Abstract
Background:
Invasive micropapillary carcinoma (IMPC) is a variant of breast carcinoma with a higher propensity for lymph node metastases compared with invasive ductal carcinoma (IDC).
Methods:
Retrospective analysis of 636 IMPC and 297 735 IDC cases in the Surveillance, Epidemiology and End Results database comparing disease-specific survival (DSS) and overall survival (OS) between IMPC and IDC.
Results:
A higher percentage of IMPC cases (52.0%) had nodal metastases compared with IDC cases (34.6%). The 5-year DSS and OS for IMPC was 91.8% and 82.9%, respectively compared with 88.6% and 80.5% for IDC, respectively. For both IMPC and IDC, oestrogen-receptor positivity was associated with better survival, while having four or more positive lymph nodes or larger tumour size correlated with worse survival. Radiotherapy provided a survival benefit for both histological types.
Conclusions:
Despite IMPC's higher propensity for lymph node metastasis, IMPC has DSS and OS that compare favourably with IDC.
Keywords: micropapillary carcinoma, breast cancer, ductal carcinoma, prognostic factors, survival, histological special type, radiotherapy
Invasive micropapillary carcinoma (IMPC) of the breast, first described in 1980 (Fisher et al, 1980) and further characterised in 1993 (Petersen, 1993; Siriaunkgul and Tavassoli, 1993), is an uncommon (<2% of cases) histological special type of invasive breast carcinoma. IMPC is described as small nests of tumour cells that appear as micropapillae surrounded by clear stromal spaces not lined by endothelial cells (Kim et al, 2005). IMPC has a high propensity for metastatic spread to the axillary lymph nodes, with reported rates of 46–95% higher than invasive ductal carcinoma (IDC) (Paterakos et al, 1999; Yamaguchi et al, 2010). Therefore, given this aggressive initial presentation of IMPC, it is presumed to have a worse prognosis than IDC. We have previously reported over 600 cases of IMPC listed in the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) database and showed that the 5-year disease-specific survival (DSS) and overall survival (OS) compare favourably with previously published survival rates of IDC (Chen et al, 2013). Here, we directly compare two cohorts of IMPC and IDC patients from the same time period while presenting additional 21 months of follow-up from our initial study.
Materials and methods
This retrospective study analysed the SEER database (November 2012 update) using methods previously described (Chen et al, 2013). We searched for cases of female breast cancer diagnosed between 2001 and 2008. Patients with unknown data for a parameter of interest or in situ disease only (stage 0) were excluded from the analysis. Patients with metachronous tumours or tumour recurrence (3422 cases) were counted only once for survival analyses with follow-up considered from the earliest tumour occurrence during the study period. Patients with synchronous primaries (2266 cases) had each tumour considered separately for survival analysis. Staging was based on American Joint Committee on Cancer 6th edition, as encoded in the SEER database. Poorly differentiated and anaplastic histological grades were considered grade 3 disease (Bloom–Richardson). Borderline oestrogen receptor (ER) or progesterone receptor (PR) status was considered unknown. The data-use policies of the SEER database were strictly adhered to in this study. R statistical software (R Core Team, 2013) with the packages ‘survival' and ‘survplot' were used for survival analysis. A P-value<0.05 was considered statistically significant.
Results
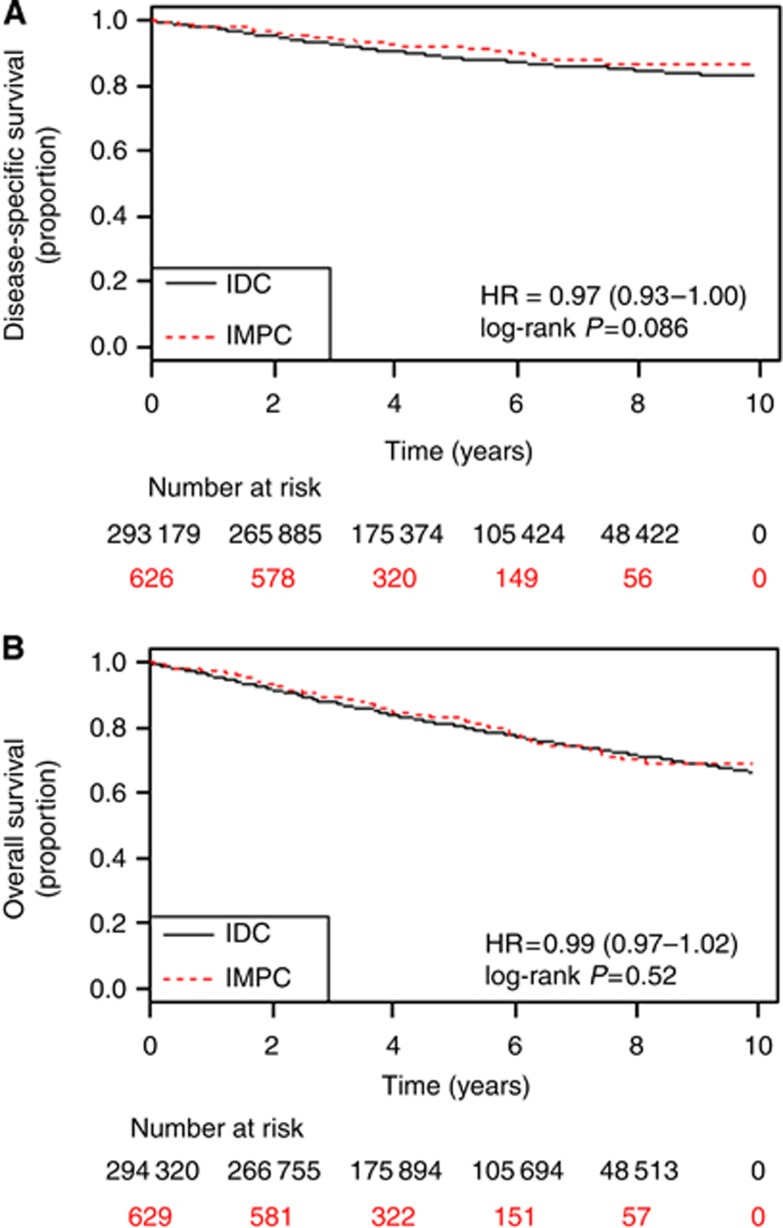
A total of 636 cases from 627 patients with IMPC and 297 735 cases from 292 052 patients with IDC were identified in the SEER database between 2001 and 2008 and included in this study. Eight patients presented with synchronous IMPC and IDC tumours. The median follow-up for the IMPC and IDC groups was 48 months and 56 months, respectively, while mean follow-up for IMPC and IDC groups was 53.2 months and 59.6 months, respectively. The 5-year DSS and OS for IMPC was 91.8% and 82.9%, respectively compared with 88.6% and 80.5%, respectively for IDC, with no significant difference between the curves (Figure 1).
Figure 1.
Disease-specific (A) and overall survival (B) by histology; Abbreviations: HR=hazard ratio; IDC=invasive ductal carcinoma; IMPC=invasive micropapillary carcinoma.
Patient characteristics
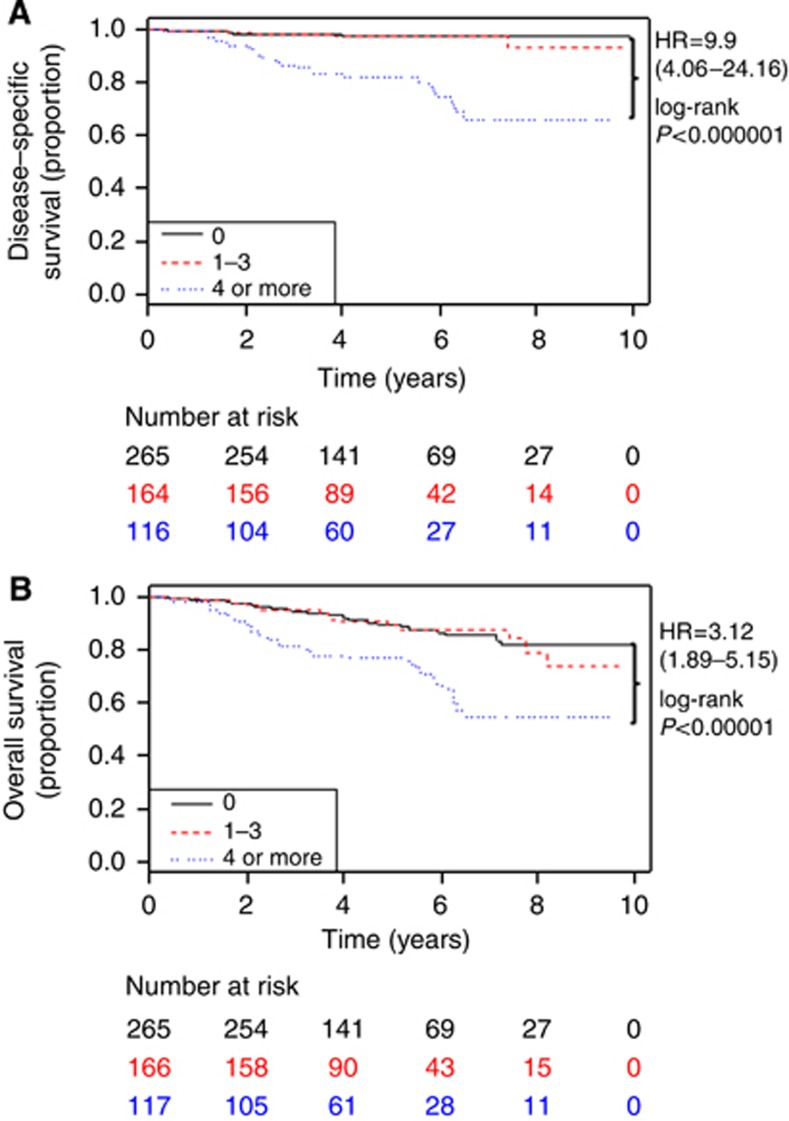
The characteristics of these patients are listed in Table 1, with similar age at diagnosis and slightly more ethnically diverse population for IMPC patients. Furthermore, IMPC patients had higher rate of known ER positivity (P<0.00001) and progesterone receptor positivity (P=0.0049) compared with IDC patients. IMPC patients with ER-positive tumours had a favourable hazard ratio (HR) for both DSS (HR 0.40, 95% confidence interval (CI) (0.22–0.75), P=0.0027) and OS (HR 0.69, 95% CI (0.43–1.10), P=0.12) compared with ER-negative tumours (Supplementary Figure 1). A similar effect was seen in IMPC patients with progesterone receptor-positive tumours and in IDC patients with hormone-receptor-positive tumours compared with hormone-receptor-negative tumours (data not shown). Of those with lymph node examinations, a median of six lymph nodes (interquartile range (IQR) 2–13.75) was examined in IMPC patients with a median of one positive lymph node (IQR 0–3). In contrast, IDC patients had a median of five lymph nodes (IQR 2–12) examined with a median of zero positive lymph nodes (IQR 0–1). IMPC patients had a higher incidence of nodal metastases (52.0%) compared with IDC patients (34.6%). Furthermore, IMPC patients had a larger proportion with 1–3 as well as four or more positive lymph nodes than IDC patients (P<0.00001). IMPC patients with four or more positive lymph nodes had worse DSS (HR 9.9, 95% CI (4.06–24.16), P<0.000001) and OS (HR 3.12, 95% CI (1.89–5.15) P<0.00001) compared with node-negative patients (Figure 2), with a similar phenomenon in IDC patients (data not shown). However, IMPC patients with 1–3 positive lymph nodes had DSS and OS similar to node-negative patients, in contrast to IDC patients (data not shown). Patients with four or more positive lymph nodes had no difference in DSS when compared between histologies (data not shown). Neither histological grade nor tumour location contributed significantly to the prognosis of IMPC. When comparing staging at presentation, IMPC patients had more T3 or T4 tumours, a higher percentage of N2 or N3 nodal involvement, but had similar rates of distant metastases compared with IDC patients (2.9% vs 3.9%, P=0.19). Larger tumour size in IMPC patients (T2 or T3) was associated with worse survival when compared with T1 tumours (data not shown).
Table 1. Patient characteristics.
| IMPC (%) | IDC (%) | P-value | |
|---|---|---|---|
|
Age at diagnosis |
Mean 61.3 years |
Mean 60.5 years |
0.11 |
| Ethnicitya |
|
|
0.053 |
| White | 494 (79.3) | 238 765 (82.2) | |
| Black | 69 (11.1) | 29 933 (10.3) | |
| Asian Pacific Islander | 59 (9.5) | 20 383 (7.0) | |
| American Indian | 1 (0.2) | 1392 (0.5) | |
| Unknown |
4 |
1579 |
|
| Oestrogen receptor |
|
|
<0.00001 |
| Positive | 501 (84.1) | 201 171 (75.1) | |
| Negative | 95 (15.9) | 66 535 (24.9) | |
| Unknown |
40 |
30 029 |
|
| Progesterone receptor |
|
|
<0.005 |
| Positive | 412 (70.2) | 16 8173 (63.9) | |
| Negative | 175 (29.8) | 95 134 (36.1) | |
| Unknown |
49 |
34 428 |
|
| Positive lymph nodes |
|
|
<0.00001 |
| None | 265 (48.0) | 168 696 (65.4) | |
| 1–3 | 169 (30.6) | 61 132 (23.7) | |
| 4 or more | 118 (21.4) | 28 294 (11.0) | |
| Unknown |
84 |
39 613 |
|
| Histological grade |
|
|
<0.000001 |
| 1 | 60 (10.0) | 51 591 (18.1) | |
| 2 | 294 (49.2) | 116 332 (40.9) | |
| 3 | 244 (40.8) | 116 541 (41.0) | |
| Unknown |
38 |
13 271 |
|
| Tumour location |
|
|
0.06 |
| Upper outer | 192 (35.4) | 102 176 (40.5) | |
| Upper inner | 77 (14.2) | 32 111 (12.7) | |
| Lower outer | 52 (9.6) | 20 036 (7.9) | |
| Lower inner | 41 (7.6) | 16 989 (6.7) | |
| Multiple | 148 (27.3) | 62 009 (24.6) | |
| Other | 32 (5.9) | 19 087 (7.6) | |
| Unknown |
94 |
45 327 |
|
| TNM classification |
|
|
<0.005 |
| T0 | 1 (0.2) | 118 (0.0) | |
| Tis | 0 (0.0) | 10 (0.0) | |
| T1 | 372 (62.2) | 175 346 (64.9) | |
| T2 | 165 (27.6) | 76 646 (28.4) | |
| T3 | 42 (7.0) | 10 480 (3.9) | |
| T4 | 18 (3.0) | 7635 (2.8) | |
| Unknown | 38 | 27 500 | |
| N0 | 315 (51.4) | 184 065 (65.9) | <0.00001 |
| N1 | 174 (28.4) | 65 152 (23.3) | |
| N2 | 59 (9.6) | 18 868 (6.8) | |
| N3 | 65 (10.6) | 11 303 (4.0) | |
| Unknown | 23 | 18 347 | |
| M0 | 611 (97.1) | 280 482 (96.1) | 0.19 |
| M1 | 18 (2.9) | 11 258 (3.9) | |
| Unknown |
7 |
5995 |
|
| Stage |
|
|
<0.00001 |
| I | 248 (40.3) | 138 668 (49.2) | |
| II | 242 (39.3) | 103 835 (36.8) | |
| III | 101 (16.4) | 27 458 (9.7) | |
| IV | 25 (4.1) | 11 983 (4.3) | |
| Unknown |
20 |
15 791 |
|
| Surgery type |
|
|
<0.05 |
| None | 34 (5.3) | 16 223 (5.5) | |
| Breast-conserving surgery | 312 (49.1) | 160 732 (54.2) | |
| Mastectomy | 290 (45.6) | 119 847 (40.4) | |
| Unknown |
0 |
933 |
|
| Radiation |
|
|
0.68 |
| No | 343 (53.9) | 158 119 (53.1) | |
| Yes | 293 (46.1) | 139 616 (46.9) |
Abbreviations: IDC=invasive ductal carcinoma; IMPC=invasive micropapillary carcinom; TNM, tumour nodes metastases.
Includes eight patients that had both IMPC and IDC.
Figure 2.
Degree of lymph node involvement affects disease-specific (A) and overall survival (B) in invasive micropapillary carcinoma; Abbreviation: HR, hazard ratio.
Surgical and radiation treatment
Patients with IMPC had a slightly higher mastectomy rate when compared with IDC (45.6% vs 40.6%, P=0.025). The percentage of total patients who received radiation treatment was similar between groups (46.1% for IMPC vs 46.9% for IDC, P=0.68). IDC patients had a benefit from radiation treatment (data not shown), as did IMPC patients with a trend for better DSS (HR 0.60, 95% CI (0.34–1.07), P=0.079) and better OS (HR 0.56, 95% CI (0.38–0.82), P=0.0026; Supplementary Figure 2).
Multivariate analysis
Multivariate analysis was performed to evaluate these different clinicopathological parameters. Of all the parameters studied, only the tumour size (T2 or T3 vs T1) and the use of radiation treatment remained independent predictors of either DSS or OS for IMPC patients (Supplementary Table 1).
Discussion
IMPC of the breast presents a clinical challenge for oncologists. Given its aggressive initial presentation with a significant propensity for lymph node metastasis, many consider the disease to be potentially more worrisome than IDC. This study shows that while IMPC patients tend to have a higher clinical stage at initial presentation, the rate of distant metastases is similar to that in IDC patients. When comparing with other studies of IMPC, one group has reported a 5-year DSS of 70% (Middleton et al, 1999) while others have reported 5-year OS ranging from 59–86% (Chen et al, 2008, Yu et al, 2010). Another group reported having 20 IMPC patients with distant metastases with 21 patients dying from the disease (Gokce et al, 2013). Our study reaffirms our previous report with longer follow-up and suggests that while IMPC patients have an increased rate of lymph node metastases, they may not be dying of distant metastases given the favourable DSS rates.
One major component to the survival advantage in IMPC may be ER positivity. Previous studies have reported a wide range of ER positivity in IMPC patients from 19.4 to 90.6% (Walsh and Bleiweiss, 2001; Kim et al, 2005). Our IMPC population has a higher rate than the IDC comparison group. The other significant clinical factor in this study is the degree of lymph node positivity. The lymph node positivity is lower than the range (68.8–90.5%) of other large studies of IMPC (Paterakos et al, 1999; Luna-More et al, 2000; Nassar et al, 2001; Walsh and Bleiweiss, 2001; Pettinato et al, 2004; Zekioglu et al, 2004; Chen et al, 2008; Yu et al, 2010), likely due to the high percentage of T1 and T2 tumours in our study population. Nonetheless, these results emphasise the importance of adequately examining the axilla for IMPC patients. Multiple groups have described the utility of screening for axillary lymphadenopathy in IMPC patients with ultrasonography (Adrada et al, 2009; Jones et al, 2013). IMPC patients without adequate lymph node exams may lack the prognostic data to see whether they fall into the category of four or more positive lymph nodes, although the multivariate analysis shows that tumour size, not degree of lymph node positivity, remains the most important independent predictor of survival.
The SEER database outlines the loco-regional therapies for patients (including surgery and radiation). IMPC patients had a notably slightly higher mastectomy rate compared with IDC, which may be due to the higher percentage of T3 and T4 disease in IMPC. This study shows that the percentage of radiation therapy for IMPC patients was similar to those seen in IDC patients and demonstrates a similar benefit of radiation treatment in both groups. An important caveat is that the SEER database does not describe the use of systemic therapies such as chemotherapy or endocrine therapy, so it is unclear which treatment regimens are most effective at controlling distant disease. However, unlike previous studies based on single institutions, the use of a large population-based database has the advantage of investigating IMPC and IDC patients who were treated during the same time period. This incorporates any possible treatment trends and allows for more direct comparisons between the two groups. Therefore, this study population is more diverse and potentially more generalisable when compared with retrospective studies from single institutions.
In conclusion, this study describes the largest set of IMPC patients reported in the literature and allows for direct comparison with a set of IDC patients during the same study period. This study provides additional follow-up from our previous study and confirms the initial findings presented there. Only tumour size and radiation treatment were independent predictors of survival in this population and may be used to subclassify these patients. Additional studies, especially prospective studies, are warranted to help establish clear guidelines for the management and treatment of this uncommon histological variant of breast carcinoma.
Acknowledgments
We thank Laura Riojas for administrative support during this project. This work was supported by institutional funds from Houston Methodist Hospital. Information was drawn from a centralised, de-identified database.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Adrada B, Arribas E, Gilcrease M, Yang WT. Invasive micropapillary carcinoma of the breast: mammographic, sonographic, and mri features. AJR Am J Roentgenol. 2009;193 (1:W58–W63. doi: 10.2214/AJR.08.1537. [DOI] [PubMed] [Google Scholar]
- Chen AC, Paulino AC, Schwartz MR, Rodriguez AA, Bass BL, Chang JC, Teh BS. Prognostic markers for invasive micropapillary carcinoma of the breast: a population-based analysis. Clin Breast Cancer. 2013;13 (2:133–139. doi: 10.1016/j.clbc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Chen L, Fan Y, Lang R-G, Guo X-J, Sun Y-L, Cui L-F, Liu F-F, Wei J, Zhang X-M, Fu L. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16 (2:155–163. doi: 10.1177/1066896907307047. [DOI] [PubMed] [Google Scholar]
- Fisher ER, Palekar AS, Redmond C, Barton B, Fisher B. Pathologic findings from the national surgical adjuvant breast project (protocol no. 4). vi. invasive papillary cancer. Am J Clin Pathol. 1980;73 (3:313–322. doi: 10.1093/ajcp/73.3.313. [DOI] [PubMed] [Google Scholar]
- Gokce H, Durak MG, Akin MM, Canda T, Balci P, Ellidokuz H, Demirkan B, Gorken IB, Sevinc AI, Kocdor MA, Saydam S, Harmancioglu O. Invasive micropapillary carcinoma of the breast: a clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J. 2013;19 (4:374–381. doi: 10.1111/tbj.12128. [DOI] [PubMed] [Google Scholar]
- Jones KN, Guimaraes LS, Reynolds CA, Ghosh K, Degnim AC, Glazebrook KN. Invasive micropapillary carcinoma of the breast: imaging features with clinical and pathologic correlation. AJR Am J Roentgenol. 2013;200 (3:689–695. doi: 10.2214/AJR.12.8512. [DOI] [PubMed] [Google Scholar]
- Kim M-J, Gong G, Joo HJ, Ahn S-H, Ro JY. Immunohistochemical and clinicopathologic characteristics of invasive ductal carcinoma of breast with micropapillary carcinoma component. Arch Pathol Lab Med. 2005;129 (10:1277–1282. doi: 10.5858/2005-129-1277-IACCOI. [DOI] [PubMed] [Google Scholar]
- Luna-More S, Casquero S, Perez-Mellado A, Rius F, Weill B, Gornemann I. Importance of estrogen receptors for the behavior of invasive micropapillary carcinoma of the breast. review of 68 cases with follow-up of 54. Pathol Res Pract. 2000;196 (1:35–39. doi: 10.1016/S0344-0338(00)80019-9. [DOI] [PubMed] [Google Scholar]
- Middleton LP, Tressera F, Sobel ME, Bryant BR, Alburquerque A, Grases P, Merino MJ. Infiltrating micropapillary carcinoma of the breast. Mod Pathol. 1999;12 (5:499–504. [PubMed] [Google Scholar]
- Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001;14:836–841. doi: 10.1038/modpathol.3880399. [DOI] [PubMed] [Google Scholar]
- Paterakos M, Watkin WG, Edgerton SM, Moore DH, Thor AD. Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol. 1999;30 (12:1459–1463. doi: 10.1016/s0046-8177(99)90168-5. [DOI] [PubMed] [Google Scholar]
- Petersen J. Breast carcinomas with an unexpected inside-out growth pattern. rotation of polarization associated with angioinvasion. Pathol Res Pract. 1993;189:780. [Google Scholar]
- Pettinato G, Manivel CJ, Panico L, Sparano L, Petrella G. Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol. 2004;121 (6:857–866. doi: 10.1309/XTJ7-VHB4-9UD7-8X60. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria; 2013. [Google Scholar]
- Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993;6 (6:660–662. [PubMed] [Google Scholar]
- Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001;32 (6:583–589. doi: 10.1053/hupa.2001.24988. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Tanaka M, Kondo K, Yokoyama T, Kaneko Y, Yamaguchi M, Ogata Y, Nakashima O, Kage M, Yano H. Characteristic morphology of invasive micropapillary carcinoma of the breast: an immunohistochemical analysis. Jpn J Clin Oncol. 2010;40 (8:781–787. doi: 10.1093/jjco/hyq056. [DOI] [PubMed] [Google Scholar]
- Yu JI, Choi DH, Park W, Huh SJ, Cho EY, Lim YH, Ahn JS, Yang JH, Nam SJ. Differences in prognostic factors and patterns of failure between invasive micropapillary carcinoma and invasive ductal carcinoma of the breast: matched case-control study. Breast. 2010;19 (3:231–237. doi: 10.1016/j.breast.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Zekioglu O, Erhan Y, Ciris M, Bayramoglu H, Ozdemir N. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004;44 (1:18–23. doi: 10.1111/j.1365-2559.2004.01757.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.