Abstract
We present novel dissolving and hydrogel-forming microneedle arrays that can be applied in a one-step fashion and are intended to enhance delivery of photosensitisers and photosensitiser precursors.
Microneedles (280 μm in length) were prepared from aqueous blends of 20% w/w poly(methylvinylether/maelic acid) and either crosslinked with glycerol by esterification to form hydrogels upon skin insertion or allowed to dissolve rapidly when inserted into skin. Drug-loaded patches containing either 19 mg cm−2 of 5-aminolevulinic acid (ALA) or mesotetra (N-methyl-4-pyridyl) porphine tetra tosylate (TMP) were attached to the MN during drug delivery experiments.
Both MN types were mechanically robust, with even compression forces of 20.0 N only causing height reductions of around 14%. Application forces as low as 8.0 N per array allowed over 95% of the MN in each type of array to penetrate excised neonatal porcine skin and the MN penetrated to depths of approximately 280 μm, well beyond the stratum corneum barrier. MN significantly enhanced delivery of both ALA and TMP across dermatomed neonatal porcine skin in vitro, with the hydrogel-forming system comparable to the dissolving system for ALA delivery (approximately 3000 nmol cm−2 over 6 hours), but superior for delivery of the much larger TMP molecule (approximately 14 nmol cm−2 over 24 hours, compared to 0.15 nmol cm−2).
This technology has the potential to improve convenience and efficacy of photodynamic therapy of neoplastic skin lesions. Consequently, we are currently planning animal studies, to be followed by preliminary human evaluations. Manufacturing scale-up is currently ongoing, with a view to patient benefit within 3-5 years.
Keywords: Microneedle, hydrogel, photodynamic, porphyrin, aminolevulinic acid
INTRODUCTION
To date, the porphyrin precursor 5-aminolevulinic acid (ALA) and its methyl ester (MAL) have been the most widely used agents in topical photodynamic therapy (PDT) (1,2). However, owing to the poor penetration of ALA and MAL into skin, ALA- and MAL-based PDT is inappropriate for difficult-to-treat deep skin neoplasias, such as nodular basal cell carcinoma due to poor drug penetration from topically-applied creams and solutions (1,2). An alternative strategy is to use pre-formed photosensitisers, which can be activated at longer wavelengths, facilitating enhanced light penetration into skin. This means irradiation time can be reduced relative to red light illumination ofALA/MAL-induced protoporphyrin IX.However, owing to their relatively high molecular weights, these compounds cannot be effectively administered topically (1,2). Systemic injection of preformed photosensitisers often leads to prolonged cutaneous photosensitivity, which is undesirable (1,2).
Microneedle (MN) arrays are minimally-invasive devices that painlessly, and without causing bleeding, penetrate the skin’s stratum corneum barrier to enhance intradermal and transdermal drug delivery (3). Since we first described the ability of MN pre-treatment of skin to enhance intradermal delivery of both ALA and hydrophilic preformed photosensitisers (4-6), numerous clinical studies have demonstrated the enhanced efficacy of PDT following lesion pre-treatment with MN (7-11). The MN allow hyperkeratotic debris overlying deeper skin neoplasias to be effectively bypassed, thus reducing, or eliminating, the need for curettage. This improves efficiency and reduces patient distress, while enhancing efficacy. However, pre-treatment of skin using silicon or metal MN can in itself be problematic, since silicon has dubious biocompatibility and broken silicon or metal microneedles could cause skin problems (3). In addition, the two-step procedure, whereby skin is treated with MN and then a drug-loaded formulation is applied, can be inconvenient. Since MN leave holes in the skin that often cannot be seen by the naked eye, it can be difficult to determine whether the complete area to be treated with PDT has been punctured. Finally, neither silicon nor metal MN are self-disabling and, hence, constitute a potential sharps hazard post-removal. Accordingly, suitable disposal precautions must be taken (3).
We have recently described (12-16) MN prepared from polymeric materials that either dissolve rapidly in skin interstitial fluid following insertion to deliver a drug cargo, or swell in skin, forming continuous, unblockable conduits between drug reservoirs and the viable skin (Figure 1). Upon removal from skin, both systems are found to be self-disabling, in that the dissolving MN are no longer intact and the hydrogel-forming MN are soft and cannot be reinserted. Accordingly, disposal is not problematic. Since these novel MN systems contain the drug to be delivered, a one-step skin puncture/drug delivery process is possible. We have already demonstrated successful delivery of a hydrophobic preformed photosensitiser encapsulated in nanoparticles (17) using polymeric MN (18). In the present study, we investigate, for the first time, the potential of dissolving and hydrogel-forming MN to enhance delivery of both ALA and a hydrophilic preformed photosensitiser. Meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate was chosen as the model here, since we have previously found it to be useful in in vitro PDT (19-22) and the need for good water solubility for efficient delivery using MN, which create aqueous pores in skin.
Figure 1.
Schematic illustration of the mechanism of drug delivery from dissolving and hydrogel-forming microneedle arrays with attached bioadhesive patch-type drug reservoirs.
Materials and Methods
Chemicals
5-aminolevulinic acid, hydrochloride salt (ALA) was obtained from Crawford Pharmaceuticals, Milton Keynes, UK. Meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate (TMP) was obtained from Frontier Scientific Europe Ltd, Carnforth, Lancashire, UK. Acetylacetone, formaldehyde 37% w/w solution in water, thionyl chloride, tripropyleneglycol methyl ether (Dowanol™ TPM), and sodium dodecyl sulphate were obtained from Sigma Aldrich, Dorset, UK. Gantrez® AN-139, a copolymer of methy vinyl ether and maleic anhydride (PMVE/MAH) was provided by Ashland, Kidderminster, UK. All other chemicals used were of analytical reagent grade.
Fabrication of master template silicon MN arrays
Silicon MN arrays to be used as master templates in micromoulding of hydrogel-forming and dissolving MN arrays were microfabricated using a previously-reported approach in 7x7 arrays, 5.6 mm in length by 4.8 mm in breadth. (4,5,12). The aspect ratio of these MNs was 3:2 (height:base diameter) with a height of 280 Nm, a diameter of 240 Nm at the base and an interspacing of 750 Nm between rows of MNs, as shown in Figure 2.
Figure 2.
Diagrammatic representation of the steps involved in the preparation of silicone moulds. A Silicon MN array is attached to a Teflon® slab, which is in turn fixed to an aluminium stub. Silicone is then added to the mould and centrifuged at 3500 rpm for 15 minutes. After curing overnight, the contents of the aluminium container are removed pressing a metal rod against the aluminium stub. The silicone mould is carefully peeled away from the silicon master array. Aqueous gel is transferred to the silicone mould. The mould is centrifuged at 3500 rpm for 15 minutes. Upon drying, the silicone mould is carefully peeled away from the formed polymeric MN array.
Fabrication of hydrogel-forming and dissolving MN arrays
Using the above silicon MN arrays as master templates, silicone elastomer micromoulds were prepared (Figure 2), as described previously (4,5,12) from LSR9-9508-30 (Polymer Systems Technology, High Wycombe, England). Dissolving polymeric MN were prepared from aqueous blends containing 20% w/w poly(methylvinylether/maleic acid) (PMVE/MA), formed by aqueous hydrolysis of the anhydride, as described previously (23). Using a graduated pastette, the silicone mould was filled with polymer solution (0.5 g). The mould was allowed to dry for 48 hours under ambient conditions. Hydrogel-forming MN were prepared in a similar fashion from aqueous blends containing 20% w/w PMVE/MA and 6% w/w glycerol (PMVE/MA-G MNs). After drying, the mould was placed in an oven for 48 hours at 60°C to crosslink the copolymer by ester formation (15,16). The “side-walls” formed by polymer adherence to the inside of the mould during drying of the MN arrays were carefully removed using a hot scalpel blade, as described previously (13,15,16), to give a flat baseplate.
Mechanical testing of MN arrays
Hydrogel-forming MN were subjected to standard mechanical tests using a TA-XT2 Texture Analyser (Stable Microsystems, Haslemere, UK) in compression mode, as described previously (13). Briefly, MN arrays were visualised before testing using a light microscope (GXMGE-5 digital microscope, Laboratory Analysis Ltd, Devon, UK). MN arrays were then carefully placed on the flat stainless steel baseplate of the Texture Analyser with the needles pointing upwards. A flat-faced probe with a diameter of 11.0 mm was lowered at a speed of 0.5 mm s−1. Upon contact with the MN array, the probe continued to travel at a speed of 0.5 mm s−1 until the required force had been exerted. Once the target force was reached, the probe was moved upwards at a speed of 0.5 mm s−1. MN arrays were then viewed again under the light microscope.
Skin insertion studies
Neonatal porcine skin, a good model for human skin (24,25), was obtained from stillborn piglets and immediately (<24 hours after birth) excised, trimmed to a thickness of 700 Sm using a dermatome (Integra Life Sciences™, Padgett Instruments, NJ, USA) and frozen in liquid nitrogen vapour, as previously described (13,15,16). Skin was then stored in aluminium foil at −20°C for no more than 7 days prior to use.
Skin was mounted on the baseplate of the Texture Analyser using cyanoacrylate adhesive (Loctite Ltd, Dublin, Ireland) while the MN were this time attached to the probe using double-sided tape (3M, Carrickmines, Ireland). The probe then moved downwards as described above until the required force had been exerted. Once the target force was reached, the probe was moved upwards at a speed of 0.5 mm s−1. The number of MN in an array that had penetrated the skin’s stratum corneum barrier was counted following visualisation of the pores formed in skin using optical coherence tomography (OCT) (EX1301 OCT microscope, Michelson Diagnostics, Kent, UK), as described previously (26). OCT also allowed measurement of the depth of MN insertion for each application force, since the MN are transparent and accordingly can be left in place during OCT studies to mimic their intended use. OCT data files were exported to Image J® (National Institutes of Health, Bethesda, MD, USA) for measurement of insertion depth and false colours were applied using Ability Photopaint® (Ability Software International, Horley, UK) for presentation purposes, with the MN outlined in white for clarity and the stratum corneum outlined in yellow.
Preparation of ALA and TMP-loaded bioadhesive patches
Bioadhesive path-type drug reservoirs were prepared using the casting method described previously (15,23). ALA films were cast from aqueous blends containing 20% w/w PMVE/MA and 10% w/w tripropylene glycol methyl ether (TPM). TMP films were prepared from blends containing 10% w/w PMVE/MA, 10% w/w TPM and 5% w/w NaCl. The NaCl was added to competitively prevent binding of the cationic TMP to any remaining ionisable acid groups in the PMVE/MA during release by forming the sodium salt of the polyacid. To the appropriate mass of blend, the required amount of ALA or TMP was added to give films with a constant drug loading of 19 mg cm−2. All films were placed in heat-sealed poly(ester) foils and stored at −20°C until required.
Evaluation of ALA and TMP skin penetration
MN-mediated of ALA across dermatomed (350 μm approx) skin was investigated in vitro using the modified Franz cell model previously described (13,15,16). Bioadhesive patches were attached to the upper side of the MN arrays using 10 μl of water to initiate adhesion. Then, using finger pressure, the MN array/patch combination was pressed into the centre of the skin. With the patches in place, the punctured skins were carefully mounted onto the Franz cell model, ensuring that the punctured area was directly over the orifice of the receptor compartment. Non-adhesive putty (BlueTac®) was used to keep the MN in place. Finally, laboratory film (Parafilm®, Camlab, Cambridge, UK) was then used to provide occlusive conditions in the donor compartment. Bioadhesive patches were applied to the skin with no MN as controls. In these control experiments, the drug-loaded bioadhesive patches were applied to skin by gentle finger pressure and using 10 μl of water to ensure bioadhesive attachment to the stratum corneum.
Using a long needle, samples (0.10 ml) were removed from the receptor compartment at defined time intervals and ALA analysis was performed by HPLC following derivatisation with acetylacetone and formaldehyde, as described previously (27). The penetration of TMP across skin was determined fluorimetrically, as described previously (5). MN arrays were examined post-removal using an ECLIPSE TE300 inverted microscope with a DXM1200 digital still camera (Nikon, UK Limited). Fluorescence images were captured at ×100 magnification after 333 ms exposure through the Cy5 filter block (excitation wavelength of 620/60 nm, emission wavelength 700/75 nm).
Statistical analysis
Where appropriate, statistical analyses of the results were performed with a one-way analysis of variance (ANOVA), where p < 0.05 was taken to represent a statistically significant difference. When there was a statistically significant difference, post-hoc Tukey’s HSD multiple comparison test was then performed. Statistical Package for the Social Sciences, SPSS 17.0 version 2.0 (SPSS, Inc., Chicago, IL), was used for all analyses.
RESULTS AND DISCUSSION
As Figure 3A shows, increasing the force of compression increased the percentage reduction in MN height, with no significant differences noted between the two types of array. Importantly, neither MN type shattered during compression, even when forces of 20.0 N were employed. Instead, the MN were distorted in shape at the tips, yet remained upright. OCT analysis showed that, as force of application was increased, there was a progressive increase in the number of MN penetrating the stratum corneum barrier, such that at an application force of 6.0 N, more than 90% of the MN in an array had penetrate the skin’s outermost barrier (Figure 3B). Again, no statistically-significant differences were noted between the two array types, regardless of the application force employed. Figure 3C shows that, as application force was increased, insertion depth increased progressively, with an 8.0 N force producing insertion depths of almost 80% of the MN shaft length (approximately 220 μm), which is in line with our previous work with other MN designs (13,16,26).
Figure 3.
Influence of compression force on reduction of MN height for dissolving and hydrogel-forming MN arrays (A). Optical coherence tomographic studies showing influence of application force on the percentage of dissolving and hydrogel-forming MN in an array penetrating excised full thickness neonatal porcine skin in vitro (B) and on the penetration depth of each MN type in the same skin model (C).
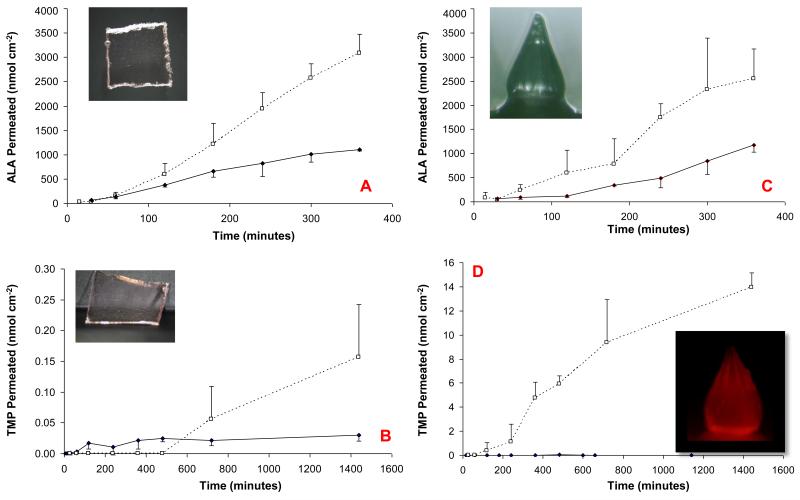
The permeation profile of ALA from the bioadhesive patch across excised dermatomed neonatal porcine skin is shown in Figure 4A. It is obvious that an appreciable amount of ALA does penetrate across intact skin. Puncturing the skin with dissolving PMVE/MA MNs resulted in a significant increase in the amount of ALA in the receiver compartment after 6 hours (p < 0.05). Figure 4B shows that intact skin was almost impermeable to TMP penetration over the duration of the experiment. MN puncture resulted in a significant increase in the level of TMP being determined in the receiver compartment. However, it should be noted that the molar amounts of TMP crossing the punctured skin were much lower that the amounts seen for ALA. In the cases of both drugs, all MN had dissolved when the patches were removed from skin at the end of the release experiments.
Figure 4.
In vitro skin penetration studies.
ALA permeation from a bioadhesive patch across intact (—♦—) and MN-punctured dermatomed neonatal porcine skin (--□--). The formulation was tailored to contain 19 mg ALA cm−2. Dissolving PMVE/MA MN were inserted using finger pressure and left in situ (A).
TMP permeation from a bioadhesive patch across intact (—♦—) and MN-punctured dermatomed neonatal porcine skin (--□--). The formulation was tailored to contain 19 mg TMP cm−2. Dissolving PMVE/MA MNs were inserted using finger pressure and left in situ (B).
ALA permeation from a bioadhesive patch across intact (—♦—) and MN-punctured dermatomed neonatal porcine skin (--□--). The formulation was tailored to contain 19 mg ALA cm−2. Hydrogel-forming MN were inserted using finger pressure and left in situ (C).
TMP permeation from a bioadhesive patch across intact (—♦—) and MN-punctured dermatomed neonatal porcine skin (--□--). The formulation was tailored to contain 19 mg TMP cm−2 and contained 5% w/w NaCl. Hydrogel-forming MN were inserted using finger pressure and left in situ (D).
All results represent means ± S.D., n=3 in each case. The inset images show the MN device upon removal from skin at the end of the experiment. It is clear that the hydrogel-forming MN are removed from skin completely intact and that the red-coloured TMP diffuses through the MN matrices. In contrast, the dissolving MN had completely dissolved upon removal from skin.
The permeation profile of ALA across excised dermatomed neonatal porcine skin is illustrated in Figure 4C. Puncturing the skin with hydrogel-forming MN was shown to result in approximately a 2-fold increase in ALA delivery. Statistical analysis revealed that this increase in ALA delivery was significant (p < 0.05). Figure 4D illustrates that the amount of TMP penetrating skin are generally undetectable. However, puncture with the hydrogel-forming MN resulted in a statistically significant increase in TMP penetration (p < 0.05). It was notable that, for both drugs, the MN were appreciably swollen when removed from skin, yet remained mechanically-robust and did not break or tear during skin removal. The concentration gradient of TMP within the matrices of the swollen MN was clearly visible using light and fluorescence microscopy.
No significant differences in skin permeation were seen between MN types for ALA. However, the hydrogel-forming MN delivered considerably more TMP across then skin that their dissolving equivalents. This was unsurprising, given that TMP has a much higher molecular weight than ALA (approx. 1400 vs approx. 167) (28) and that the dissolving MN are likely to form a thick gel in the puncture holes, which may impair transport of the larger molecule. Indeed, we have seen a similar phenomenon with peptide delivery from dissolving MN before (14). In contrast, hydrogels possess very open structures comprised of up to 99% water (29), meaning diffusion of macromolecules through swollen MN is not typically problematic. In fact, we have successfully delivered even large proteins through such MN, both in vitro and in vivo (15,16).
In the present study, we have shown that the photosensitiser precursor ALA and the large preformed photosensitiser TMP have their delivery across skin significantly enhanced by one-step application of polymeric MN arrays. TMP delivery was much more marked from the hydrogel-forming system, which offers another advantage over its dissolving counterpart in that the MN are removed intact, thus leaving no polymer behind in the skin. Deposition of an FDA-approved copolymer with a long history of safe use, such as PMVE/MA, in skin is unlikely to have a detrimental effect on patient health, given that only a small number of MN-enhanced PDT treatments are likely to be required at the same site. However, the deposited copolymer may scatter the incident light during irradiation, thus complicating the treatment or reducing its efficacy (30). Accordingly, we are currently pursuing development of the hydrogel-forming system, with PDT studies of subcutaneously-implanted animal tumours the next step, before exploratory photosensitiser accumulation studies in human volunteers, such as those described previously (6). The ultimate aim is to improve therapeutic outcomes for patients with deep skin neoplasias, such as nodular basal cell carcinomas (BCCs), through MN-mediated enhanced PDT. Delivery of preformed photosensitisers in this way will be particularly beneficial, since it is well known that regions within nodular BCCs synthesise relatively low concentrations of protoporphyrin IX following ALA/MAL delivery (1,2).Given that we have recently obtained substantial commercial and grant (31) support for scale-up manufacture of these hydrogel-forming MN, we expect the first patients to benefit within 3-5 years.
ACKNOWLEDGEMENTS
This study was supported by BBSRC grant numbers BB/FOF/287 and BB/E020534/1 and Wellcome Trust grant number WT094085MA.
REFERENCES
- 1.Darlenski R, Fluhr JW. Photodynamic therapy in dermatology: past, present, and future. J. Biomed. Opt. 2013;18:1117–1121. doi: 10.1117/1.JBO.18.6.061208. [DOI] [PubMed] [Google Scholar]
- 2.Kohl E, Karrer S. New developments in photodynamic therapy. Hautarzt. 2013;64:363–369. doi: 10.1007/s00105-012-2513-x. [DOI] [PubMed] [Google Scholar]
- 3.Donnelly RF, Thakur RRS, Morrow DIJ, Woolfson AD. Microneedle-mediated Transdermal and Intradermal Drug Delivery. Wiley-Blackwell; Oxford: 2012. [Google Scholar]
- 4.Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, Juzeniene A, Iani V, McCarthy HO, Moan J. Microneedle-mediated Intradermal Delivery of 5-Aminolevulinic acid: Potential for Enhanced Topical Photodynamic Therapy. J. Cont. Rel. 2008;129:154–162. doi: 10.1016/j.jconrel.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, Juzeniene A, Iani V, McCarthy HO, Moan J. Microneedle Arrays Permit True Intradermal Delivery of a Preformed Photosensitiser. Photochem. Photobiol. 2009;85:195–204. doi: 10.1111/j.1751-1097.2008.00417.x. [DOI] [PubMed] [Google Scholar]
- 6.Mikolajewska P, Donnelly RF, Garland MJ, Morrow DIJ, Iani V, Moan J, Juzeniene A. Microneedle pre-treatment of human skin improves 5-aminolevulininc acid (ALA) and 5-aminolevulinic acid methyl ester (MAL) induced PpIX production for topical photodynamic therapy without increase in pain or erythema. Pharm. Res. 2010;27:2213–2220. doi: 10.1007/s11095-010-0227-2. [DOI] [PubMed] [Google Scholar]
- 7.Lev-Tov H, Larsen L, Zackria R, Chahal H, Eisen D, Sivamani R. Microneedle pretreatment may reduce incubation time in photodynamic therapy J. Invest. Dermatol. 2013;133:S180–S180. [Google Scholar]
- 8.Kolde G, Rowe E, Meffert H. Effective photodynamic therapy of actinic keratoses and Bowen’s disease using microneedle perforation. Brit. J. Dermatol. 2013;168:450–452. doi: 10.1111/j.1365-2133.2012.11153.x. [DOI] [PubMed] [Google Scholar]
- 9.Kolde G, Rowe E, Meffert H. Optimization of the photodynamic Therapy by Microneedle-Perforation. J. Dtsch. Dermatol. Ges. 2011;9(Special Issue Supplement: 5):7–7. [Google Scholar]
- 10.Lee JW, Yoo KH, Kim BJ, Kim MN. Photodynamic therapy with methyl 5-aminolevulinate acid combined with microneedle treatment in patients with extensive alopecia areata. Clin. Exp. Dermatol. 2010;35:548–549. doi: 10.1111/j.1365-2230.2009.03695.x. [DOI] [PubMed] [Google Scholar]
- 11.Clementoni MT, B-Roscher M, Munavalli GS. Photodynamic Photorejuvenation of the Face With a Combination of Microneedling, Red Light, and Broadband Pulsed Light. Laser. Surg. Med. 2010;42(Special Issue):150–159. doi: 10.1002/lsm.20905. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly RF, Morrow DIJ, Thakur RRS, Migalska K, McCarron PA, O’Mahony C, Woolfson AD. Processing Difficulties and Instability of Carbohydrate Microneedle Arrays. Drug. Dev. Ind. Pharm. 2009;35:1242–1254. doi: 10.1080/03639040902882280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnelly RF, Majithiya R, Thakur RRS, Morrow DIJ, Garland MJ, Demir YK, Migalska K, Ryan E, Gillen D, Scott CJ, Woolfson AD. Design and physicochemical characterisation of optimised polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm. Res. 2011;28:41–57. doi: 10.1007/s11095-010-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migalska K, Morrow DIJ, Garland MJ, Thakur RRS, Woolfson AD, Donnelly RF. Laser-engineered dissolving microneedle arrays for transdermal macromolecular drug delivery. Pharm. Res. 2011;28:1919–1930. doi: 10.1007/s11095-011-0419-4. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly RF, Thakur RRS, Garland MJ, Migalska K, Majithiya R, McCrudden CM, Kole PL, Mahmood TMT, McCarthy HO, Woolfson AD. Hydrogel-forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012;22:4879–4890. doi: 10.1002/adfm.201200864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donnelly RF, Thakur RRS, McCrudden MTC, Zaid-Alkilani A, O’Mahony C, Armstrong K, McLoone N, Kole P, Woolfson AD. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. Int. J. Pharm. 2013;451:76–91. doi: 10.1016/j.ijpharm.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarron PA, Donnelly RF, Marouf W. Celecoxib-loaded poly(D,L-lactide-co-glycolide) nanoparticles prepared using a novel and controllable combination of diffusion and emulsification steps as part of the salting-out procedure. J. Microencapsul. 2006;23:480–498. doi: 10.1080/02652040600682390. [DOI] [PubMed] [Google Scholar]
- 18.Donnelly RF, Morrow DIJ, Fay F, Scott CJ, Abdelghany S, Thakur RRS, Garland MJ, Woolfson AD. Microneedle-mediated intradermal nanoparticle delivery: Potential for enhanced local administration of hydrophobic pre-formed photosensitisers. Photodiagn. Photodyn. 2010;7:222–231. doi: 10.1016/j.pdpdt.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly RF, McCarron PA, Cassidy CM, Elborn JS, Tunney MM. Delivery of photosensitisers and light through mucus: Investigations into the potential use of photodynamic therapy for treatment of Pseudomonas aeruginosa cystic fibrosis pulmonary infection. J. Cont. Rel. 2007;117:217–226. doi: 10.1016/j.jconrel.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Donnelly RF, Cassidy CM, Loughlin RG, Brown A, Tunney MM, Jenkins MG, McCarron PA. Delivery of Methylene Blue and meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate from crosslinked poly(vinyl alcohol) hydrogels: A potential means of photodynamic therapy of infected wounds. J. Photochem. Photobiol. B: Biol. 2009;96:223–231. doi: 10.1016/j.jphotobiol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Cassidy CM, Tunney MM, Caldwell DL, Andrews GP, Donnelly RF. Development of a colon specific photosensitiser delivery system: Potential for use in treatment of colonic infections. Photochem. Photobiol. 2011;87:867–876. doi: 10.1111/j.1751-1097.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 22.Abdelghany SM, Schmid D, Deacon J, Jaworski J, Fay F, McLoughlin K, Gormley J, Burrows J, Longley D, Donnelly RF, Scott CJ. Enhanced antitumor activity of the photosensitizer Meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate (TMP) through encapsulation in antibody targeted chitosan/alginate nanoparticles. Biomacromol. 2013;14:302–310. doi: 10.1021/bm301858a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarron PA, Woolfson AD, Donnelly RF, Andrews GP, Zawislak A, Price JH. Influence of Plasticiser Type and Storage Conditions on the Properties of Poly(methyl vinyl ether-co-maleic anhydride) Bioadhesive Films. J. Appl. Polym. Sci. 2004;91:1576–1589. [Google Scholar]
- 24.Fourtanier A, AND C. Berrebi. Miniature pig as an animal model to study photoaging. Photochem. Photobiol. 1989;50:771–784. doi: 10.1111/j.1751-1097.1989.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 25.Woolfson AD, McCafferty DF, McCallion CR, McAdams, E. E, Anderson, J J. Moisture-activated, electrically conducting bioadhesive hydrogels as interfaces for bioelectrodes: Effect of film hydration on cutaneous adherence in wet environments. J. Appl. Polym. Sci. 1995;58:1291–1296. [Google Scholar]
- 26.Donnelly RF, Garland MJ, Morrow DIJ, Migalska K, Thakur RRS, Majithiya R, Woolfson AD. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Cont Rel. 2010;147:333–341. doi: 10.1016/j.jconrel.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly RF, Morrow DIJ, McCarron PA, Juzenas P, Woolfson AD. Pharmaceutical Analysis of 5-Aminolevulinic acid in Solution and in Tissues. Journal of Photochemi Photobiol. 2006;82:59–71. doi: 10.1016/j.jphotobiol.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Meso-tetra (N-methyl-4-pyridyl) porphine tetra tosylate (TMP) product data sheet. Frontier Scientific Europe Ltd; Carnforth, Lancashire, UK: 2004. [Google Scholar]
- 29.Thakur RRS, McCarron PA, Woolfson AD, Donnelly RF. Investigation of swelling and network parameters of poly (ethylene glycol)-crosslinked poly (methyl vinyl ether-co-maleic acid) hydrogels. Eur.Polym J. 2009;45:1239–1249. [Google Scholar]
- 30. [Accessed 19th October 2013]; http://www.malvern.com.
- 31. [Accessed 19th October 2013]; http://www.bbsrc.ac.uk/news/health/2013/131009-f-how-do-microneedles-deliver-drugs.aspx.