Abstract
Background:
An elevated neutrophil-to-lymphocyte ratio (NLR) is associated with poor outcome in various tumours. Its prognostic utility in patients with urothelial carcinoma of the bladder (UCB) undergoing radical cystectomy (RC) is yet to be fully elucidated.
Methods:
A cohort of patients undergoing RC for UCB in a tertiary referral centre between 1992 and 2012 was analysed. Neutrophil-to-lymphocyte ratio was computed using complete blood counts performed pre-RC, or before neo-adjuvant chemotherapy where applicable. Time-dependent receiver operating characteristic curves were used to determine the optimal cutoff point for predicting recurrence-free survival (RFS), cancer-specific survival (CSS) and overall survival (OS). The predictive ability of NLR was assessed using Kaplan–Meier analyses and multivariable Cox proportional hazards models. The likelihood-ratio test was used to determine whether multivariable models were improved by including NLR.
Results:
The cohort included 424 patients followed for a median of 58.4 months. An NLR of 3 was determined as the optimal cutoff value. Patients with an NLR⩾3.0 had significantly worse survival outcomes (5y-RFS: 53% vs 64%, log-rank P=0.013; 5y-CSS: 57% vs 75%, log-rank P<0.001; 5y-OS: 43% vs 64%, log-rank P<0.001). After adjusting for disease-specific predictors, an NLR ⩾3.0 was significantly associated with worse RFS (HR=1.49; 95% CI=1.12–2.0, P=0.007), CSS (HR=1.88; 95% CI=1.39–2.54, P<0.001) and OS (average HR=1.67; 95% CI=1.17–2.39, P=0.005). The likelihood-ratio test confirmed that prognostic models were improved by including NLR.
Conclusions:
Neutrophil-to-lymphocyte ratio is an inexpensive prognostic biomarker for patients undergoing RC for UCB. It offers pre-treatment prognostic value in addition to established prognosticators and may be helpful in guiding treatment decisions.
Keywords: urinary bladder neoplasms, neutrophils, lymphocytes, biological markers, prognosis, treatment outcome, cystectomy, inflammation
Radical cystectomy (RC) with pelvic lymph node dissection is the standard treatment for muscle-invasive (MI) urothelial carcinoma of the bladder (UCB) and is recommended for patients with non-muscle-invasive (NMI) UCB with high risk of progression (Clark et al, 2013). Despite curative intent, disease recurs in a significant proportion of patients and 5-year survival rates of only 40–60% have consistently been reported (Gakis et al, 2013).
More aggressive treatment options, such as early RC in patients with high-risk NMI UCB or RC in combination with neo-adjuvant chemotherapy (NAC) or adjuvant chemotherapy (AC), have been shown to improve outcomes (Raj et al, 2011; Meeks et al, 2012; Leow et al, 2013; Sternberg et al, 2013). However, employing aggressive strategies unselectively to all patients carries the risk of overtreatment in patients with favourable prognoses. Improved risk stratification will individualise the use of such approaches. At this time, however, risk stratification based on clinicopathological data alone is unlikely to be sufficient for optimal treatment decision-making (Ficarra et al, 2005; Shariat et al, 2007; Canter et al, 2011). Thus, novel prognostic markers are needed to improve stratification, and, eventually outcomes, of patients with UCB.
Inflammation has an important role in the development and progression of many malignancies (Grivennikov et al, 2010; Hanahan and Weinberg, 2011). Putative mechanisms include the increased supply of factors that promote carcinogenesis and tumour progression by cells of the innate immune systems (that is, neutrophils) and decreased antitumoural response by immune cells of the adaptive system (that is, lymphocytes; Hanahan and Weinberg, 2011). The neutrophil-to-lymphocyte ratio (NLR), which can easily be calculated from routine complete blood counts (CBCs) with differentials, is an emerging marker of host inflammation and has been shown to be an independent prognosticator for a variety of solid malignancies (Proctor et al, 2012; Guthrie et al, 2013; Templeton et al, 2014). However, there is sparse data on the prognostic role of NLR in patients with UCB (Gondo et al, 2012; Demirtas et al, 2013; Krane et al, 2013).
The objective of our investigation was to evaluate the association between pre-treatment NLR and survival in patients undergoing RC for UCB in a cohort of patients from a tertiary care centre.
Materials and Methods
Patients and data sources
Using our institutional database, patients who underwent RC between 1 January 1992 and 31 December 2012 were retrospectively identified. Patients were excluded if CBCs with differentials were unavailable for analysis (n=14), or if they had a history of conditions that may have influenced blood cell lines (connective tissue disease: n=4, malignant lymphoma: n=3, leukaemia: n=2, and human immunodeficiency virus infection: n=1). Patients undergoing RC for salvage therapy following failed chemoradiation (n=20) were excluded because of the potential influence of prior chemotherapy on blood cell lines. Patients with non-urothelial cancers (n=9), or for primary prostatic urothelial carcinoma (n=5), were also excluded in order to maintain a homogenous cohort. Electronic hospital chart review was performed to collect clinical parameters including blood work results. Mortality data were obtained through the Princess Margaret Cancer Centre Cancer Registry. Institutional research ethics board approval was obtained.
Primary study exposure
The date of initiation of treatment for each patient was defined as the date of RC or the date of initiation of NAC for patients who received NAC. All patients were seen for medical assessment before the initiation of treatment. Generally, NLR was calculated using neutrophil and lymphocyte counts from a routine CBC with differentials performed on the same day as these visits (median of 6 days (interquartile range (IQR): 2–10 days) before initiation of treatment). Review of the pre-treatment clinic notes did not reveal any symptoms or signs of infections that may have influenced the NLR.
Outcome measures
Patients were generally seen at 6–8 weeks after the RC, and otherwise every 3–6 months early on for periodic physical examination, imaging to rule out hydronephrosis or tumour recurrence, and urethroscopy if indicated. Follow-up subsequently became less intensive based on individual physician's practice patterns and clinical suspicion. The outcome measures were recurrence-free survival (RFS), cancer-specific survival (CSS) and overall survival (OS) measured in months from the date of initial treatment.
Statistical analysis
Statistical analyses were performed using SAS v9.3 (SAS Institute Inc, Cary, NC, USA). Clinical characteristics were compared between patients with NLR values above and below the optimal cutoff point (see below) using the Wilcoxon rank-sum test for continuous variables and Pearson's χ2 test for categorical variables.
In the literature, there is heterogeneity in the NLR cutoff points used (Guthrie et al, 2013). Therefore, in order to determine the optimal cutoff point for clinical use, time-dependent receiver-operating characteristic (ROC) curves were created for each outcome measure at 12, 24, 36, 48 and 60 months (Heagerty and Zheng, 2005; Lu and Liu, 2006). NLR values between 1.5 and 6 were considered in 0.5 increments. The optimal NLR value for each outcome at a given time point was identified by minimising the distance from the ROC curve to the top left corner of the ROC plot (and thus optimising both sensitivity and specificity; Perkins and Schisterman, 2006).
Kaplan–Meier analyses with log-rank tests were then used to compare survival outcomes between patients with NLR values above vs below the optimal cutoff point. To determine how NLR can influence risk stratification in the pre-treatment and post-cystectomy settings among patients with localised disease (without evidence of nodal disease), we performed additional Kaplan–Meier analyses stratifying by clinical and pathological stage, respectively. In these analyses, patients receiving NAC or AC were excluded to have a more clear impression of how NLR values have an impact on the natural history of disease.
Univariate and multivariable Cox proportional hazards models were built for each survival outcome. Multivariable models adjusted for a priori defined patient-related risk factors (age, gender and Charlson comorbidity index), tumour-related variables (pathological T-stage and lymphovascular invasion), treatment-related parameters (year of RC, use of NAC or AC and surgical margin status) and haematologic parameters (haemoglobin and platelet counts). AC was operationalised as a time-varying covariate to address survivor treatment bias (Austin et al, 2006). A robust sandwich covariance matrix estimator was used to account for clustering of outcomes by surgeon (Lin and Wei, 1989). The additional value to the models provided by NLR was evaluated using the likelihood-ratio test to compare models for each survival outcome with and without NLR. Statistical model assumptions, including the proportional hazards assumption, were tested (Hess, 1995).
In order to ensure that our use of a cutoff point did not introduce bias (Royston et al, 2006), we performed a sensitivity analysis analysing NLR as a continuous variable with log-transformation (because of its skewed distribution). Given that other studies have not included patients receiving NAC (Gondo et al, 2012), we also performed a sensitivity analysis excluding such patients. All tests were two-sided with P-values <0.05 considered statistically significant.
Results
The final study cohort consisted of 424 patients with a median follow-up of 58.4 months (IQR: 21.3–94.5 months). The cohort characteristics are described in Table 1. Overall, 138 patients (32.6%) had cancer recurrence and 178 (42%) died, of which 110 (25.9%) died of UCB.
Table 1. Cohort characteristics.
| Total n=424 | NLR ⩾3 n=216 | NLR <3 n=208 | P-value | ||
|---|---|---|---|---|---|
|
Patient characteristics | |||||
| Age in years | 70.1 (60.6–76.3) | 71.5 (61.9–77.3) | 68.9 (59.5–75.5) | 0.086 | |
| Female sex, n | 99 (23.4) | 43 (20.7) | 56 (25.9) | 0.20 | |
| Haemoglobin (g l−1) | 132.5 (116–145) | 125 (109–140) | 137.5 (125–148.5) | <0.001a | |
| WBC ( × 109 l−1) | 7.6 (6.0–9.1) | 8.7 (7.05–10.4) | 6.6 (5.5–8.2) | <0.001a | |
| Platelets ( × 109 l−1) | 252 (202–303) | 274 (218–329) | 236 (193–282.5) | <0.001a | |
| Heavy smoking (30⩾ pack-years), n | 134 (31.6) | 64 (30.8) | 70 (32.4) | 0.72 | |
| Charlson comorbidity indexb |
|
6 (5–7) |
6 (5–8) |
6 (4.5–7) |
0.010a |
|
Disease characteristics | |||||
| Hydronephrosis, n (%) | 119 (28.1) | 64 (30.8) | 55 (25.5) | 0.22 | |
| Concurrent CIS, n (%) | 190 (44.8) | 85 (40.9) | 105 (48.6) | 0.11 | |
| T-stage, n (%) | pT0 | 25 (5.9) | 9 (4.3) | 16 (7.4) | 0.004a |
| pTa/T1/T2 CIS | 205 (48.4) | 87 (41.8) | 118 (54.6) | ||
| pT3/T4 | 194 (45.7) | 112 (53.9) | 82 (38.0) | ||
| N-stage, n (%) | pN0 | 287 (67.7) | 144 (69.2) | 143 (66.2) | 0.68 |
| pN-pos | 116 (27.4) | 53 (25.5) | 63 (29.2) | ||
| pNx | 21 (4.9) | 11 (5.3) | 10 (4.6) | ||
| Positive surgical margin, n (%) | 333 (7.8) | 16 (7.7) | 17 (7.9) | 0.95 | |
| Lymphovascular invasion |
|
143 (33.7) |
77 (37.0) |
66 (30.6) |
0.16 |
|
Other treatment characteristics | |||||
| Total node count | 12 (7–19) | 12 (7–19) | 13 (8–19) | 0.22 | |
| NAC/primary chemotherapyc, n | 29 (6.8) | 15 (7.2) | 14 (6.5) | 0.77 | |
| Adjuvant chemotherapyc, n | 87 (20.5) | 34 (16.4) | 53 (24.5) | 0.037a | |
| Salvage chemotherapyc, n | 55 (13.0) | 27 (13.0) | 28 (13.0) | >0.99 | |
Abbreviations: CIS=carcinoma in situ; NAC: neo-adjuvant chemotherapy; NLR=neutrophil-to-lymphocyte ratio; WBC=white blood count.
All data presented as median (interquartile range) or number (percent).
A significant difference is indicated between the groups NLR ⩾3.0 and <3.
Sum of co-morbidity score (including malignancy) and age score.
Generally, chemotherapy was cisplatin-based. Patients with renal insufficiency received carboplatin-based chemotherapy.
In time-dependent ROC curve analyses, an NLR cutoff point of 3 minimised the distance from the ROC curve to the top left of the plot for 14 out of 15 time points across the three outcome measures (Supplementary Table 1). Given that this cutoff point was among those used by other studies (Guthrie et al, 2013), we proceeded to use this as the optimal cutoff point in our study.
There were 216 (50.9%) patients who had an NLR value ⩾3 (Table 1). These patients had significantly lower haemoglobin values, higher platelet counts and a higher Charlson comorbidity index and were less likely to receive AC. They were more likely to have pT3–4 disease (53.9% vs 38%); however, there was no significant difference in pN stage.
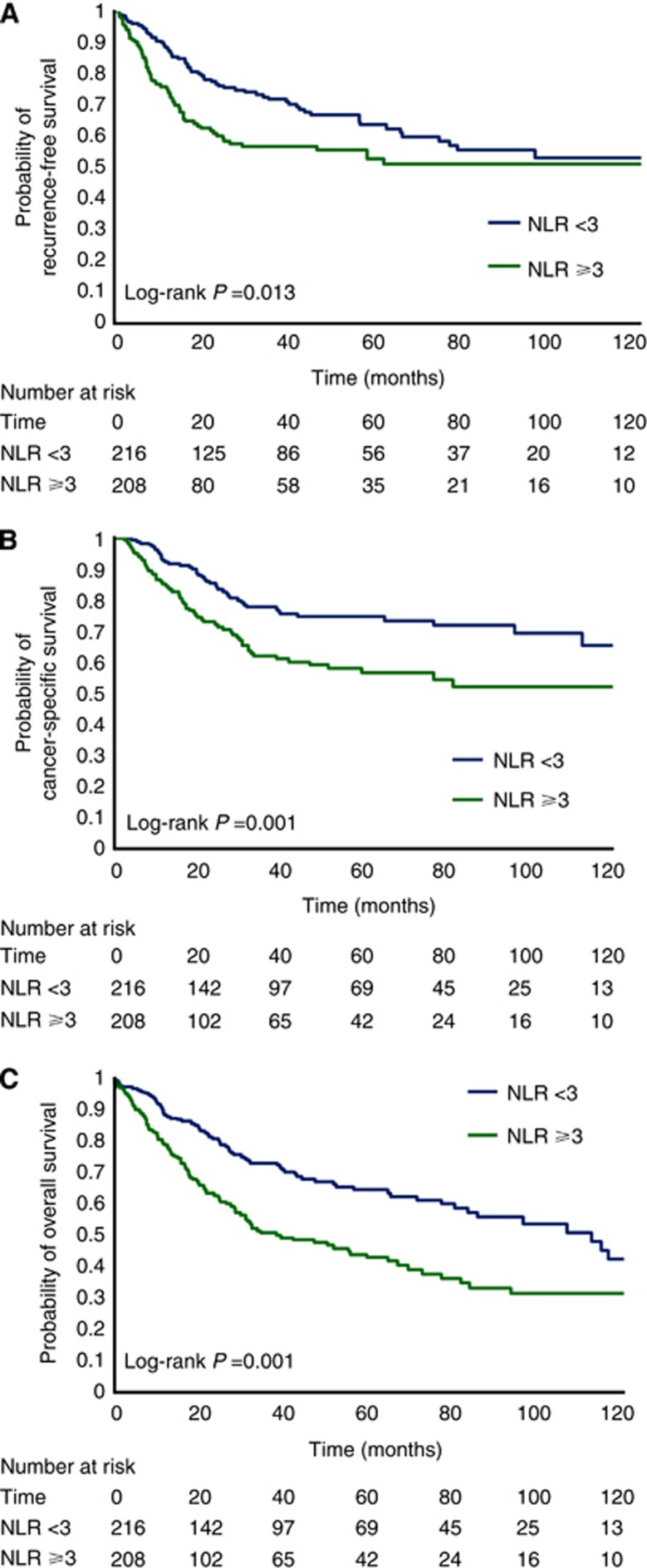
In univariate Kaplan–Meier analyses, NLR ⩾3 vs <3 was associated with increased probability of recurrence (5y-RFS: 53% vs 64%, log-rank P=0.013, Figure 1A), cancer-specific mortality (5y-CSS: 57% vs 75%, log-rank P<0.001, Figure 1B) and overall mortality (5y-OS: 43% vs 64%, log-rank P<0.001; Figure 1C).
Figure 1.
(A–C) Kaplan–Meier curves for recurrence-free survival (A), cancer-specific survival (B) and overall survival (C) for patients with an NLR <3 and ⩾3.
In univariate Cox models, an NLR ⩾3 was associated with increased risk of recurrence (hazard ratio (HR)=1.53, 95% confidence interval (CI)=1.23–1.89, P<0.001, Table 2), cancer-specific mortality (HR=1.88, 95% CI=1.52–2.33, P<0.001, Table 3) and overall mortality (HR=1.80, 95% CI=1.48–2.20, P<0.001, Table 4).
Table 2. Cox proportional hazard models for recurrence-free survival.
| |
Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
| NLR (⩾3 vs <3) |
1.53 (1.23–1.89) |
<0.001 |
1.49 (1.12–2.00) |
0.007 |
| Age |
1.01 (1.00–1.02) |
0.22 |
1.00 (0.98–1.03) |
0.93 |
| Gender (female vs male) |
0.96 (0.68–1.34) |
0.79 |
0.87 (0.60–1.27) |
0.48 |
| Charlson comorbidity index |
1.08 (0.99–1.18) |
0.078 |
1.04 (0.98–1.11) |
0.17 |
| Haemoglobin (per 10 units) |
0.89 (0.86–0.93) |
<0.001 |
0.97 (0.95–1.00) |
0.014 |
| Platelets (per 100 units) |
1.24 (1.10–1.38) |
<0.001 |
1.17 (1.10–1.25) |
<0.001 |
| T-stage (pT3–4 vs pT0–2) |
2.75 (1.99–3.80) |
<0.001 |
1.51 (0.97–2.34) |
0.067 |
| N-Stage (N+ vs N0/Nx) |
3.01 (2.68–3.38) |
<0.001 |
2.16 (1.82–2.57) |
<0.001 |
| Positive surgical margin |
2.07 (1.33–3.22) |
0.001 |
1.99 (1.28–3.10) |
0.002 |
| Year of radical cystectomy |
1.00 (0.97–1.03) |
0.93 |
1.00 (0.97–1.03) |
>0.99 |
| Lymphovascular invasion |
2.91 (1.88–4.52) |
<0.001 |
1.82 (1.13–2.95) |
0.014 |
| NAC/primary chemotherapy |
0.93 (0.43–2.01) |
0.86 |
0.90 (0.41–1.97) |
0.79 |
| Adjuvant chemotherapy | 1.56 (1.21–2.00) | <0.001 | 0.89 (0.55–1.42) | 0.61 |
Abbreviations: CI=confidence interval; HR=hazard ratio; NAC=neo-adjuvant chemotherapy; NLR=neutrophil-to-lymphocyte ratio.
Table 3. Cox proportional hazard models for cancer-specific survival.
| |
Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
| NLR (⩾3 vs <3) |
1.88 (1.52–2.33) |
<0.001 |
1.88 (1.39–2.54) |
<0.001 |
| Age |
1.01 (0.99–1.04) |
0.19 |
1.00 (0.97–1.04) |
0.88 |
| Gender (female vs male) |
1.17 (0.95–1.46) |
0.14 |
1.10 (0.87–1.40) |
0.41 |
| Charlson Comorbidity Index |
1.16 (1.06–1.28) |
0.002 |
1.16 (1.09–1.24) |
<0.001 |
| Haemoglobin (per 10 units) |
0.87 (0.82–0.91) |
<0.001 |
0.96 (0.92–0.99) |
0.013 |
| Platelets (per 100 units) |
1.28 (1.14–1.44) |
<0.001 |
1.19 (1.03–1.37) |
0.019 |
| T-stage (pT3–4 vs pT0–2) |
3.12 (2.18–4.47) |
<0.001 |
1.65 (1.06–2.56) |
0.026 |
| N-stage (N+ vs N0/Nx) |
3.21 (2.14–4.81) |
<0.001 |
2.26 (1.38–3.70) |
0.001 |
| Positive surgical margin |
1.98 (1.03–3.82) |
0.041 |
1.61 (0.79–3.31) |
0.19 |
| Year of radical cystectomy |
0.98 (0.92–1.04) |
0.50 |
0.96 (0.90–1.03) |
0.30 |
| Lymphovascular invasion |
3.09 (1.98–4.82) |
<0.001 |
1.91 (1.10–3.34) |
0.023 |
| NAC/primary chemotherapy |
1.47 (0.66–3.25) |
0.34 |
1.33 (0.59–2.98) |
0.50 |
| Adjuvant chemotherapy | 1.36 (1.01–1.83) | 0.041 | 0.95 (0.49–1.86) | 0.89 |
Abbreviations: CI=confidence interval; HR=hazard ratio; NAC=neo-adjuvant chemotherapy; NLR=neutrophil-to-lymphocyte ratio.
Table 4. Cox proportional hazard models for overall survival.
| |
Unadjusted |
Adjusted |
||
|---|---|---|---|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
| NLR (⩾3 vs <3) |
1.80 (1.48–2.20) |
<0.001 |
1.67a (1.17–2.39) |
0.005 |
| Age |
1.03 (1.02–1.05) |
<0.001 |
1.01 (0.99–1.03) |
0.20 |
| Gender (female vs male) |
0.95 (0.81–1.11) |
0.48 |
0.89 (0.73–1.07) |
0.21 |
| Charlson Comorbidity Index |
1.28 (1.18–1.39) |
<0.001 |
1.23 (1.16–1.31) |
<0.001 |
| Haemoglobin (per 10 units) |
0.85 (0.82–0.87) |
<0.001 |
0.93 (0.89–0.97) |
<0.001 |
| Platelets (per 100 units) |
1.21 (1.11–1.31) |
<0.001 |
1.21 (1.14–1.28) |
<0.001 |
| T-stage (pT3–4 vs pT0–2) |
2.44 (1.58–3.77) |
<0.001 |
1.41 (0.82–2.42) |
0.21 |
| N-stage (N+ vs N0/Nx) |
2.22 (1.67–2.94) |
<0.001 |
1.59 (1.14–2.22) |
0.006 |
| Positive surgical margin |
2.00 (0.99–4.04) |
0.054 |
1.83 (0.94–3.60) |
0.078 |
| Year of radical cystectomy |
0.97 (0.93–1.01) |
0.15 |
0.95 (0.92–0.98) |
0.004 |
| Lymphovascular invasion |
2.65 (2.06–3.41) |
<0.001 |
2.08 (1.18–3.68) |
0.012 |
| NAC/primary chemotherapy |
1.44 (0.67–3.06) |
0.35 |
1.31 (0.81–2.13) |
0.28 |
| Adjuvant chemotherapy | 0.91 (0.62–1.33) | 0.61 | 0.84 (0.48–1.49) | 0.55 |
Abbreviations: CI=confidence interval; HR=hazard ratio; NAC=neo-adjuvant chemotherapy; NLR=neutrophil-to-lymphocyte ratio.
HR represents average HR across follow-up time. However, the HR varies as a function of time (see Figure 2 for details).
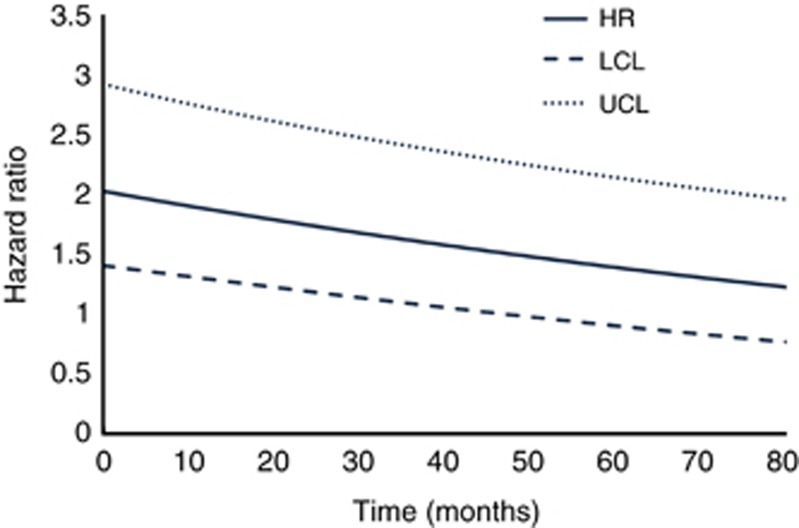
Upon adjusting for confounders using multivariable models, NLR remained significantly associated with increased risk of recurrence (HR=1.49, 95% CI=1.12–2, P=0.007, Table 2), cancer-specific mortality (HR=1.88, 95% CI=1.39–2.54, P<0.001, Table 3) and overall mortality (average HR=1.67, 95% CI=1.17–2.39, P=0.005, Table 4). Of note, the proportional hazards assumption was satisfied for the models for RFS and CSS but not for OS. This would suggest that the HR for the association between NLR and OS is not constant but varies as a function of time. Therefore, the HR presented in Table 4 represents an average value across the study period. As others have presented such findings (Lipscombe et al, 2013), Figure 2 shows how the HR changes as a function of time from initial treatment (with the corresponding model that includes an NLR × time interaction term shown in Supplementary Table 2). Neutrophil-to-lymphocyte ratio has the strongest association with increased risk of overall mortality early on and then gradually decreases. This association remained statistically significant up to ∼50 months from the start of treatment.
Figure 2.
Instantaneous hazard ratio vs time with corresponding confidence intervals for OS LCL=lower 95% confidence limit, UCL=upper 95% confidence limit.
Associations between NLR and increased risk of adverse survival outcomes remained statistically significant when NLR was analysed as a log-transformed continuous variable, as well as in the sensitivity analysis excluding patients who received NAC (data not shown). Using the likelihood-ratio test and comparing multivariable Cox models with and without NLR, it was determined that NLR significantly improved models for RFS (P=0.013), CSS (P=0.001) and OS (P=0.003).
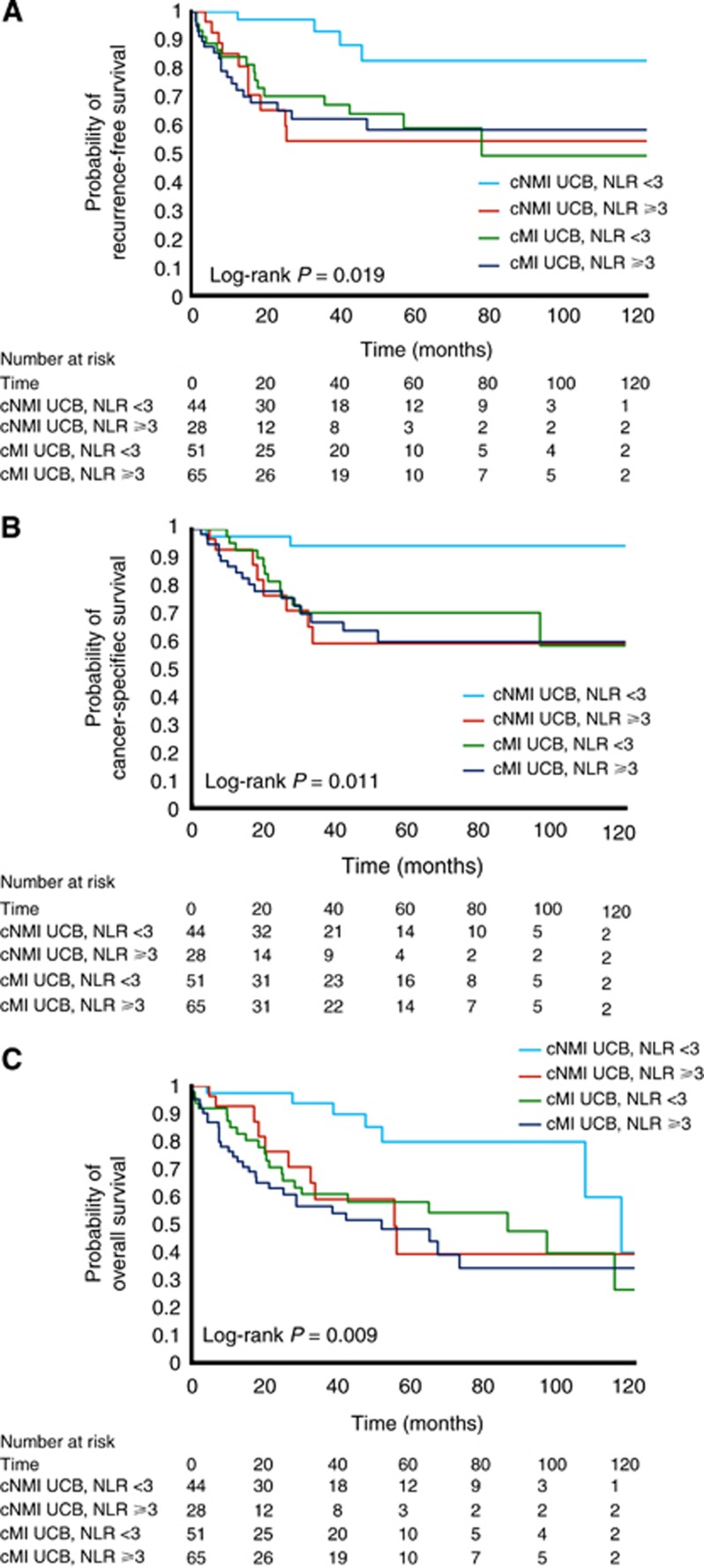
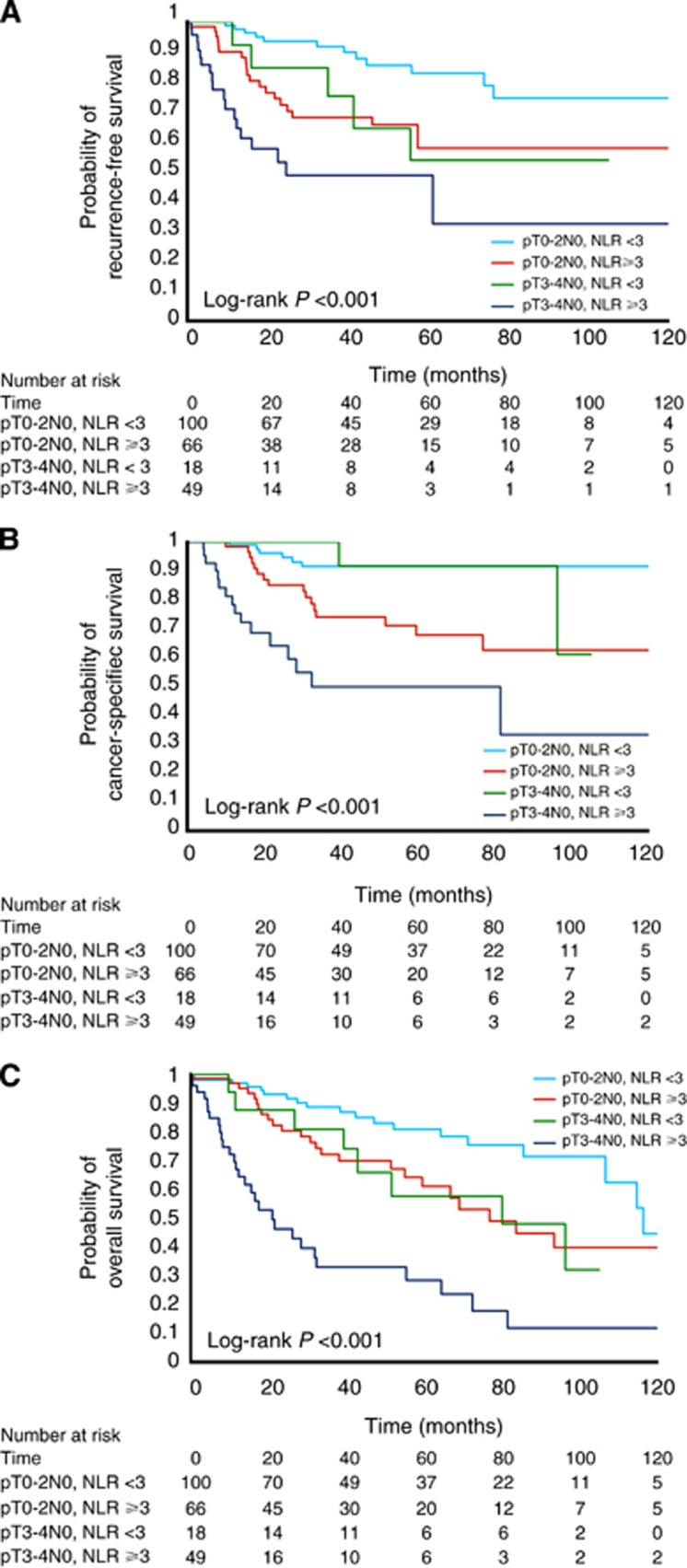
Lastly, we performed exploratory analyses to assess the potential prognostic impact of using NLR when risk stratifying patients into two scenarios. For these analyses, patients receiving NAC or AC were excluded to better reflect the natural history of disease and avoid confounding from adjunctive treatment. The first scenario assessed patients without clinical evidence of nodal disease (cN0), where risk stratification may guide initial management in the pre-treatment setting. We sought to determine whether NLR further stratified patients beyond their clinical stage, analysing its impact on clinically NMI UCB vs MI UCB disease subgroups. In these Kaplan–Meier analyses (Figures 3A–C), NLR added valuable prognostic information. Patients with clinical NMI UCB appeared to separate into two groups, with those with clinical NMI UCB and NLR ⩾3 manifesting survival outcomes comparable to clinical MI UCB. The second scenario was the postoperative setting among patients who were pN0 (where a decision must be made regarding the use of AC). NLR further stratified patients within pT-stage categories (Figures 4A–C). Notably among patients with organ-confined (pT0–pT2) disease, NLR identified a subset of patients who were at increased risk of adverse oncologic outcomes.
Figure 3.
(A–C) Kaplan–Meier curves for recurrence-free survival (A), cancer-specific survival (B) and overall survival (C) for patients without clinical evidence of nodal disease (cN0) and clinically NMI bladder cancer (BC) or MI BC and an NLR <3 or ⩾3, respectively.
Figure 4.
(A–C) Kaplan–Meier curves for recurrence-free survival (A), cancer-specific survival (B) and overall survival (C) for patients with organ-confined bladder cancer (pT0–2 pN0) or non-organ-confined bladder cancer (pT3–4 pN0) and an NLR <3 or ⩾3, respectively.
Discussion
The host inflammatory response has gained increasing attention in oncology research. Infiltrating cells of the immune system are constituents of virtually all neoplasms (Hanahan and Weinberg, 2011). While initially thought to represent an antitumoural response, immune cells, particularly those of the innate immune system, also exhibit effects that promote carcinogenesis and cancer progression (Grivennikov et al, 2010; Hanahan and Weinberg, 2011). Proposed mechanisms include increased supply of growth factors, survival factors, pro-angiogenic factors, extracellular matrix-modifying enzymes (which can facilitate invasion and metastasis) and inductive signals that may lead to epithelial-to-mesenchymal transition (Hanahan and Weinberg, 2011). Thus, there is a biological rationale for using NLR, the ratio of circulating neutrophils (immune cells of the innate system) to lymphocytes (immune cells of the adaptive system), as a measure of the systemic host response when evaluating the association between inflammation and cancer outcomes.
The prognostic role of NLR has been evaluated in numerous epidemiologic studies of various cancer types. Higher NLR has been found to be consistently associated with more advanced stage and more aggressive tumour behaviour (Guthrie et al, 2013; Templeton et al, 2014). However, data regarding the association of NLR and prognosis for UCB after RC are still scarce. To date, only three small studies have been published in this population (Gondo et al, 2012; Demirtas et al, 2013; Krane et al, 2013). Gondo et al (2012) were the first to describe an association between higher NLR (>2.5) and CSS in a cohort of 189 patients undergoing RC. Demirtas et al (2013) (201 patients) reported no association between NLR (>2.5) and OS, whereas Krane et al (2013) (68 patients) found that an elevated NLR (>2.5) was an independent predictor of extravesical disease and worse OS. In the latter study, however, 10 patients received NAC and it is unclear how their calculated NLR based on immediate preoperative blood work may have been affected.
To the best of our knowledge, our study has the largest sample size investigating the independent prognostic ability of NLR in patients undergoing RC for UCB. It is the first of its kind to show that pre-treatment NLR is an independent prognostic factor for RFS, CSS and OS. Among patients receiving NAC, we used CBCs collected before the initiation of chemotherapy to eliminate this potential confounder. In addition, a sensitivity analysis excluding patients receiving NAC was performed to confirm robustness of the findings.
There is heterogeneity in reported thresholds used to define an elevated NLR in the literature (range 2–7.7; Templeton et al, 2014). This may reflect variations in the host response for different disease sites and stages, or may reflect the different approaches used when determining cutoff values. Not all studies used an accepted method for cutoff point determination, and in some instances the rationale for the cutoff point decision was not described (Templeton et al, 2014). All three previously mentioned studies in the RC population used an optimal NLR cutoff point of 2.5 (Gondo et al, 2012; Demirtas et al, 2013; Krane et al, 2013). Gondo et al (2012) used the cutoff point that generated the lowest P-value in Kaplan–Meier analyses. It is unclear, however, whether this cutoff point was associated with optimal sensitivity and specificity for adverse oncologic outcomes in their study population. One of the other studies chose 2.5 as their cutoff point for consistency with Gondo et al (2012) (Krane et al, 2013), whereas the third study did not elaborate on the rationale for their cutoff point value (Demirtas et al, 2013).
We used time-dependent ROC curves to determine the optimal cutoff point for NLR. Whereas ROC curves are conventionally used for binary outcomes to identify points of optimal sensitivity and specificity, this approach was adapted for survival analyses (Heagerty and Zheng, 2005; Lu and Liu, 2006). In our study, 3 was determined optimal cutoff point. We felt that it was important to identify an a priori optimal cutoff point both for practical purposes, and to minimise bias. Even so, there is likely a continuous association between NLR and risk of adverse oncologic outcomes. This warranted a sensitivity analysis using NLR as a log-transformed continuous variable to ensure that we did not introduce any cutoff point bias (Royston et al, 2006).
Lastly, our exploratory analyses indicate that NLR may better risk-stratify patients in the pre- and postoperative settings in order to guide treatment strategies. In patients with clinically NMI UCB, there is a high risk of under-staging and a high risk of disease progression to MI UCB (Shariat et al, 2007; Thomas et al, 2012; Chamie et al, 2013). The NLR may be helpful to identify patients most likely to benefit from early RC. Similarly, NLR may improve postoperative risk stratification to guide the use of AC. However, this was not the primary objective of this study and further work is needed to identify the clinical scenarios in which NLR may be helpful.
There are limitations to our study. First, this is a retrospective, single-institution observational study. Second, our study included patients across a long recruitment period, during which practice patterns might have changed. We addressed this by including year of cystectomy in the multivariable model. Third, we did not measure NLR after RC and therefore cannot investigate whether post-RC improvement of NLR has a predictive value. Finally, we are unable to determine whether the outcomes following NAC or AC are different among those patients with high vs low NLR because of the limited number of patients receiving NAC or AC in our cohort.
In conclusion, NLR is an inexpensive haematologic test based on commonly measured parameters that predicts RFS, CSS and OS in patients with UCB undergoing RC, independent of well-established patient-related and tumour-related predictors. Whereas our results suggest that NLR may have a role as a prognostic biomarker in the pre-RC and post-RC settings, further studies are needed to maximise the clinical utility of NLR.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Austin PC, Mamdani MM, van Walraven C, Tu JV. Quantifying the impact of survivor treatment bias in observational studies. J Eval Clin Prac. 2006;12 (6:601–612. doi: 10.1111/j.1365-2753.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- Canter D, Long C, Kutikov A, Plimack E, Saad I, Oblaczynski M, Zhu F, Viterbo R, Chen DY, Uzzo RG, Greenberg RE, Boorjian SA. Clinicopathological outcomes after radical cystectomy for clinical T2 urothelial carcinoma: further evidence to support the use of neoadjuvant chemotherapy. BJU Int. 2011;107 (1:58–62. doi: 10.1111/j.1464-410X.2010.09442.x. [DOI] [PubMed] [Google Scholar]
- Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, Konety BR, Saigal CS, Urologic Diseases in America P Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119 (17:3219–3227. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel TM, Lele SM, Michalski J, Pagliaro LC, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Richie JP, Sexton WJ, Shipley WU, Small EJ, Spiess PE, Trump DL, Wile G, Wilson TG, Dwyer M, Ho M, National Comprehensive Cancer N Bladder cancer. J Natl Compr Canc Netw. 2013;11 (4:446–475. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- Demirtas A, Sabur V, Akinsal EC, Demirci D, Ekmekcioglu O, Gulmez I, Tatlisen A. Can neutrophil-lymphocyte ratio and lymph node density be used as prognostic factors in patients undergoing radical cystectomy. TheScientificWorldJournal. 2013;2013:703579. doi: 10.1155/2013/703579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarra V, Dalpiaz O, Alrabi N, Novara G, Galfano A, Artibani W. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int. 2005;95 (6:786–790. doi: 10.1111/j.1464-410X.2005.05401.x. [DOI] [PubMed] [Google Scholar]
- Gakis G, Efstathiou J, Lerner SP, Cookson MS, Keegan KA, Guru KA, Shipley WU, Heidenreich A, Schoenberg MP, Sagaloswky AI, Soloway MS, Stenzl A, International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder C ICUD-EAU International Consultation on Bladder Cancer 2012: radical cystectomy and bladder preservation for muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63 (1:45–57. doi: 10.1016/j.eururo.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Gondo T, Nakashima J, Ohno Y, Choichiro O, Horiguchi Y, Namiki K, Yoshioka K, Ohori M, Hatano T, Tachibana M. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology. 2012;79 (5:1085–1091. doi: 10.1016/j.urology.2011.11.070. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140 (6:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Cri Rev Oncol Hematol. 2013;88 (1:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144 (5:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61 (1:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14 (15:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- Krane LS, Richards KA, Kader AK, Davis R, Balaji KC, Hemal AK. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol. 2013;27 (8:1046–1050. doi: 10.1089/end.2012.0606. [DOI] [PubMed] [Google Scholar]
- Leow JJ, Martin-Doyle W, Rajagopal PS, Patel CG, Anderson EM, Rothman AT, Cote RJ, Urun Y, Chang SL, Choueiri TK, Bellmunt J.2013Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials Eur Urole-pub ahead of print 28 August 2013doi: 10.1016/j.eururo.2013.08.033 [DOI] [PubMed]
- Lin D, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84 (408:1074–1078. [Google Scholar]
- Lipscombe LL, Chan WW, Yun L, Austin PC, Anderson GM, Rochon PA. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia. 2013;56 (3:476–483. doi: 10.1007/s00125-012-2793-9. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu C.2006. Using the Time Dependent ROC Curve to Build Better Survival Model in SAS. NorthEast SAS Users Group Conferences (NESUG); Philadelphia, 17–20 September 2006.
- Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T, Sternberg CN, Sonpavde G. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62 (3:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163 (7:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MJ, McMillan DC, Morrison DS, Fletcher CD, Horgan PG, Clarke SJ. A derived neutrophil to lymphocyte ratio predicts survival in patients with cancer. Br J Cancer. 2012;107 (4:695–699. doi: 10.1038/bjc.2012.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj GV, Karavadia S, Schlomer B, Arriaga Y, Lotan Y, Sagalowsky A, Frenkel E. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117 (2:276–282. doi: 10.1002/cncr.25429. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25 (1:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Palapattu GS, Karakiewicz PI, Rogers CG, Vazina A, Bastian PJ, Schoenberg MP, Lerner SP, Sagalowsky AI, Lotan Y. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51 (1:137–149. doi: 10.1016/j.eururo.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Sternberg CN, Bellmunt J, Sonpavde G, Siefker-Radtke AO, Stadler WM, Bajorin DF, Dreicer R, George DJ, Milowsky MI, Theodorescu D, Vaughn DJ, Galsky MD, Soloway MS, Quinn DI, International Consultation on Urologic Disease-European Association of Urology Consultation on Bladder C ICUD-EAU International Consultation on Bladder Cancer 2012: chemotherapy for urothelial carcinoma-neoadjuvant and adjuvant settings. Eur Urol. 2013;63 (1:58–66. doi: 10.1016/j.eururo.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106 (6:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- Thomas F, Rosario DJ, Rubin N, Goepel JR, Abbod MF, Catto JW. The long-term outcome of treated high-risk nonmuscle-invasive bladder cancer: time to change treatment paradigm. Cancer. 2012;118 (22:5525–5534. doi: 10.1002/cncr.27587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.