Abstract
Bone morphogenetic proteins (BMPs) are members of the transforming growth factor beta superfamily. Several members of this family have been shown to transduce their signals through binding to type I and type II serine-(threonine) kinase receptors. Here we report the cDNA cloning and characterization of a human type II receptor for BMPs (BMPR-II), which is distantly related to DAF-4, a BMP type II receptor from Caenorhabditis elegans. In transfected COS-1 cells, osteogenic protein (OP)-1/BMP-7, and less efficiently BMP-4, bound to BMPR-II. BMPR-II bound ligands only weakly alone, but the binding was facilitated by the presence of previously identified type I receptors for BMPs. Binding of OP-1/BMP-7 to BMPR-II was also observed in nontransfected cell lines. Moreover, a transcriptional activation signal was transduced by BMPR-II in the presence of type I receptors after stimulation by OP-1/BMP-7.
Full text
PDF

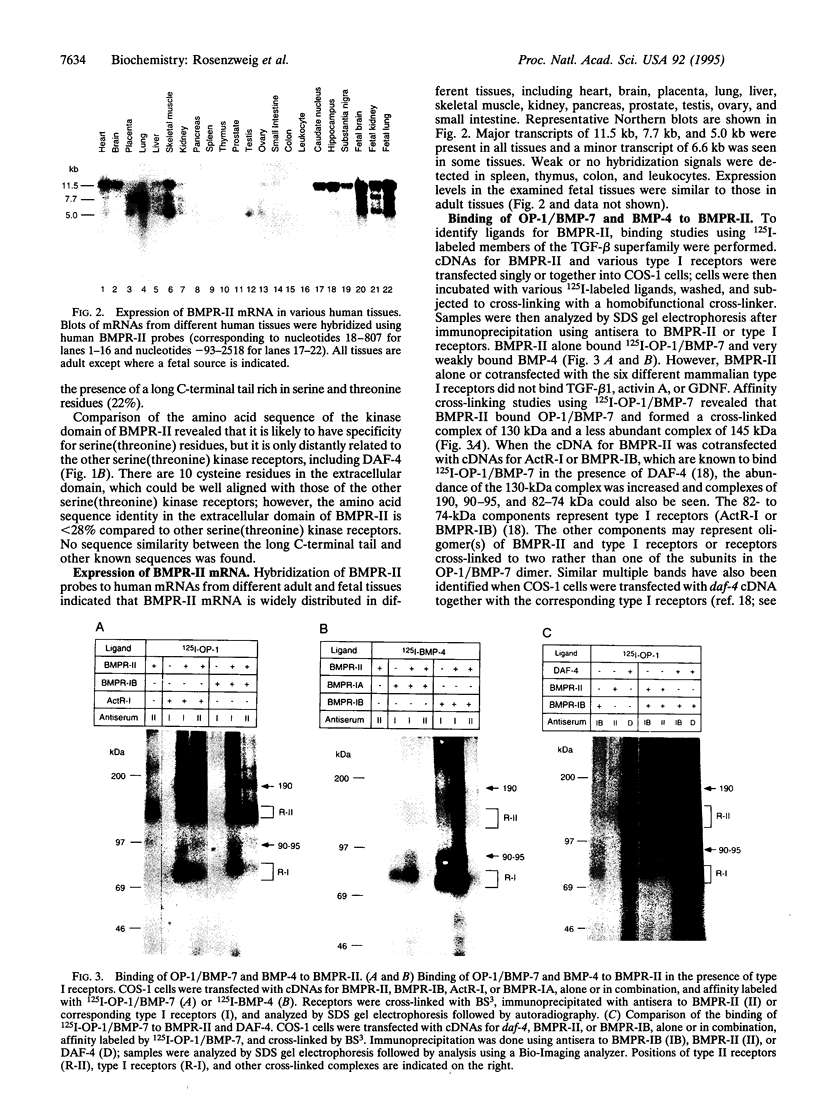
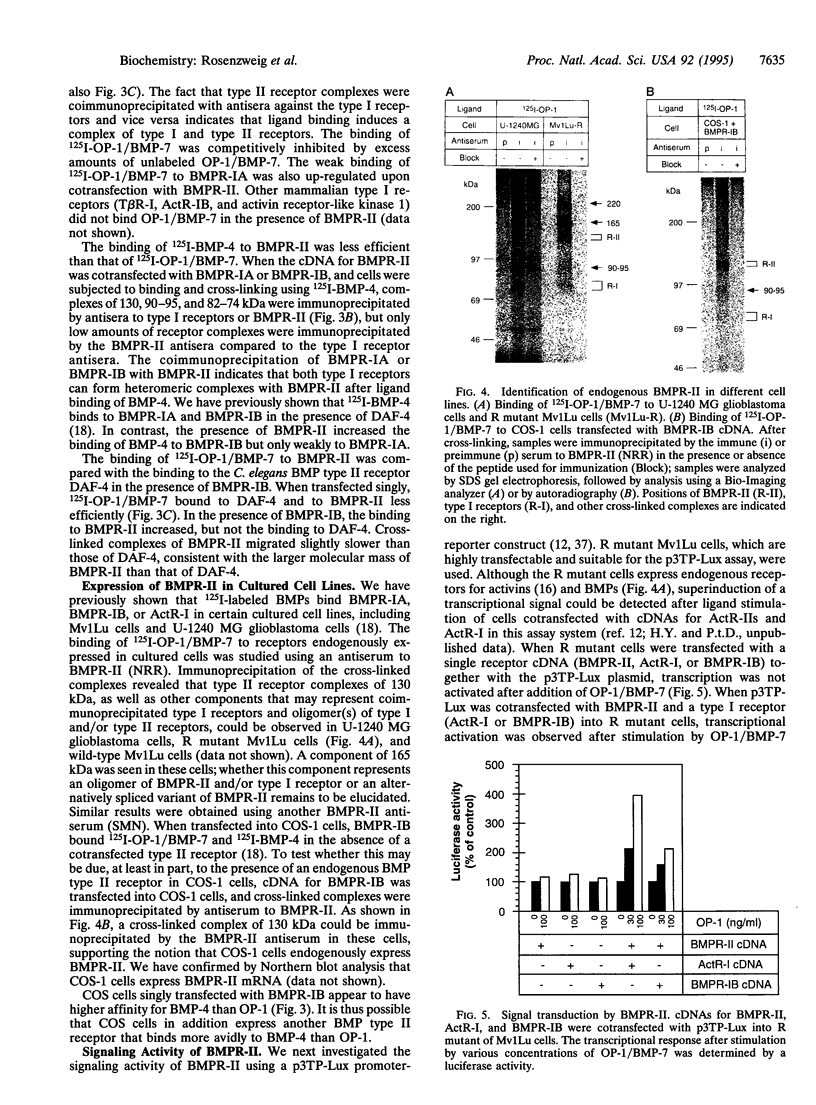

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attisano L., Cárcamo J., Ventura F., Weis F. M., Massagué J., Wrana J. L. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993 Nov 19;75(4):671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- Attisano L., Wrana J. L., Cheifetz S., Massagué J. Novel activin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threonine kinase receptors. Cell. 1992 Jan 10;68(1):97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Baarends W. M., van Helmond M. J., Post M., van der Schoot P. J., Hoogerbrugge J. W., de Winter J. P., Uilenbroek J. T., Karels B., Wilming L. G., Meijers J. H. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the müllerian duct. Development. 1994 Jan;120(1):189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- Bassing C. H., Yingling J. M., Howe D. J., Wang T., He W. W., Gustafson M. L., Shah P., Donahoe P. K., Wang X. F. A transforming growth factor beta type I receptor that signals to activate gene expression. Science. 1994 Jan 7;263(5143):87–89. doi: 10.1126/science.8272871. [DOI] [PubMed] [Google Scholar]
- Childs S. R., Wrana J. L., Arora K., Attisano L., O'Connor M. B., Massagué J. Identification of a Drosophila activin receptor. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9475–9479. doi: 10.1073/pnas.90.20.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárcamo J., Weis F. M., Ventura F., Wieser R., Wrana J. L., Attisano L., Massagué J. Type I receptors specify growth-inhibitory and transcriptional responses to transforming growth factor beta and activin. Mol Cell Biol. 1994 Jun;14(6):3810–3821. doi: 10.1128/mcb.14.6.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L., Howes G., Price B. M., Smith J. C. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992 Jun;115(2):573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Ebner R., Chen R. H., Shum L., Lawler S., Zioncheck T. F., Lee A., Lopez A. R., Derynck R. Cloning of a type I TGF-beta receptor and its effect on TGF-beta binding to the type II receptor. Science. 1993 May 28;260(5112):1344–1348. doi: 10.1126/science.8388127. [DOI] [PubMed] [Google Scholar]
- Estevez M., Attisano L., Wrana J. L., Albert P. S., Massagué J., Riddle D. L. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993 Oct 14;365(6447):644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- Franzén P., ten Dijke P., Ichijo H., Yamashita H., Schulz P., Heldin C. H., Miyazono K. Cloning of a TGF beta type I receptor that forms a heteromeric complex with the TGF beta type II receptor. Cell. 1993 Nov 19;75(4):681–692. doi: 10.1016/0092-8674(93)90489-d. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Thies R. S., Song J. J., Celeste A. J., Melton D. A. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994 Oct 7;79(1):169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Gullick W. J., Downward J., Waterfield M. D. Antibodies to the autophosphorylation sites of the epidermal growth factor receptor protein-tyrosine kinase as probes of structure and function. EMBO J. 1985 Nov;4(11):2869–2877. doi: 10.1002/j.1460-2075.1985.tb04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Harland R. M. The transforming growth factor beta family and induction of the vertebrate mesoderm: bone morphogenetic proteins are ventral inducers. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10243–10246. doi: 10.1073/pnas.91.22.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. M., Lyons K. M., Lapan P. M., Wright C. V., Hogan B. L. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992 Jun;115(2):639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Kawabata M., Chytil A., Moses H. L. Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor. J Biol Chem. 1995 Mar 10;270(10):5625–5630. doi: 10.1074/jbc.270.10.5625. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Koenig B. B., Cook J. S., Wolsing D. H., Ting J., Tiesman J. P., Correa P. E., Olson C. A., Pecquet A. L., Ventura F., Grant R. A. Characterization and cloning of a receptor for BMP-2 and BMP-4 from NIH 3T3 cells. Mol Cell Biol. 1994 Sep;14(9):5961–5974. doi: 10.1128/mcb.14.9.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990 Oct 25;265(30):18518–18524. [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993 May 21;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Massagué J., Attisano L., Wrana J. L. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994 May;4(5):172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991 Jun 14;65(6):973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- Mathews L. S., Vale W. W., Kintner C. R. Cloning of a second type of activin receptor and functional characterization in Xenopus embryos. Science. 1992 Mar 27;255(5052):1702–1705. doi: 10.1126/science.1313188. [DOI] [PubMed] [Google Scholar]
- Miyazono K., ten Dijke P., Yamashita H., Heldin C. H. Signal transduction via serine/threonine kinase receptors. Semin Cell Biol. 1994 Dec;5(6):389–398. doi: 10.1006/scel.1994.1046. [DOI] [PubMed] [Google Scholar]
- Nistér M., Libermann T. A., Betsholtz C., Pettersson M., Claesson-Welsh L., Heldin C. H., Schlessinger J., Westermark B. Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res. 1988 Jul 15;48(14):3910–3918. [PubMed] [Google Scholar]
- Paralkar V. M., Weeks B. S., Yu Y. M., Kleinman H. K., Reddi A. H. Recombinant human bone morphogenetic protein 2B stimulates PC12 cell differentiation: potentiation and binding to type IV collagen. J Cell Biol. 1992 Dec;119(6):1721–1728. doi: 10.1083/jcb.119.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perides G., Hu G., Rueger D. C., Charness M. E. Osteogenic protein-1 regulates L1 and neural cell adhesion molecule gene expression in neural cells. J Biol Chem. 1993 Nov 25;268(33):25197–25205. [PubMed] [Google Scholar]
- Reddi A. H. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994 Oct;4(5):737–744. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., Maliakal J. C., Hauschka P. V., Jones W. K., Sasak H., Tucker R. F., White K. H., Coughlin J. E., Tucker M. M., Pang R. H. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992 Oct 5;267(28):20352–20362. [PubMed] [Google Scholar]
- Suzuki A., Thies R. S., Yamaji N., Song J. J., Wozney J. M., Murakami K., Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K., Mathews L. S., Vale W. W. Cloning and characterization of a transmembrane serine kinase that acts as an activin type I receptor. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11242–11246. doi: 10.1073/pnas.90.23.11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Reddi A. H. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8793–8797. doi: 10.1073/pnas.86.22.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J. M. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1(4):267–280. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- di Clemente N., Wilson C., Faure E., Boussin L., Carmillo P., Tizard R., Picard J. Y., Vigier B., Josso N., Cate R. Cloning, expression, and alternative splicing of the receptor for anti-Müllerian hormone. Mol Endocrinol. 1994 Aug;8(8):1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Ichijo H., Franzén P., Schulz P., Saras J., Toyoshima H., Heldin C. H., Miyazono K. Activin receptor-like kinases: a novel subclass of cell-surface receptors with predicted serine/threonine kinase activity. Oncogene. 1993 Oct;8(10):2879–2887. [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Ichijo H., Franzén P., Laiho M., Miyazono K., Heldin C. H. Characterization of type I receptors for transforming growth factor-beta and activin. Science. 1994 Apr 1;264(5155):101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Yamashita H., Sampath T. K., Reddi A. H., Estevez M., Riddle D. L., Ichijo H., Heldin C. H., Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994 Jun 24;269(25):16985–16988. [PubMed] [Google Scholar]