Abstract
Background:
The addition of carbogen and nicotinamide (CON) to radiotherapy (RT) improves overall survival in invasive bladder cancer. We explored whether expression of the hypoxia marker hypoxia-inducible factor-1α (HIF-1α) alone or in combination with other markers predicted benefit from CON.
Methods:
A retrospective study was carried out using material from patients with high-grade invasive bladder carcinoma enrolled in the BCON phase III trial of RT alone or with CON (RT+CON). HIF-1α expression was studied in 137 tumours using tissue microarrays and immunohistochemistry. Data were available from other studies for carbonic anhydrase IX and glucose transporter 1 protein and gene expression and tumour necrosis.
Results:
Patients with high HIF-1α expression had improved 5-year local relapse-free survival with RT+CON (47%) compared with RT alone (21% hazard ratio (HR) 0.48, 95% CI 0.26–0.8, P=0.02), no benefit was seen with low HIF-1α expression (HR 0.81, 95% CI 0.43–1.50, P=0.5). Combinations of markers including necrosis also predicted benefit but did not improve on prediction using necrosis alone.
Conclusions:
HIF-1α may be used to predict benefit from CON in patients with bladder cancer but does not improve on use of necrosis.
Keywords: bladder, hypoxia, HIF-1α, CAIX, GLUT1, biomarkers, BCON
Bladder cancer is common, with over 10 000 new diagnoses in the United Kingdom in 2010 (Kreimer et al, 2005; CRUK, 2013). Conventional treatment for muscle-invasive disease involves radiotherapy (RT) or radical cystectomy, which have similar survival rates (Dunst et al, 2001). Five-year overall survival (OS) is only around 58% for men and 50% for women (Klussmann et al, 2003). There is therefore a need for improved treatment strategies. The BCON (bladder carbogen and nicotinamide) phase III clinical trial showed that addition of carbogen and nicotinamide (CON) to RT improved OS (Hoskin et al, 2010). Adding gemcitabine (Choudhury et al, 2011) or fluorouracil and mitomycin C (James et al, 2012) to RT also improves outcomes. Biomarkers are required to predict benefit from the different approaches to individualise treatment.
Hypoxic tumours benefit most from hypoxia modification (Kaanders et al, 2002; Rischin et al, 2006; Janssens et al, 2012; Toustrup et al, 2012). As direct measurement of hypoxia is invasive and impractical, analysis of pathological features and surrogate hypoxia markers may be preferable. In support, we showed previously that tumour necrosis is an independent prognostic factor in invasive bladder cancer, and both necrosis and expression of the hypoxia-inducible enzyme carbonic anhydrase IX (CAIX) predicted benefit from hypoxia modification (Eustace et al, 2013a). In contrast, a 26-gene hypoxia signature did not predict benefit from RT+CON in BCON (Eustace et al, 2013b).
In this study, we explored hypoxia-inducible factor (HIF). HIF-1 is a transcription factor that transactivates genes implicated in cancer development, including contributors to angiogenesis and anaerobic metabolism (Shweiki et al, 1992; Elbert et al, 1996). The subunit hypoxia-inducible factor-1α (HIF-1α), which accumulates in response to cellular hypoxia, correlates with stage, grade and metastatic potential of bladder cancers (Wang et al, 1995; Deniz et al, 2010). Moreover, HIF-1α and the downstream proteins CAIX and glucose transporter 1 (GLUT1) are all independent prognostic factors in bladder cancer (Hoskin et al, 2003; Palit et al, 2005; Theodoropoulos et al, 2005; Ord et al, 2007; Chai et al, 2008; Deniz et al, 2010).
As tumour necrosis represents an attractive prognostic and predictive factor that is relatively simple to assess, it remains unclear whether the addition of hypoxia markers will improve our ability to predict benefit from CON. We hypothesised that as HIF-1α has an important role in mediating the cellular response to tumour hypoxia, it may improve patient stratification. Therefore, we performed a retrospective study to explore whether expression of combinations of necrosis, HIF-1α, CAIX and GLUT1 predict benefit from hypoxia modification. We also compared the predictive ability of quantitative CAIX or GLUT1 gene expression with immunohistochemistry. Samples were taken from patients enrolled in the BCON trial. REMARK guidelines for prognostic tumour marker studies were followed (McShane et al, 2005).
Materials and Methods
Patients and tissue samples
A retrospective cohort study was carried out in 137 patients with high-grade, non-metastatic transitional cell carcinoma of the bladder. Patients participated in the BCON phase III trial and were randomised between November 2000 and April 2006. The study was approved by the local research ethics committee (LREC 09/H1013/24) and informed consent for sample collection and analysis was obtained.
Trial protocol and sample acquisition were described previously (Hoskin et al, 2010; Eustace et al, 2013a). In brief, patients were randomised to RT alone or RT plus carbogen (2% CO2+98% O2) and nicotinamide (40 or 60 mg kg−1). Pre-treatment tissue samples were obtained via transurethral resection of the bladder tumour and formalin-fixed and paraffin-embedded blocks were constructed.
Immunohistochemistry
Methods for tissue microarray construction were previously described (Eustace et al, 2013a). Immunohistochemistry was carried out for CAIX and GLUT1 as per a previous protocol (Hoskin et al, 2003). HIF-1α staining was carried out using the Bond-Max Automated staining system (Leica Biosystems, Newcastle, UK). Samples were de-waxed and rehydrated, followed by antigen retrieval at pH 9.0 for 40 min at 100 °C. Endogenous peroxidase was blocked using 3% hydrogen peroxide solution. Primary antibody (mouse monoclonal HIF-1α, BD Biosciences 610959, Oxford, UK) was diluted to a 1 : 20 solution with diluent and incubated with samples for 15 min at room temperature. A negative control of IgG1 (Dako X0931, Cambridge, UK) was also used. Post-primary rabbit anti-mouse link reagent was applied (Bond Polymer Refine Detection System, Leica DS9800, Newcastle, UK), and samples were incubated for 8 min at room temperature. The anti-rabbit polymer-HRP detection reagent (Bond Polymer Refine Detection System, Leica) was then added and samples were incubated at room temperature for a further 8 min. 3,3′-diaminobenzidine tetrahydrochloride was added, and after a further 10 min of incubation samples were counterstained with haematoxylin.
Immunohistochemical analysis
Data for necrosis, CAIX and GLUT1 were available from another study (Eustace et al, 2013a). HIF-1α, GLUT1 and CAIX expression were determined using an H-score: a combination of the intensity (0–3) and percentage of cells stained, with a range of 0–300. Only nuclear expression of HIF-1α was scored. For CAIX, scoring included both nuclear and cytoplasmic staining. Supplementary Figure 1 shows examples of staining for HIF-1α, CAIX and GLUT1. For HIF-1α, cores were scored twice by the same scorer (BH) on different days. Cores (10%) were scored independently by a consultant histopathologist (HD). Scorers were blinded to clinical outcome data. Duplicate scores and independent histopathologist scores correlated well (Spearman ρ>0.91). There was no statistically significant intra-observer (P=0.47) or inter-observer (P=0.06) variability in histological scores. Where scores were discordant, the score of the consultant histopathologist was used. Median HIF-1α, CAIX and GLUT1 scores were 19 (range 0–199), 2.0 (range 0–208.4) and 106 (range 0–300), respectively. Median HIF-1α H-scores were used to provide an objective cutoff and facilitate comparison with other studies. Owing to the distribution of scores, cutoff values were 0 for CAIX and 100 for GLUT1. High CAIX (>0) was seen in 79 (58%) patients and high GLUT1 (>100) in 65 (47%) patients.
Qualitative PCR and gene analysis
Data for quantitative gene expression of CAIX (n=111) and SLC2A1 (n=147) were available from a previous study (Eustace et al, 2013b). Methods for RNA extraction, cDNA synthesis and gene quantification were previously described (Eustace et al, 2013b).
Statistical analysis
Analyses were performed using SPSS (IBM, version 12, Portsmouth, UK) and Prism (Graphpad, version 6, La Jolla, CA, USA). Five-year OS time was taken as time from randomisation to any cause of death; patients still alive were censored to date of the last follow-up or at 5 years, depending on which was earlier. Local relapse-free survival (LRFS) was taken as time to tumour recurrence in bladder, locoregional failure or death from any cause. Those alive and free of local disease were censored at their last follow-up. Patients with persistent muscle-invasive disease or with no cystoscopy post treatment had their time set to zero. Survival estimates were performed using the Kaplan–Meier analysis; data were compared using the Mantel–Cox log-rank test. Hazard ratios (HRs) and 95% confidence intervals for OS and LRFS were obtained using Cox regression analysis. Differences in treatment effect according to HIF-1α expression were addressed using stratum-specific treatment variables. Correlations were assessed using Spearman's correlation, and variability analysed using the Wilcoxon matched pairs test. All P-values were two sided and agreed statistical significance was 0.05. No corrections were made for multiple testing and P-values should be interpreted accordingly. The χ2-test with Yates correction was used to compare proportions across the levels of categorical variables.
Results
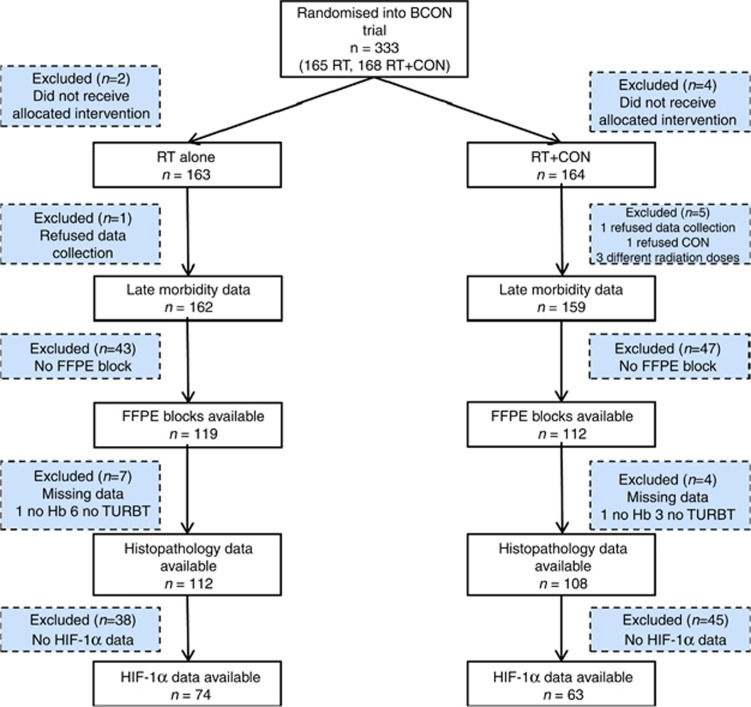
Figure 1 shows the study CONSORT diagram. Analysis of HIF-1α expression was possible in a subset of 137 patients enrolled in the BCON trial. HIF-1α data were not obtained for some patients because of poor stain uptake or TMA degradation. Data for CAIX and GLUT1 expression were available for 138 and 127 patients, respectively. Table 1 shows the distribution of clinicopathological parameters by HIF-1α expression. Patients with high tumour expression of HIF-1α also had high expression of CAIX (P=0.004) and GLUT1 (P=0.006), and tended to have more necrosis (P=0.07).
Figure 1.
Study CONSORT diagram. Data for HIF-1α expression were available for 137 patients enrolled in the BCON trial.
Table 1. Distribution of clinicopathological features by HIF-1α expression.
| Variable | HIF-1α<19 | HIF-1α⩾19 | P |
|---|---|---|---|
|
Gender | |||
| Male | 54 (47) | 60 (53) | 0.18 |
| Female |
15 (65) |
8 (35) |
|
|
Age (years) | |||
| <75 | 38 (58) | 27 (42) | 0.1 |
| ⩾75 |
31 (43) |
41 (57) |
|
|
Stage | |||
| 1 | 2 (40) | 3 (60) | 0.91 |
| 2 | 51 (50) | 50 (50) | |
| 3 | 14 (54) | 12 (46) | |
| 4a |
2 (40) |
3 (60) |
|
|
TURBT | |||
| Complete | 24 (46) | 28 (54) | 0.31 |
| Partial | 22 (47) | 25 (53) | |
| Biopsy |
21 (62) |
13 (38) |
|
|
Necrosis | |||
| Absent | 38 (59) | 26 (41) | 0.07 |
| Present |
31 (42) |
42 (58) |
|
|
Growth pattern | |||
| Papillary | 7 (50) | 7 (50) | 0.4 |
| Solid | 37 (56) | 29 (44) | |
| Both |
25 (44) |
32 (56) |
|
|
CIS | |||
| Absent | 45 (46) | 52 (54) | 0.21 |
| Present |
24 (60) |
16 (40) |
|
|
Hb (g dl−1) | |||
| <14 | 43 (57) | 33 (63) | 0.15 |
| ⩾14 |
26 (43) |
35 (57) |
|
|
CAIX | |||
| 0 | 34 (65) | 18 (35) | 0.004 |
| >0 |
30 (38) |
49 (62) |
|
|
GLUT1 | |||
| <100 | 36 (60) | 24 (40) | 0.006 |
| ⩾100 | 22 (34) | 43 (66) | |
Abbreviations: CAIX=carbonic anhydrase IX; CIS=carcinoma in situ; Hb=haemoglobin; HIF-1α=hypoxia-inducible factor-1α; TURBT=transurethral resection of bladder tumour.
Analyses for OS and LRFS were performed, but owing to similarity in results, data for LRFS only are presented. Univariate and multivariate prognostic analyses for patients receiving the standard treatment of RT alone showed that neither HIF-1 α (n=74), CAIX (n=73) nor GLUT1 (n=70) were prognostic in patients receiving RT alone (P>0.29). Age (P=0.04) and necrosis (P=0.01) were the only prognostic factors in the univariate analysis, but neither was significant in multivariate analysis.
Multivariate analyses were performed on a smaller cohort of 133 patients, as those with missing data for any clinicopathological or immunohistochemical variable were excluded. Forward stepwise analysis including both treatment arms stratified by HIF-1α expression showed that age (1.65, 95% CI 1.06–2.57, P=0.03) and treatment in the presence of high HIF-1α (HR 0.49, 95% CI 0.27–0.90, P=0.02) were statistically significant prognostic factors (Table 2). Treatment was not a significant prognostic factor in those with low HIF-1α expression (P=0.55).
Table 2. Multivariate forward stepwise analysis stratified by HIF-1α expression.
| Step | Variable | n | LRFS HR | 95% CI | P | OS HR | 95% CI | P |
|---|---|---|---|---|---|---|---|---|
| 1 |
HIF-1α: treatment |
|
|
|
|
|
|
|
| |
RT alone or HIF-1α<19+CON |
97 |
|
|
|
|
|
|
| |
HIF-1α>19+CON |
36 |
0.49 |
0.27–0.90 |
0.02 |
0.49 |
0.27–0.91 |
0.02 |
| 2 |
Age |
|
|
|
|
|
|
|
| |
<75 |
62 |
|
|
|
|
|
|
| ⩾75 | 71 | 1.65 | 1.06–2.57 | 0.03 | 1.64 | 1.06–2.56 | 0.03 |
Abbreviations: CI=confidence interval; HIF-1α=hypoxia-inducible factor-1α; HR=hazard ratio; LRFS=local relapse-free survival; OS=overall survival.
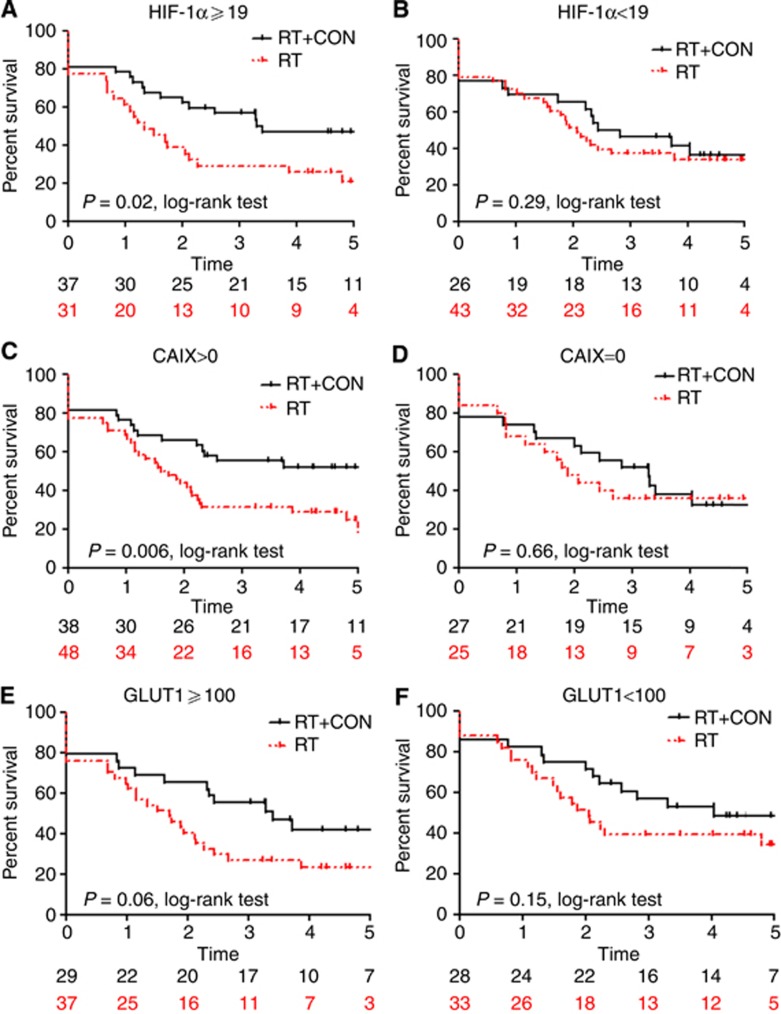
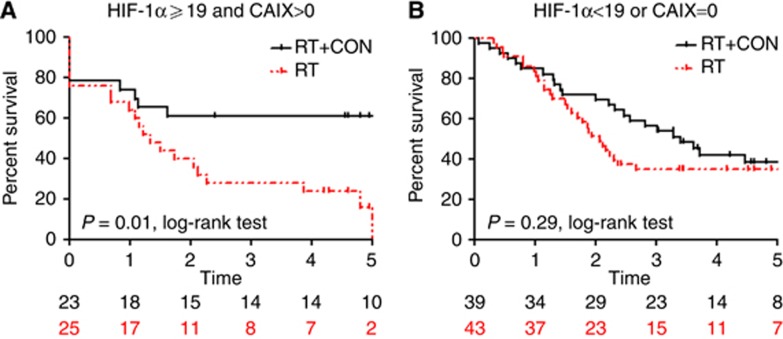
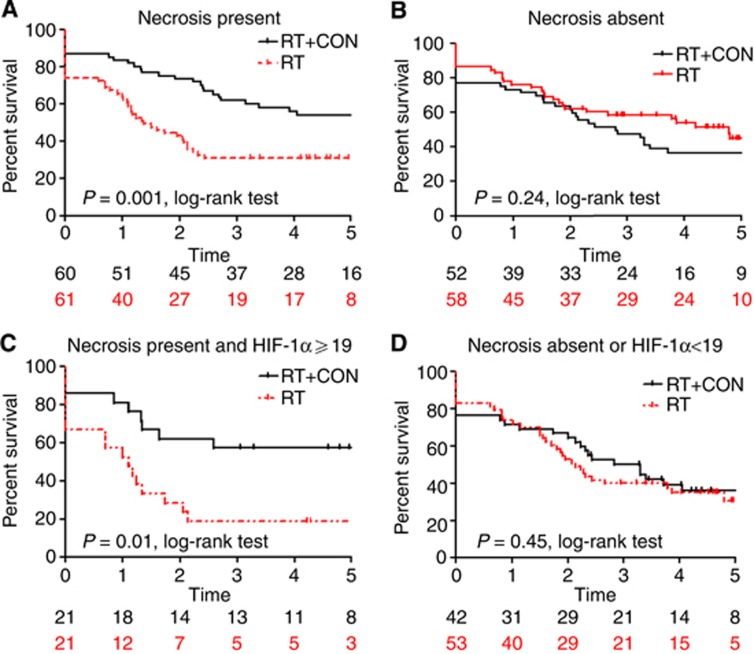
Kaplan–Meier survival analyses are presented in Figures 2, 3, 4. Hazard ratios for RT+CON compared with RT alone for patients with high or low HIF-1α, CAIX and GLUT1 expression are presented in Table 3. Also shown are HRs for combinations of these markers and combinations with necrosis.
Figure 2.
Kaplan–Meier survival plots for local relapse-free survival after radiotherapy alone or with carbogen and nicotinamide and stratified according to (A) HIF-1α score ⩾19 or (B) <19, (C) CAIX score >0 or (D)=0, or (E) GLUT1 ⩾100 or (F) <100. Log-rank P-values and number of patients at risk in each yearly interval are shown.
Figure 3.
Kaplan–Meier survival plots for local relapse-free survival after radiotherapy alone or with carbogen and nicotinamide and stratified according to (A) HIF-1α score ⩾19 and CAIX score >0 or (B) <19 or=0. Log-rank P-values and number of patients at risk in each yearly interval are also shown.
Figure 4.
Kaplan–Meier survival plots for local relapse-free survival after radiotherapy alone or with carbogen and nicotinamide and stratified according to (A) necrosis present, (B) necrosis absent, (C) necrosis present and HIF-1α >19 and (D) necrosis absent or HIF-1α<19. Log-rank P-values and number of patients at risk in each yearly interval are also shown.
Table 3. Hazard ratios for 5-year local recurrence-free survival after radiotherapy plus carbogen and nicotinamide compared with radiotherapy alone.
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
|
HIF-1α ⩾19 |
0.48 |
0.26–0.80 |
0.02 |
| HIF-1α <19 |
0.81 |
0.43−1.50 |
0.5 |
|
CAIX >0 |
0.47 |
0.28–0.81 |
0.006 |
| CAIX=0 |
0.81 |
0.43−1.50 |
0.5 |
| GLUT1 ⩾100 |
0.56 |
0.31−1.03 |
0.06 |
| GLUT <100 |
0.62 |
0.32−1.20 |
0.15 |
|
HIF-1α ⩾19+CAIX >0 |
0.38 |
0.18–0.79 |
0.01 |
| HIF-1α <19 or CAIX=0 |
0.74 |
0.43−1.28 |
0.29 |
|
CAIX >0+GLUT1 ⩾0 |
0.47 |
0.23–0.95 |
0.03 |
| CAIX=0 or GLUT1 <100 |
0.64 |
0.35−1.15 |
0.13 |
| HIF-1α ⩾19+GLUT1 ⩾100 |
0.49 |
0.23−1.05 |
0.06 |
| HIF-1α <19 or CAIX=0 |
0.69 |
0.40−1.21 |
0.2 |
|
Necrosis present |
0.46 |
0.29–0.73 |
0.001 |
| Necrosis absent |
1.37 |
0.81−2.30 |
0.24 |
|
Necrosis present+HIF-1α ⩾19 |
0.36 |
0.16–0.79 |
0.01 |
| Necrosis absent or HIF-1α <19 |
0.82 |
0.49−1.38 |
0.45 |
|
Necrosis present+CAIX >0 |
0.47 |
0.25–0.89 |
0.02 |
| Necrosis absent or CAIX=0 |
0.77 |
0.43−1.38 |
0.38 |
|
Necrosis present+GLUT1 ⩾100 |
0.37 |
0.17–0.80 |
0.01 |
| Necrosis absent+GLUT1 <100 | 0.89 | 0.50−1.58 | 0.69 |
Abbreviation: CAIX=carbonic anhydrase IX; CI=confidence interval; GLUT1=glucose transporter 1; HIF-1α=hypoxia-inducible factor-1α; HR=hazard ratio.
Hazard ratios for necrosis were assessed in the 137 patients with available HIF-1α data. Statistically significant differences are highlighted in bold.
Five-year LRFS was 46% in patients receiving RT+CON and 38% in those receiving RT alone. In patients with high HIF-1α expression, there was a statistically significant improvement in 5-year LRFS in those receiving RT+CON (47%) compared with RT alone (21%) (P=0.02). Similarly, in those with high CAIX expression, 5-year LRFS was improved with RT+CON (52%) vs RT alone (25%) (P=0.006). Patients with combinations of high HIF-1α and CAIX expression or high CAIX and GLUT1 expression had improved 5-year LRFS with RT+CON (61% and 47%, respectively) compared with RT alone (16% and 18%, respectively, P=0.01 and 0.03). Patients with necrosis plus high HIF-1α, CAIX or GLUT1 expression all had significant improvements in LRFS with RT+CON (57%, 54% and 66%, respectively) vs RT alone (19%, 28% and 25%, respectively, P=0.01, 0.02 and 0.01).
There was no improvement in LRFS in patients with high expression of the CAIX gene receiving RT+CON compared with RT (HR 0.92, 95% CI 0.49–1.75, P=0.81). Similarly, no benefit from CON was seen in those with high GLUT1 gene expression (HR 0.66, 95% CI 0.38–1.14, P=0.14). There was no correlation between CAIX gene and protein expression (r=−0.02) and only weak correlation between GLUT1 gene and protein expression (r=0.39).
Discussion
Hypoxia is of critical importance in cancer treatment. It contributes to tumour progression, invasiveness, metastasis and resistance to both chemotherapy and RT (Gray et al, 1953; Deschner and Gray, 1959; Brown, 2000; Harris, 2002). In bladder cancer, there is evidence that hypoxia modification improves locoregional control after RT (Overgaard and Horsman, 1996) and the BCON trial showed significant improvements in OS, risk of death and local relapse for patients receiving RT+CON (Hoskin et al, 2010). Furthermore, significant improvements in 5-year regional control with RT+CON were observed in patients with hypoxic laryngeal cancers; those with well-oxygenated tumours received no benefit (Janssens et al, 2012). Our findings support the notion that patients without evidence of tumour hypoxia do not receive benefit from hypoxia modification. For such patients, alternative methods of radiosensitisation such as concurrent gemcitabine (Choudhury et al, 2011) or fluorouracil and mitomycin C (James et al, 2012) should be considered. There is a clear need to stratify patients according to the presence of tumour hypoxia. Future work is planned to investigate the relationship between necrosis and outcomes in patients enrolled in the BC2001 trial that randomised to RT alone or with 5-fluorouracil and mitomycin. The latter study will investigate whether patients with necrosis in their tumours benefit from the addition of fluorouracil and mitomycin C to RT.
Direct measurement of hypoxia via Eppendorf electrodes is difficult in bladder cancer owing to tumour inaccessibility, and while pimonidazole binding is a widely accepted surrogate it is invasive and impractical. Our previous work showed that tumour necrosis is an attractive and easily assessable factor that may be used to predict benefit from hypoxia modification in bladder cancer (Eustace et al, 2013a). Coagulative tumour necrosis, the subtype studied here is thought to be specific to tumour hypoxia. In addition, analysis of hypoxia-associated proteins is attractive because of the simplicity of obtaining paraffin-embedded material. Before HIF-1α, CAIX or GLUT1 can be used clinically they must be validated as intrinsic markers of tumour hypoxia. In support, their expression correlates with pO2 and co-localises with pimonidazole in cervical cancer (Airley 2001, Haugland 2002, Jankovic 2006 and Dellas 2008). There is also significant co-localisation of CAIX and GLUT with pimonidazole in bladder cancer Hoskin et al, 2003. Although there are no current data correlating HIF-1α expression with pimonidazole binding in bladder cancer, our work did suggest that patients with high HIF-1α expression also tended to have higher expression of CAIX and GLUT1.
Analysis of a larger cohort of the BCON patients showed tumour necrosis is a prognostic factor in bladder cancer (Eustace et al, 2013a). The loss of significance in multivariate analysis in the current analysis is due to reduced patient numbers owing to adding another variable. Although our results suggest that HIF-1α, GLUT1 and CAIX are not prognostic, this is not consistent with other studies and may be due to differences in analytical techniques, sample heterogeneity, the limitations of TMA analysis or most likely the small number of patients in the RT only arm (Hoskin et al, 2003; Theodoropoulos et al, 2005).
Tumour necrosis was strongly predictive of benefit from CON and is clinically very appealing owing to its simplicity and wide availability. HIF-1α is a hypoxia-inducible marker, which we show for the first time predicts benefit from CON. Kaplan–Meier analyses showed that CAIX was also predictive in the cohort studied here, as we reported previously with a larger group (Eustace et al, 2013a). The findings that combined expression of HIF-1 or GLUT1 with CAIX retained predictive ability supports the notion that tumour hypoxia may be best assessed using multiple markers. Furthermore, analysis of necrosis in combination with HIF-1α, CAIX or GLUT1 may be a viable option, as this improved the predictive value of HIF-1α and CAIX. However, it is important to note that combinations of hypoxia-specific markers alone or with necrosis did not improve upon the prognostic or predictive ability of necrosis alone. A limitation of our study was the lack of a validation cohort to confirm our findings, but unfortunately there is no other trial that randomised patients to RT alone or with CON.
Previous work established a hypoxic gene signature that may predict response to hypoxia-modifying therapy (Eustace et al, 2013b). The signature predicted response to CON in laryngeal cancers but not in bladder tumours, which may reflect highly conserved genetic alterations specific to bladder cancer (Eustace et al, 2013b). Our findings that CAIX gene expression did not correlate with protein expression may offer some explanation as to why the signature was not predictive. The finding differs from our observations in head and neck cancers, where CAIX protein and gene expression correlated (Winter et al, 2007). At present immunohistochemical analysis of protein expression seems more favourable than gene expression analysis in bladder cancer, but neither of them seem to improve upon the utility, simplicity and availability of tumour necrosis.
BCON is currently a routine treatment for bladder cancer in some UK centres. Our data suggest that BCON is more effective in those with evidence of necrosis or hypoxia marker expression. Although HIF-1α could be used to predict benefit from CON, it does not improve on the simple assessment of necrosis alone.
Acknowledgments
This work was supported by, Cancer Research UK (C2094/A11365), the MRC Biomarker grant (G0801525) and Experimental Cancer Medicine Centre funding (C1467/A7286).
Author contributions
BAH carried out the HIF work, the analyses and wrote the paper; AE, AC, HRV, JJL and CMW supervised the project; HD carried out pathology assessments; RS oversaw or carried out statistical analyses; all authors read the paper and provided feedback.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res. 2001;7 (4:928–934. [PubMed] [Google Scholar]
- Brown JM. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol Med Today. 2000;6 (4:157–162. doi: 10.1016/s1357-4310(00)01677-4. [DOI] [PubMed] [Google Scholar]
- Chai CY, Chen WT, Hung WC, Kang WY, Huang YC, Su YC, Yang CH. Hypoxia-inducible factor-1alpha expression correlates with focal macrophage infiltration, angiogenesis and unfavourable prognosis in urothelial carcinoma. J Clin Pathol. 2008;61 (5:658–664. doi: 10.1136/jcp.2007.050666. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Swindell R, Logue JP, Elliott PA, Livsey JE, Wise M, Symonds P, Wylie JP, Ramani V, Sangar V, Lyons J, Bottomley I, McCaul D, Clarke NW, Kiltie AE, Cowan RA. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011;29 (6:733–738. doi: 10.1200/JCO.2010.31.5721. [DOI] [PubMed] [Google Scholar]
- CRUK 2013Cancer incidence—UK statistics Cancer Research UK: London; (updated 2010; cited 9 April 2013). Available from http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/incidence/ . [Google Scholar]
- Dellas K, Bache M, Pigorsch SU, Taubert H, Kappler M, Holzapfel D, Zorn E, Holzhausen HJ, Haensgen G. Prognostic impact of HIF-1alpha expression in patients with definitive radiotherapy for cervical cancer. Strahlenther Onkol. 2008;184 (3:169–174. doi: 10.1007/s00066-008-1764-z. [DOI] [PubMed] [Google Scholar]
- Deniz H, Karakok M, Yagci F, Guldur ME. Evaluation of relationship between HIF-1alpha immunoreactivity and stage, grade, angiogenic profile and proliferative index in bladder urothelial carcinomas. Int Urol Nephrol. 2010;42 (1:103–107. doi: 10.1007/s11255-009-9590-5. [DOI] [PubMed] [Google Scholar]
- Deschner EE, Gray LH. Influence of oxygen tension on x-ray-induced chromosomal damage in Ehrlich ascites tumor cells irradiated in vitro and in vivo. Radiation Res. 1959;11 (1:115–146. [PubMed] [Google Scholar]
- Dunst J, Rodel C, Zietman A, Schrott KM, Sauer R, Shipley WU. Bladder preservation in muscle-invasive bladder cancer by conservative surgery and radiochemotherapy. Semin Surg Oncol. 2001;20 (1:24–32. doi: 10.1002/ssu.1013. [DOI] [PubMed] [Google Scholar]
- Elbert B, Gleadle J, O'Rourke JF, Bartlett SM, Poulton J, Ratcliffe PJ. Isoenzyme-specific regulation of genes involved in energy metabolism by hypoxia: similarities with the regulation of erythropoietin. J Biochem. 1996;313:809–814. doi: 10.1042/bj3130809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace A, Irlam JJ, Taylor J, Denley H, Agrawal S, Choudhury A, Ryder D, Ord JJ, Harris AL, Rojas AM, Hoskin PJ, West CM. Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol. 2013;108:40–47. doi: 10.1016/j.radonc.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustace A, Mani N, Span PN, Irlam JJ, Taylor J, Betts GN, Denley H, Miller CJ, Homer JJ, Rojas AM, Hoskin PJ, Buffa FM, Harris AL, Kaanders JH, West CM. A 26-gene hypoxia signature predicts benefit from hypoxia-modifying therapy in laryngeal cancer but not bladder cancer. Clin Cancer Res. 2013;19:4879–4888. doi: 10.1158/1078-0432.CCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol. 1953;26 (312:638–648. doi: 10.1259/0007-1285-26-312-638. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2 (1:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Haugland HK, Vukovic V, Pintilie M, Fyles AW, Milosevic M, Hill RP, Hedley DW. Expression of hypoxia-inducible factor-1alpha in cervical carcinomas: correlation with tumor oxygenation. Int J Radiat Oncol Biol Phys. 2002;53 (4:854–861. doi: 10.1016/s0360-3016(02)02815-8. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol. 2010;28 (33:4912–4918. doi: 10.1200/JCO.2010.28.4950. [DOI] [PubMed] [Google Scholar]
- Hoskin PJ, Sibtain A, Daley FM, Wilson GD. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: relationship with vascularity and proliferation as predictors of outcome of ARCON. Br J Cancer. 2003;89 (7:1290–1297. doi: 10.1038/sj.bjc.6601260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, Crundwell M, Sizer B, Sreenivasan T, Hendron C, Lewis R, Waters R, Huddart RA. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366 (16:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- Jankovic B, Aquino-Parsons C, Raleigh JA, Stanbridge EJ, Durand RE, Banath JP, MacPhail SH, Olive PL. Comparison between pimonidazole binding, oxygen electrode measurements, and expression of endogenous hypoxia markers in cancer of the uterine cervix. Cytometry B Clin Cytom. 2006;70 (2:45–55. doi: 10.1002/cyto.b.20086. [DOI] [PubMed] [Google Scholar]
- Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P, Chin A, Marres HA, de Bree R, van der Kogel AJ, Hoogsteen IJ, Bussink J, Span PN, Kaanders JH. Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol. 2012;30 (15:1777–1783. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, de Wilde PC, Bussink J, Raleigh JA, van der Kogel AJ. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62 (23:7066–7074. [PubMed] [Google Scholar]
- Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, Eckel HE, Pfister HJ, Fuchs PG. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162 (3:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14 (2:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97 (16:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- Ord JJ, Agrawal S, Thamboo TP, Roberts I, Campo L, Turley H, Han C, Fawcett DW, Kulkarni RP, Cranston D, Harris AL. An investigation into the prognostic significance of necrosis and hypoxia in high grade and invasive bladder cancer. J Urol. 2007;178 (2:677–682. doi: 10.1016/j.juro.2007.03.112. [DOI] [PubMed] [Google Scholar]
- Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6 (1:10–21. doi: 10.1053/SRAO0060010. [DOI] [PubMed] [Google Scholar]
- Palit V, Phillips RM, Puri R, Shah T, Bibby MC. Expression of HIF-1alpha and Glut-1 in human bladder cancer. Oncol Rep. 2005;14 (4:909–913. doi: 10.3892/or.14.4.909. [DOI] [PubMed] [Google Scholar]
- Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, Peters LJ, Trans-Tasman Radiation Oncology Group Study 98.02 Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol. 2006;24 (13:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359 (6398:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos VE, Lazaris AC, Kastriotis I, Spiliadi C, Theodoropoulos GE, Tsoukala V, Patsouris E, Sofras F. Evaluation of hypoxia-inducible factor 1alpha overexpression as a predictor of tumour recurrence and progression in superficial urothelial bladder carcinoma. BJU Int. 2005;95 (3:425–431. doi: 10.1111/j.1464-410X.2005.05314.x. [DOI] [PubMed] [Google Scholar]
- Toustrup K, Sorensen BS, Lassen P, Wiuf C, Alsner J, Overgaard J, Danish H, Neck Cancer G. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102 (1:122–129. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92 (12:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SC, Buffa FM, Silva P, Miller C, Valentine HR, Turley H, Shah KA, Cox GJ, Corbridge RJ, Homer JJ, Musgrove B, Slevin N, Sloan P, Price P, West CM, Harris AL. Relation of a hypoxia metagene derived from head and neck cancer to prognosis of multiple cancers. Cancer Res. 2007;67 (7:3441–3449. doi: 10.1158/0008-5472.CAN-06-3322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.