defina LF, Keebler MW, Didehbani N, Lu H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Frontiers in Aging Neuroscience. 2013;5:75. doi: 10.3389/fnagi.2013.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HS, Mudar RA, Bartz KE, Martin-Cook K, Kraut M, Hart J., Jr Loss of differentiation in event-related potentials (ERPs) to reflect a feature-based object memory retrieval process in patients with Mild Cognitive Impairment. Neurology. 2012 Apr 23;78:P01.193. doi: 10.1212/WNL.78.1_MeetingAbstracts.P01.193. 2012. Meeting Abstracts 1. [DOI] [Google Scholar]
- Coulson S, Van Petten C. Conceptual integration and metaphor: An event-related potential study. Memory & Cognition. 2002;30(6):958–968. doi: 10.3758/bf03195780. [DOI] [PubMed] [Google Scholar]
- Debruille JB, Renoult L. Effects of semantic matching and of semantic category on reaction time and N400 that resist numerous repetitions. Neuropsychologia. 2009;47(2):506–517. doi: 10.1016/j.neuropsychologia.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Michelson CA, Franklin MS. Separating the visual sentence N400 effect from the P400 sequential expectancy effect: cognitive and neuroanatomical implications. Brain Research. 2010;1355:126–140. doi: 10.1016/j.brainres.2010.07.099. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principal components analysis of event-related potential datasets. In: Handy T, editor. Event-Related Potentials: A Methods Handbook. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Federmeier KD, Kutas M. Aging in context: Age-related changes in context use during language comprehension. Psychophysiology. 2005;42(2):133–141. doi: 10.1111/j.1469-8986.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, van Petten C, Schwartz TJ, Kutas M. Sounds, words, sentences: Age-related changes across levels of language processing. Psychology and Aging. 2003;18(4):858–872. doi: 10.1037/0882-7974.18.4.858. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Brier MR, Hart JJ, Kraut MA. Space–time–frequency analysis of EEG data using within-subject statistical tests followed by sequential PCA. Neuroimage. 2009;45(1):109–121. doi: 10.1016/j.neuroimage.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Freunberger R, Klimesch W, Griesmayr B, Sauseng P, Gruber W. Alpha phase coupling reflects object recognition. Neuroimage. 2008;42(2):928–935. doi: 10.1016/j.neuroimage.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Galdo-Alvarez S, Lindín M, Díaz F. The effect of age on event-related potentials (ERP) associated with face naming and with the tip-of-the-tongue (TOT) state. Biological Psychology. 2009;81(1):14–23. doi: 10.1016/j.biopsycho.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kutas M. An electrophysiological study of scene effects on object identification. Cognitive Brain Research. 2003;16(2):123–144. doi: 10.1016/S0926-6410(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Grieder M, Crinelli RM, Koenig T, Wahlund LO, Dierks T, Wirth M. Electrophysiological and behavioral correlates of stable automatic semantic retrieval in aging. Neuropsychologia. 2012;50(1):160–171. doi: 10.1016/j.neuropsychologia.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Giaquinto S, Ranghi F, Butler S. Stability of word comprehension with age. an electrophysiological study. Mechanisms of Ageing and Development. 2007;128(11–12):628–636. doi: 10.1016/j.mad.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. Journal of Neuroscience. 2006;26(18):4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature Reviews Neuroscience. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbin TJ, Marsh GR, Harvey MT. Differences in the late components of the event-related potential due to age and to semantic and non-semantic tasks. Electroencephalography and Clinical Neurophysiology. 1984;59(6):489–496. doi: 10.1016/0168-5597(84)90007-8. [DOI] [PubMed] [Google Scholar]
- Hart J, Jr, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, et al. Neuroimaging of cognitive dysfunction and depression in aging retired national football league players: A cross-sectional study. JAMA Neurology. 2013;70(3):326–335. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart J, Jr, Maguire MJ, Motes M, Mudar RA, Chiang H, Womack KB, Kraut MA. Semantic memory retrieval circuit: Role of pre-SMA, caudate, and thalamus. Brain and Language. 2013;126(1):89–98. doi: 10.1016/j.bandl.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Hart JJ, Anand R, Zoccoli S, Maguire M, Gamino J, Tillman G, et al. Neural substrates of semantic memory. Journal of the International Neuropsychological Society. 2007;13(5):865–880. doi: 10.1017/S135561770707110X. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Neural subsystems for object knowledge. Nature. 1992;359(6390):60–64. doi: 10.1038/359060a0. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Hill H, Strube M, Roesch-Ely D, Weisbrod M. Automatic vs. controlled processes in semantic priming — differentiation by event-related potentials. International Journal of Psychophysiology. 2002;44(3):197–218. doi: 10.1016/s0167-8760(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(2):179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Large, colorful, or noisy? attribute- and modality-specific activations during retrieval of perceptual attribute knowledge. Cognitive, Affective & Behavioral Neuroscience. 2001;1(3):207–221. doi: 10.3758/cabn.1.3.207. [DOI] [PubMed] [Google Scholar]
- Kemmer L, Coulson S, De Ochoa E, Kutas M. Syntactic processing with aging: An event-related potential study. Psychophysiology. 2004;41(3):372–384. doi: 10.1111/1469-8986.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Pulvermüller F. Conceptual representations in mind and brain: Theoretical developments, current evidence and future directions. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(7):805–825. doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kiefer M. Repetition-priming modulates category-related effects on event-related potentials: Further evidence for multiple cortical semantic systems. Journal of Cognitive Neuroscience. 2005;17(2):199–211. doi: 10.1162/0898929053124938. [DOI] [PubMed] [Google Scholar]
- Kiefer M. Perceptual and semantic sources of category-specific effects: Event-related potentials during picture and word categorization. Memory & Cognition. 2001;29(1):100–116. doi: 10.3758/bf03195745. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Cherry B, Pitcock JA, Anand R, Li J, Vestal L, et al. The semantic object retrieval test (SORT) in amnestic mild cognitive impairment. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2007;20(1):62–67. doi: 10.1097/WNN.0b013e3180335f7d. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Cherry B, Pitcock JA, Vestal L, Henderson VW, Hart J., Jr The semantic object retrieval test (SORT) in normal aging and alzheimer disease. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology. 2006;19(4):177–184. doi: 10.1097/01.wnn.0000213922.41008.22. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Pitcock JA, Calhoun V, Li J, Freeman T, Hart J., Jr Neuroanatomic organization of sound memory in humans. Journal of Cognitive Neuroscience. 2006;18(11):1877–1888. doi: 10.1162/jocn.2006.18.11.1877. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Calhoun V, Pitcock JA, Cusick C, Hart J., Jr Neural hybrid model of semantic object memory: Implications from event-related timing using fMRI. Journal of the International Neuropsychological Society. 2003;9(7):1031–1040. doi: 10.1017/S135561770397007X. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Kremen S, Moo LR, Segal JB, Calhoun V, Hart J., Jr Object activation in semantic memory from visual multimodal feature input. Journal of Cognitive Neuroscience. 2002;14(1):37–47. doi: 10.1162/089892902317205302. [DOI] [PubMed] [Google Scholar]
- Kraut MA, Moo LR, Segal JB, Hart J., Jr Neural activation during an explicit categorization task: Category- or feature-specific effects? Cognitive Brain Research. 2002;13(2):213–220. doi: 10.1016/s0926-6410(01)00117-3. [DOI] [PubMed] [Google Scholar]
- Kröger S, Rutter B, Hill H, Windmann S, Hermann C, Abraham A. An ERP study of passive creative conceptual expansion using a modified alternate uses task. Brain Research. 2013;1527:189–198. doi: 10.1016/j.brainres.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR. Neural mechanisms of language comprehension: Challenges to syntax. Brain Research. 2007;1146:23–49. doi: 10.1016/j.brainres.2006.12.063. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Caplan D, Holcomb PJ. Electrophysiological distinctions in processing conceptual relationships within simple sentences. Cognitive Brain Research. 2003;17(1):117–129. doi: 10.1016/S0926-6410(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP) Annual Review of Psychology. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in Cognitive Sciences. 2000;4(12):463–470. doi: 10.1016/S1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Iragui V. The N400 in a semantic categorization task across 6 decades. Electroencephalography and Clinical Neurophysiology. 1998;108(5):456–471. doi: 10.1016/s0168-5597(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207(4427):203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Lau EF, Phillips C, Poeppel D. A cortical network for semantics: [de]constructing the N400. Nature Reviews Neuroscience. 2008;9(12):920–33. doi: 10.1038/nrn2532. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17(4):677–689. doi: 10.1037/0882-7974.17.4.677. [DOI] [PubMed] [Google Scholar]
- Li S, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Research article transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science (Wiley-Blackwell) 2004;15(3):155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Lima SD, Hale S, Myerson J. How general is general slowing? evidence from the lexical domain. Psychology and Aging. 1991;6(3):416–425. doi: 10.1037/0882-7974.6.3.416. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potential components. Oxford University Press; 2012. [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. Cambridge, Mass: MIT Press; 2005. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annual Review of Psychology. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology. 2001;11(2):194–201. doi: 10.1016/S0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Nobre AC, Bentin S, Spencer DD. Language-related field potentials in the anterior-medial temporal lobe: I. intracranial distribution and neural generators. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15(2):1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson WB, Holcomb PJ. An electrophysiological investigation of semantic priming with pictures of real objects. Psychophysiology. 1999;36(1):53–65. doi: 10.1017/S0048577299971196. [DOI] [PubMed] [Google Scholar]
- McRae K, Cree GS, Seidenberg MS, McNorgan C. Semantic feature production norms for a large set of living and nonliving things. Behavior Research Methods. 2005;37(4):547–559. doi: 10.3758/bf03192726. [DOI] [PubMed] [Google Scholar]
- Meyer AM, Federmeier KD. Event-related potentials reveal the effects of aging on meaning selection and revision. Psychophysiology. 2010;47(4):673–686. doi: 10.1111/j.1469-8986.2010.00983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Federmeier KD. The effects of context, meaning frequency, and associative strength on semantic selection: Distinct contributions from each cerebral hemisphere. Brain Research. 2007;1183:91–108. doi: 10.1016/j.brainres.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudrik L, Lamy D, Deouell LY. ERP evidence for context congruity effects during simultaneous object–scene processing. Neuropsychologia. 2010;48(2):507–517. doi: 10.1016/j.neuropsychologia.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Nobre AC, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: II. effects of word type and semantic priming. The Journal of Neuroscience. 1995;15(2):1090–1099. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan, Jefferies Krist A, ElizabethVisser MayaLambon Ralph, Matthew A. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. Journal of Cognitive Neuroscience. 2013;25(11):1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of visual, auditory, and abstract semantics. Neuroimage. 2002;15(4):917–926. doi: 10.1006/nimg.2001.1016. [DOI] [PubMed] [Google Scholar]
- Pergola G, Bellebaum C, Gehlhaar B, Koch B, Schwarz M, Daum I, Suchan B. The involvement of the thalamus in semantic retrieval: A clinical group study. Journal of Cognitive Neuroscience. 2013;25(6):872–886. doi: 10.1162/jocn_a_00364. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson RJ, et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37(2):127–152. [PubMed] [Google Scholar]
- Pulvermüller F. How neurons make meaning: Brain mechanisms for embodied and abstract-symbolic semantics. Trends in Cognitive Sciences. 2013;17(9):458–470. doi: 10.1016/j.tics.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Renoult L, Wang X, Mortimer J, Debruille JB. Explicit semantic tasks are necessary to study semantic priming effects with high rates of repetition. Clinical Neurophysiology. 2012;123(4):741–754. doi: 10.1016/j.clinph.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Segal JB, Williams R, Kraut MA, Hart JJ. Semantic memory deficit with a left thalamic infarct. Neurology. 2003;61(2):252–254. doi: 10.1212/01.wnl.0000073145.08816.e2. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–703. doi: 10.1111/1469-8986.ep11026949. [DOI] [PubMed] [Google Scholar]
- Sitnikova T, Holcomb PJ, Kiyonaga KA, Kuperberg GR. Two neurocognitive mechanisms of semantic integration during the comprehension of visual real-world events. Journal of Cognitive Neuroscience. 2008;20(11):2037–2057. doi: 10.1162/jocn.2008.20143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Kraut MA, Lesser RP, Hart J., Jr Interactions between thalamic and cortical rhythms during semantic memory recall in human. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):6440–6443. doi: 10.1073/pnas.092514899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JS, Brier MR, Hart J, Jr, Ferree TC. Removing an intersubject variance component in a general linear model improves multiway factoring of event-related spectral perturbations in group EEG studies. Human Brain Mapping. 2013;34(3):651–664. doi: 10.1002/hbm.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab TY, Brown C, Hagoort P. Understanding ambiguous words in sentence contexts: Electrophysiological evidence for delayed contextual selection in broca’s aphasia. Neuropsychologia. 1998;36(8):737–761. doi: 10.1016/s0028-3932(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill S, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Van Petten C, Luka BJ. Prediction during language comprehension: Benefits, costs, and ERP components. International Journal of Psychophysiology. 2012;83(2):176–190. doi: 10.1016/j.ijpsycho.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Luka BJ. Neural localization of semantic context effects in electromagnetic and hemodynamic studies. Brain and Language. 2006;97(3):279–293. doi: 10.1016/j.bandl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Whitney Carin, Kirk Marie, O’Sullivan Jamie, Ralph Matthew A Lambon, Jefferies Elizabeth. Executive semantic processing is underpinned by a large-scale neural network: Revealing the contribution of left prefrontal, posterior temporal, and parietal cortex to controlled retrieval and selection using TMS. Journal of Cognitive Neuroscience. 2012;24(1):133–147. doi: 10.1162/jocn_a_00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiswede D, Koranyi N, Müller F, Langner O, Rothermund K. Validating the truth of propositions: Behavioral and ERP indicators of truth evaluation processes. Social Cognitive & Affective Neuroscience. 2013;8(6):647–653. doi: 10.1093/scan/nss042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlotko EW, Lee C, Federmeier KD. Language of the aging brain: Event- related potential studies of comprehension in older adults. Language and Linguistics Compass. 2010;4(8):623–638. doi: 10.1111/j.1749-818X.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, Sen NM, Chong H, Riis JL, McGinnis SM, Holcomb PJ, Daffner KR. ERP correlates of item recognition memory: Effects of age and performance. Brain Research. 2009;1250:218–231. doi: 10.1016/j.brainres.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.