1. Introduction
As childhood ends, a period of expansive brain reorganization begins. A decline in synaptic density is observed in the healthy human cortex starting near ages 9 or 10 and continuing into early adulthood. This decline is reflected in a number of neural measures. MRI studies—including a longitudinal study (Gogtay et al., 2004) —have shown significant decreases in cortical and subcortical grey matter across adolescent development (Steen et al., 1997); (Pfefferbaum et al., 1994); (Shaw et al., 2008); (Giedd et al., 1999). These changes are mirrored by declines in blood glucose metabolism assessed by PET (Chugani et al., 1987) and by reduced synchronous neural activity indexed by waking (for a review see (Segalowitz et al., In Press) and sleep (Campbell and Feinberg, 2009) (Tarokh and Carskadon, In Press-a) EEG power. A relatively steady state in these measures is not reached until early adulthood, between the ages of 19 to 25.
In addition to the maturational decline in the number of cortical synapses, adolescent development is accompanied by modified connectivity between brain regions. Stevens (Stevens, 2009) has proposed that a decline in EEG power and an increase in regional connectivity are complementary processes that support cognitive gains observed during this period. According to this model, adolescent brain development involves “discarding” unused synapses thereby decreasing the amplitude of neural activity. Concurrently, connections frequently utilized are strengthened, leading to the emergence of more complex and efficient networks. Stevens’ proposal aligns with the prominent theory of cognitive development called ‘neural constructivism,’ which postulates that age-related cognitive improvements result from increased inter-connectivity and functional specialization of neural networks (Quartz and Sejnowski, 1997).
Connectivity between brain regions is inferred from temporal associations between spatially remote neurophysiological events. One such measure of connectivity is correlation between two simultaneously recorded signals in the frequency domain, called coherence, which can be assessed in humans using fMRI and EEG. A major assumption is that strong correlation of neural activity between two distal brain regions provides evidence that those regions are connected or that a common third region drives the activity of both regions. A corollary assumption is that coherence depends on axonal anatomical linkage between brain regions; however, coherence can also reflect dynamic and transient activity between brain regions. One way to distinguish between these two possibilities is by examining coherent activity in different neurophysiological states: coherence based on anatomical linkage should be state independent, whereas dynamic and transient coherent activity is perhaps state dependent.
Several studies show positive correlations of developmental gains in performance on cognitive tests with increased coherence. For example, a study of narrative comprehension in children and teens (age range = 4.9 to 18.9 years) found an age-related increase in the number of questions answered correctly and fMRI-measured effective connectivity between Broca's area and the left superior temporal gyrus during task performance (Schmithorst et al., 2007). The authors measured effective connectivity with multivariate autoregressive modeling between regions of interest. Another study of teens ages 13.5 to 14.1 found a positive correlation between scores on the Intelligence Structure Test (IST) and waking resting (eyes closed) interhemispheric EEG coherence for the theta band between two temporal (T4 and T3) and two occipital (O1 and O2) electrodes (Anokhin et al., 1999). These studies indicate that increased coherent activity during performance of cognitive tasks and while resting has functional significance, in general showing a positive association between these measures.
One of the first large-scale studies examining waking EEG coherence as a function of development was performed by Thatcher and colleagues (Thatcher et al., 1987). This cross-sectional study examined resting EEG power in 557 children ranging in age from 2 months to early adulthood. Coherence was calculated from one-minute samples of resting awake eyes-closed EEG. The authors described an overall trend for an exponential increase in intrahemispheric coherence between frontal and occipital EEG leads (Fp1 and O1; Fp2 and O2) with increasing age, though they did not report at which frequencies this trend was observed. Several other cross-sectional resting wake EEG studies have corroborated these findings, showing a trend towards increasing intrahemispheric coherence between anterior and posterior electrodes with development in children across a range of frequency bands (Barry et al., 2004) (Gasser et al., 1988).
The interpretation of these studies is limited by several factors. In the first place, the samples are cross-sectional, and large inter-individual variability in coherence levels makes it difficult to disassociate maturational changes from individual variability or cohort effects. Furthermore, the time of day that coherence was measured and the sleep/wake history of participants were not controlled. This issue is of concern because many studies have shown that circadian factors (Dijk, 1999) (Dijk and Czeisler, 1995) and sleep/wake history influence EEG spectral power (for a review see Borbély and Achermann, 1999), although the impact of these factors on coherence has not been determined. Finally, coherence estimates in the waking EEG literature are usually based on small data segments (e.g. 1 to 2 minutes), which increases the susceptibility of these measures to variations in attention and motivation, detracting from measurement reliability. The sleep EEG provides a unique opportunity to examine brain connectivity by reducing such confounding factors as attention, motivation and cognitive abilities related to waking activities. Furthermore, sleep recordings comprise hours rather than minutes of EEG recordings, which increases the stability of the measure. Additionally, two distinct neurophysiological states can be identified in sleep.
The two states of sleep – rapid eye movement (REM) and non-REM (NREM) sleep – are determined by events in the EEG, the electrooculogram (EOG), and the electromygram (EMG) recorded continuously. NREM and REM sleep alternate during the night, with a average period of about 90 minutes in adult humans. Two prominent feature of the NREM sleep EEG are: (1) slow waves and (2) sleep spindles. Slow waves in sleep are defined as low frequency (0.6 to 4.6 Hz) waves that are high amplitude (> 75 μV peak to peak) and are at a maximum early in the night decreasing across consecutive NREM episodes. Sleep spindle oscillations occur episodically in sleep, appearing in the EEG power spectrum as a peak in the 12 – 16 Hz range. Sleep spindles are generated by thalamic reticular neurons and transmitted to the cortex via thalamocortical pathways (for a review see De Gennaro and Ferrara, 2003). REM sleep is characterized by relatively low voltage, mixed frequency EEG, bursts of rapid eye movements, and suppressed muscle tone (Rechtschaffen and Kales, 1968).
Several studies have examined power spectral characteristics of the sleep EEG as a function of adolescent development (Tarokh and Carskadon, In Press-a) (Campbell and Feinberg, 2009) (Jenni and Carskadon, 2004) (Gaudreau et al., 2001). A consistent finding is lower EEG spectral power with increasing age (Tarokh and Carskadon, In Press-a) (Campbell and Feinberg, 2009), although the age at which this decline begins remains equivocal (Campbell and Feinberg, In Press, Tarokh and Carskadon, In Press-b). The Tarokh and Carskadon study also found an increase in the frequency of the NREM sleep spindle peak occurs across this age range (Tarokh and Carskadon, In Press-a).
One group has shown that sleep EEG coherence can reliably distinguish children and adolescents with depression from those who have no psychopathology (Armitage et al., 2006). The aim of the present study was to assess how sleep EEG coherence changes from early adolescence to young adulthood using both longitudinal and cross-sectional data. To this end, we analyzed all-night sleep EEG recordings in two longitudinal adolescent cohorts and a one-time assessment of a young adult cohort. We hypothesized a positive correlation of coherence and age, though not uniformly across/between all cortical regions.
2. Experimental Procedures
2.1 Participants
Participants were recruited with flyers, mailings to previous participants, and radio and newspaper advertisements. Individuals with a current or chronic illness, evidence of learning disability, sleep disorder, personal or family history of psychopathology were excluded from the study. Additional exclusion criteria included individuals with a pattern of insufficient sleep or excessive daytime sleepiness.
Data from three age cohorts, two of which were followed-up longitudinally, are presented here. Data were collected from participants ranging in age from 9 to 23 years (Table 1). The protocol, data collection and analysis were identical across cohorts, with the exception of sleep opportunity, which varied across cohorts (but not within a cohort). Sleep opportunity may have an impact on our results; therefore we analyze the data within the two longitudinal cohorts separately and present them as two separate studies. We use the third cohort of young adults to perform a linear regression across all ages to gain a broader perspective of developmental changes in coherence from late childhood to early adulthood.
Table 1.
Participant Characteristics: Top row corresponds to the mean (standard deviations) of the age of participants at each recording sessions. Bottom row corresponds to the mean (standard deviations) of the frequency of the peak in the sigma range (11 16 Hz) of the NREM sleep EEG for each recording session.
| Children Initial | Children Follow-up | Teens Initial | Teens Follow-up | Young Adults | |
|---|---|---|---|---|---|
| Age (Years) | 10 (0.5) | 12.4 (0.6) | 15.8 (0.5) | 18.3 (0.6) | 22.3 (0.9) |
| Spindle (Hz) | 12.5 (0.4) | 12.7 (0.4) | 13 (1) | 13.4 (1.2) | 13.4 (0.4) |
2.2 Study 1: Children Cohort
The first cohort included twelve children (8 males) who were 9 or 10 years old at the time of the Initial recording session and 11 to 13 years old at the time of the Follow-Up recording (Table 1). Tanner stage, a measure of external primary and secondary sex characteristics, was determined during a brief physical exam at each assessment. Tanner stages range from 1, characterized by child like appearance, to Tanner 5, indicating attainment of adult-like appearance (Tanner, 1962). All children, with the exception of one female who was a Tanner 4, were either Tanner 1 or 2 at the time of the Initial recording session and all had advanced at least one Tanner stage at the Follow-Up session. The racial distribution of our sample was as follows: nine children were White, one was Hispanic/Latino, one was Asian/Asian American, and one was Black/African-American.
2.3 Study 2: Teens Cohort
The second cohort consisted of twelve teens (3 males) aged 15 and 16 years at their Initial assessment and followed-up two to three years later (Table 1). In this cohort, eight participants were White, three were Multiracial, and one was Black/African-American. At both sessions, alcohol use was assessed through the 1-month Time-Line Follow Back Interview (Sobell and Sobell, 1992). Only participants who reported either no experience with alcohol, experience with alcohol only in small quantities for religious observance or less than one standard drink per month on average, drinking ≤ 2 standard drinks on a given occasion and never getting drunk are included in this analysis.
2.4 Adult Cohort
The final cohort consisted of ten 20 to 23 year olds (6 males) studied one time. Six of the participants were White, two were Black/African-American, one was Hispanic/Latino, and one was Multiracial. As with the 15/16 year-old cohort, alcohol use was assessed (see above) with the same inclusion criteria.
2.5 Procedures
Each recording session was preceded by at least one week sleeping on a fixed schedule individually modified to suit each participants daytime activities, and varying by cohort to adjust for developmental differences. Children in study 1 were given a schedule of at least ten hours time in bed (TIB); study 2 teens were allotted at least nine hours TIB; young adults were given a minimum of eight and half hours TIB (Table 2). Individually scheduled TIB were the same at both assessments. Compliance to the sleep schedule was confirmed using sleep diaries, continuous wrist actigraphy, and daily phone calls to the lab's time-stamped answering machine at rise times and bedtimes. Participants were asked to abstain from alcohol and caffeine throughout the study. A breathalyzer test was performed on arrival for the in-lab nights in the two older cohorts (i.e., teens and young adults) to verify compliance. A urine test on the in-lab nights confirmed that none of the participants had taken illicit drugs.
Table 2.
Sleep Stage Variables: Means (standard deviations) expressed as a percentage of total sleep time for stage 1, stage 2, slow wave sleep (SWS), and REM sleep for all cohorts. WASO = Minutes of wake after sleep onset measured from first occurrence of 1.5 minutes of stage 1 or stage 2 to final awakening; Sleep latency = time in minutes to the first 1.5 consecutive minutes of stage 1 or first stage 2, REM latency = number of minutes to the first REM episode after sleep onset; Total sleep time = total time in minutes spent in NREM and REM sleep; Total recording time = Time from lights off to lights on; Sleep efficiency = total sleep time divided by total recording time. Statistical significance was assessed using a paired t-test and alpha was set to 0.05.
| Sleep Variable | Children | Teens | Young Adults | ||||
|---|---|---|---|---|---|---|---|
| Initial | Follow- Up | P- Value | Initial | Follow- Up | P- Value | Initial | |
| Stage 1 (%) | 5 (2) | 7 (3) | 0.04 | 6 (2) | 8 (2) | 0.04 | 10 (4) |
| Stage 2 (%) | 36 (8) | 44 (5) | 0.007 | 42 (7) | 50 (4) | 0.0007 | 52 (4) |
| SWS (%) | 40 (7) | 29 (9) | 0.0008 | 32 (7) | 23 (6) | 0.00004 | 16 (6) |
| REM (%) | 19 (4) | 20 (4) | 0.11 | 19 (3) | 20 (6) | 0.81 | 21 (3) |
| WASO (Min) | 11 (11) | 14 (14) | 0.62 | 13 (18) | 14 (28) | 0.59 | 14.5 (12) |
| Sleep Latency(Min) | 15 (9) | 17 (18) | 0.59 | 8 (5) | 9 (6) | 0.50 | 11 (11) |
| REM Latency (Min) | 163 (39) | 143 (56) | 0.17 | 171 (53) | 146 (50) | 0.44 | 124 (40) |
| Total Sleep Time (Min) | 568 (17) | 560 (22) | 0.21 | 515 (9) | 523 (44) | 0.60 | 481 (17) |
| Time in Bed (Min) | 600 (7) | 600 (<1) | 0.89 | 543 (12) | 550 (31) | 0.25 | 510 (<1) |
| Sleep Efficiency (%) | 95 (2) | 93 (4) | 0.22 | 95 (2) | 95 (5) | 0.65 | 94 (3) |
Each recording session included two consecutive nights in the lab. Fixed pre-study sleep schedules helped ensure that participants were well slept. None was taking medications. Participants slept in individual darkened bedrooms while polysomnography (PSG) was recorded. Participants adapted to sleeping in the lab and were screened on the first night for sleep-related breathing abnormalities and periodic limb movements using oral/nasal thermocouples and leg electromyogram (EMG), respectively. No sleep disorders were detected. Sleep and EEG data from the second night were analyzed for this report.
The 9/10-year-old cohort, referred to as children throughout this paper, and the 15/16-year-old cohort, referred to as teens throughout this paper, slept in the lab on two occasions separated by 22 to 33 months (mean = 29.5; SD = 2.7). We refer to the first session as Initial and the second session will be referred to as Follow-up. The 20-23-year-old cohort will be referred to as young adults throughout this paper.
2.6 Polysomnography Recordings
Recordings included right and left electrooculgram (EOG), electromyogram (EMG; mentalis, submentalis), and electrocardiogram (ECG), along with two central (C3/A2 and C4/A1) and two occipital (O1/A2 and O2/A1) referential EEG leads placed according to the international 10-20 system (Jasper, 1958). Equipment upgrades during the study led us to use two recording systems for this data set. The majority of Initial recordings in children and teens were performed using the Albert Grass Heritage System (Astromed, Grass, West Warwick, RI) with GAMMA software. EEG signals were digitized on-line (12 bit AD converter; Butterworth filter, -12 dB/octave; low-pass filter, -6dB at 35 Hz; time constant 1.0; collected and stored at a resolution of 128 Hz for the EEG). Two participants from the children cohort were recorded on the TWin system (Astromed, Grass, West Warwick, RI) using TWin AS40 bedside amplifiers. Signals were filtered off-line (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz). These Twin signals were collected digitally (storage resolution 400 Hz) and saved as EDF files. All Follow-Up recordings and all adult recordings (except for two participants who were recorded on the GAMMA system) were performed on the TWin system. Impedance values were always below 10 k[.Omega]. A calibration signal was input into both systems simultaneously to assess whether signals from the two systems were comparable. Signals from the two systems were in good agreement from 0.3 to 16 Hz; however, small discrepancies emerged at higher frequencies. Therefore, frequencies above 16 Hz were not examined.
2.7 Sleep Stage Scoring and Coherence Analysis
Sleep data were visually scored in 30-s epochs according to the criteria of Rechtschaffen and Kales (Rechtschaffen and Kales, 1968). Inter- and intra-rater reliability was at least 86%. Epochs with artifacts were rejected using a semi-automated procedure based on power in the 0.6 to 4.6 Hz and 20 to 40 Hz bands and confirmed by visual inspection (Buckelmuller et al., 2006). Coherence was computed on each 30-s epoch (Hanning window, average of six 5-s epochs) using Welch's averaged, modified periodgram method in MATLAB (The MathWorks Inc., Nantick, MA, USA). The squared coherence between two signals, x and y, at frequency f is defined as the squared cross-spectrum between the signals divided by the product of the auto-spectra of each signal:
Coherence values range from 0 to 1: a value of 1 indicates constant relative phase between signals at a frequency f; values near zero indicate that phase between signals at frequency f is random. Frequencies from 0.6 to 16 Hz were examined in 0.2 Hz steps. The two lowest frequency bins (0.2 and 0.4 Hz) were excluded due to the sensitivity of these bins to low frequency artifacts.
Intra-hemispheric coherence was calculated between the left (C3/A2 and O1/A2) and right (C4/A1 and O2/A1) central and occipital electrodes. Inter-hemispheric coherence was calculated between the central (C3/A2 and C4/A1) and occipital (O1/A2 and O2/A1) electrodes in the left and right hemispheres. Coherence between diagonal electrodes (C3/A2 and O2/A1; C4/A1 and O1/A2) was also calculated.
As expected, sleep stages changed from the Initial to the Follow-Up session in children and teens (Table 2), with significantly more slow wave sleep (SWS; NREM sleep stages 3 and 4) and less NREM stage 2 sleep at the Initial sessions (Table 2). Because SWS and stage 2 sleep affect the calculation of the NREM sleep coherence spectrum, we calculated the coherence spectra for SWS, stage 2 sleep and REM sleep separately.
2.8 Statistics: Within Cohort
Although coherence values can range from 0 to 1, they are not normally distributed, but rather the distribution is positively skewed. To account for this distribution, we applied a Fisher's z-transform to the square root of the data for all analyses. Data were plotted after the subject average data were retransformed to coherence values.
We performed a bootstrap test at each frequency bin comparing coherence at the Initial and the Follow-Up sessions to assess significance within a cohort (e.g., children). The bootstrap methodology has been applied to sleep EEG spectral data (De Gennaro et al., 2000) (Tarokh and Carskadon, 2009) and is well suited for analyzing EEG data, since it makes no assumptions regarding the distribution of the data. The principle underlying the bootstrap is to generate a random sample, called the bootstrap distribution by randomly sampling (with replacement) from the original pool of data. Because we perform a bootstrap statistic on each individual frequency bin for each channel pair, we must address the issue of multiple comparisons. Maris and Oostenveld (Maris and Oostenveld, 2007) have shown that such non-parametric tests as the bootstrap applied to EEG and MEG data control the false alarm rate since the distributions are based on the data set. Our conservative approach addresses the issue of multiple comparisons by reporting statistically significant effects only when at least three adjacent frequency bins achieve statistical significance (p < 0.05), since the probability of adjacent bins reaching statistical significance by chance is low.
2.9 Statistics: Linear Regression Across Cohorts
Changes in coherence with age spanning a broader age range were examined by combining the data from the longitudinal cohorts (children and teens) and the young adult cohort. Thus, we have coherence data at 9/10 years, 11 to 13 years, 15/16 years, 17 to 19 years and 20 to 23 years. We reduced the number of statistical tests by averaging the data into frequency bands based on the previous sleep EEG literature (Achermann and Borbely, 1998) and on the morphology of our coherence spectra: low-delta (0.8 to 1.6 Hz), delta (1.8 to 4.8 Hz), theta (5 to 8.4 Hz), alpha (8.6 to 10.8), and sigma (11 to 16 Hz). As described above, we averaged the individual subject data after applying Fisher's z-transform, and then retransform the data back before performing the linear regression. Although this analysis violates assumptions of linear regression by using both between and within subjects data, it allows us to examine the “developmental trajectory” across this age range. We used the Dubey/Armitage-Parmar procedure to correct for multiple comparisons (Armitage and Parmar, 1986, Dubey, 1985). This adjustment takes into account the mean correlation between the variables used in the regression and adjusts the p-value accordingly. Based on this procedure the adjusted p-value for statistical significance was 0.002.
3. Results
3.1 Sleep Stage Variables
Sleep stage variables are reported in Table 2. Statistically significant differences between the two assessments for the children and the teen cohorts were assessed using a paired t-test. At Follow-Up compared to the Initial session both the children and teen cohorts (study 1) had a greater percentage of stage 1 and stage 2 sleep and less slow wave sleep at Follow-Up (Table 2).
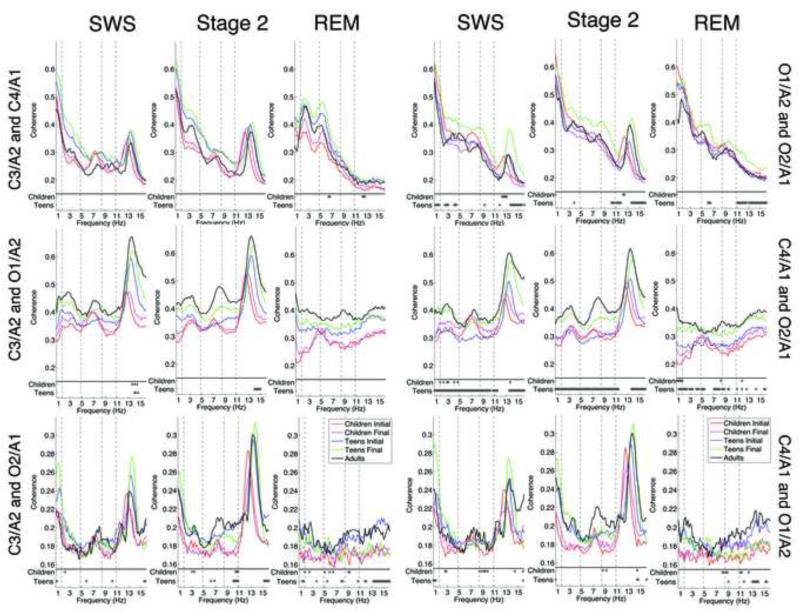
We use the bands defined for the linear regression to describe the within subjects data. Furthermore, individual frequency bins that were significantly different between assessments are marked by an asterisk for the children and teen cohorts separately (Figure 1).
Figure 1. Intra- and Inter- Hemispheric and Diagonal Coherence for SWS, Stage 2 and REM sleep.
The top plots correspond to mean interhemispheric coherence between the two central (left figure) and two occipital (right figure) electrodes, the second row correspond to left (left figure) and right (right figure) intrahemispheric coherence, and the third row is coherence between diagonal electrodes. At the bottom of each individual plot, separated from the rest of the plot by a horizontal line, are the statistical results obtained from the bootstrap analysis. Asterisks correspond to frequency bins that were statistically significantly different between the Initial and Follow-Up session for the children (Children) and teen (Teens) cohorts at p < .05.
3.2 Study 1: Children
Few consistent changes in coherence occurred from the Initial to the Follow-Up session in the children's cohort (Figure 1). With respect to interhemispheric assessment, coherence between the anterior electrodes (C3/A2 and C4/A1) showed a significant developmental increase only during REM sleep in the theta and sigma bands (6.4 – 6.6 Hz and 12.2 – 12.6 Hz). Conversely, coherence decreased between posterior electrodes (O2/A1 and O1/A2) in the spindle range during slow wave and stage 2 sleep.
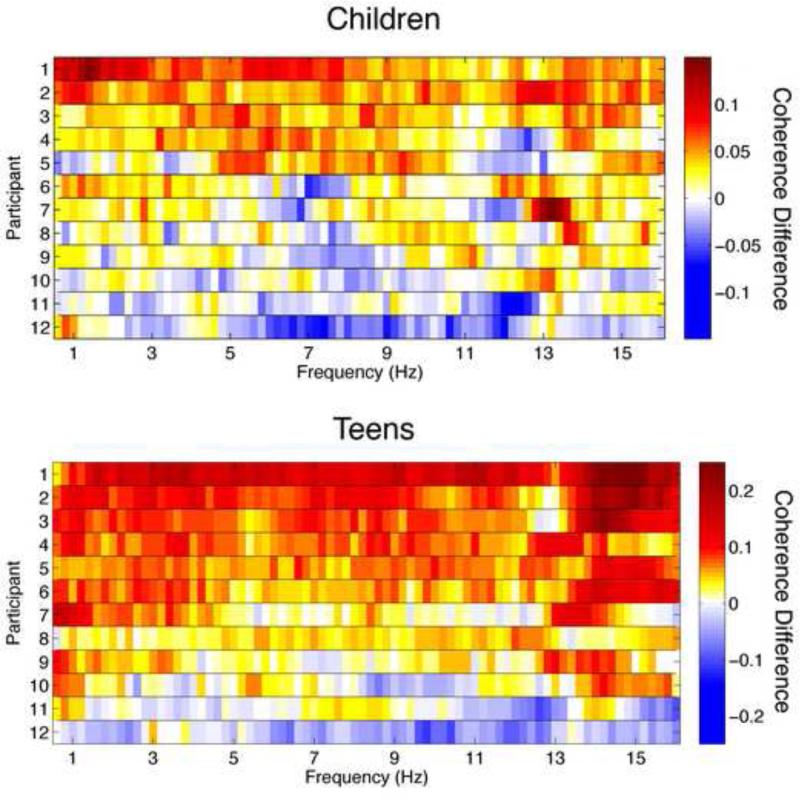
Looking within hemispheres, coherence in the right hemisphere increased with age in the low delta and delta bands during REM sleep and SWS, respectively. To demonstrate the consistency of this affect across participants, we plot the age dependent change for SWS for individual participants in Figure 2. Our analysis of diagonal electrodes revealed an increase in coherence with age in the alpha band during stage 2 and REM sleep between the left central and right occipital electrodes. Looking at the opposing diagonal electrode pair (right central and left occipital), we observed an increase in the alpha band in all states as well as a state-independent increase in the sigma band. In summary, for the children cohort, we observed few across hemispheric changes with age in any sleep stage, few changes within hemispheric coherence, and an increase in cross hemispheric coherence for one diagonal pair in the sigma and alpha bands.
Figure 2. Individual data for right intrahemispheric coherence during SWS for Children (Top) and Teens (Bottom).
Coherence at the follow-up session minus coherence at the initial session for all frequency bins and subjects are illustrated. Warm colors are indicative of increases in coherence between assessments, cool colors denote decreases in coherence and values of zero (no change in coherence) are shown in white. Note that the two plots are on different scales, and changes in coherence are larger for the teen cohort.
3.3 Study 2: Teens
Our analysis of coherence across hemispheres in the teens revealed an increase in sigma band coherence between the posterior channels (O2/A1 and O1/A2) during all states. Coherence for this electrode pair was also greater at the Follow-Up session in the low-delta and delta bands during SWS and in the theta band during REM sleep. In contrast, coherence did not change as a function of development for the anterior electrode pairs in any sleep state. Looking at coherence within hemispheres, we found extensive evidence of a developmental increase in coherence over the right hemisphere, but not the left. Thus, coherence between C4/A1 and O2/A1 increased with development in all frequency bands and across sleep states. As shown in Figure 2, the increase in coherence was present for most participants during SWS. On the other hand, coherence increases within the left hemisphere (C3/A2 and O1/A2) were limited to frequencies between 14 and 15 Hz and only during slow wave and stage 2 sleep. Our analysis of diagonal electrode pairs revealed developmental increases of coherence, though the results were less consistent. Coherence between the left central and right occipital electrode increased across all sleep states in the sigma band. Furthermore, coherence increased during stage 2 in the alpha band, in the theta and low-delta bands during REM sleep, and in the low-delta band during SWS. The other diagonal pair (C4/A1 and O1/A2) showed an increase in coherence in the sigma band for REM and stage 2, but not slow wave sleep; increases in coherence during SWS were limited to the low-delta band. The young adult data is shown for comparison purposes. In summary interhemispheric coherence increased between posterior electrodes, while intrahemispheres coherence increases were limited to the right hemisphere, and both diagonal pairs showed increases.
3.4 Re-referencing Central to Occipital Channels
To assess the impact of the reference electrode on the general finding that sleep EEG coherence increases with age, we re-referenced the central channels to the occipital channels then calculated coherence (i.e., bipolar derivations C3/O1 and C4/O2). We found a state and frequency independent increase of approximately 78% in coherence from the Initial to the Follow-up session for both cohorts (children and teens). Interpretation of this finding is difficult due to the large distance between the central and occipital electrodes, however, this analysis affirms our finding of increased coherence with maturation.
3.5 Linear Regression
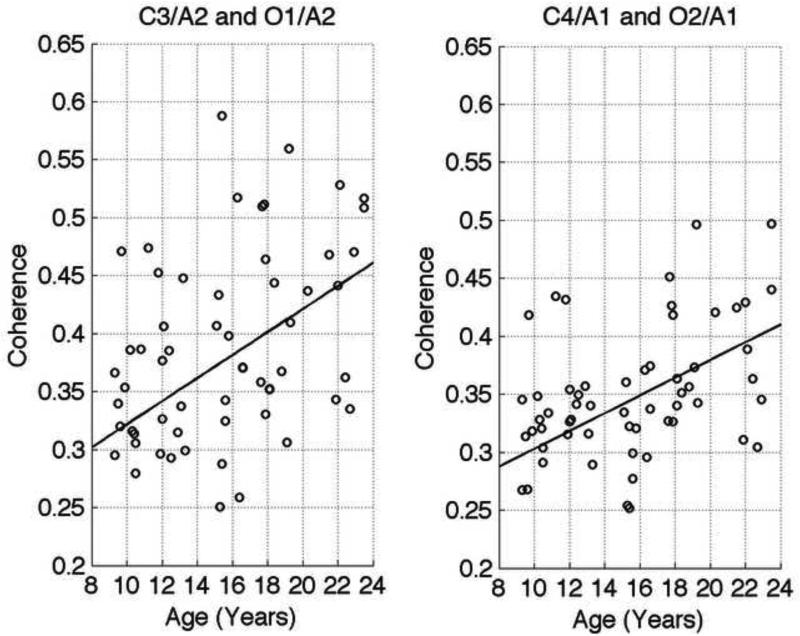
Broadening our lens, we performed a linear regression analysis of coherence across all cohorts, the details (slope, intercept, R2, and p-value) of which can be found in Table 3. Intrahemispheric coherence increased in a linear fashion in both the right (C4/A1 and O2/A1) and left (C3/A2 and O1/A2) hemispheres for most frequency bands. Figure 2 demonstrates this in the low delta band. More specifically, coherence increased over the left hemisphere in all sleep states and frequency bands with the following exceptions: the theta band in all states, the delta band in stage 2 sleep, and the alpha band during SWS. In the opposite hemisphere (C4/A1 and O2/A1), the exceptions to the significant linear increase in coherence were the theta band during REM sleep, and the theta and alpha bands during SWS.
Table 3.
Results of the Regression Analysis. Results from the linear regression on coherence change across all assessments (age) of all cohorts. Significant values (p ≤ 0.002) are highlighted in grey. SWS = slow wave sleep/ NREMS stages 3 and 4; Stage 2 = NREM sleep stage 2; REM = REM sleep; Low Delta = 0.8 to 1.6 Hz; Delta = 1.8 to 4.8 Hz, Theta = 5 to 8.4 Hz, Alpha = 8.6 to 10.8 Hz, and Sigma = 11 to 16 Hz.
| SWS | Stage 2 | REM Sleep | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | R2 | P-Value | Slope | Intercept | R2 | P-Value | Slope | Intercept | R2 | P-Value | |
| C3/O1 and O1/A2 | ||||||||||||
| Low Delta | 0.01 | 0.22 | 0.20 | 0.0004 | 0.01 | 0.19 | 0.23 | 0.0001 | 0.02 | 0.07 | 0.32 | 0.0000 |
| Delta | 0.007 | 0.27 | 0.16 | 0.002 | 0.007 | 0.26 | 0.14 | 0.004 | 0.009 | 0.18 | 0.19 | 0.0006 |
| Theta | 0.003 | 0.34 | 0.02 | 0.23 | 0.007 | 0.26 | 0.12 | 0.007 | 0.007 | 0.22 | 0.12 | 0.008 |
| Alpha | 0.006 | 0.26 | 0.10 | 0.01 | 0.01 | 0.21 | 0.25 | 0.0001 | 0.009 | 0.17 | 0.22 | 0.0002 |
| Sigma | 0.01 | 0.25 | 0.35 | 0.0000 | 0.01 | 0.30 | 0.24 | 0.0001 | 0.007 | 0.23 | 0.16 | 0.002 |
| C4/A1 and O2/A1 | ||||||||||||
| Low Delta | 0.008 | 0.23 | 0.20 | 0.0004 | 0.008 | 0.19 | 0.25 | 0.0001 | 0.01 | 0.70 | 0.44 | 0.0000 |
| Delta | 0.005 | 0.27 | 0.17 | 0.001 | 0.006 | 0.24 | 0.18 | 0.001 | 0.008 | 0.17 | 0.22 | 0.0002 |
| Theta | 0.001 | 0.32 | 0.008 | 0.50 | 0.006 | 0.23 | 0.18 | 0.001 | 0.005 | 0.21 | 0.14 | 0.004 |
| Alpha | 0.005 | 0.26 | 0.11 | 0.01 | 0.008 | 0.20 | 0.29 | 0.0000 | 0.008 | 0.16 | 0.30 | 0.0000 |
| Sigma | 0.01 | 0.25 | 0.38 | 0.0000 | 0.009 | 0.28 | 0.27 | 0.0000 | 0.007 | 0.22 | 0.20 | 0.0004 |
| C3/A2 and O2/A1 | ||||||||||||
| Low Delta | 0.001 | 0.21 | 0.01 | 0.39 | 0.003 | 0.17 | 0.17 | 0.001 | 0.002 | 0.15 | 0.22 | 0.0002 |
| Delta | 0.0002 | 0.18 | 0.001 | 0.79 | 0.002 | 0.16 | 0.08 | 0.03 | 0.002 | 0.15 | 0.28 | 0.0000 |
| Theta | 0.0001 | 0.18 | 0.0008 | 0.83 | 0.002 | 0.16 | 0.16 | 0.001 | 0.0009 | 0.16 | 0.10 | 0.01 |
| Alpha | 0.0009 | 0.16 | 0.05 | 0.08 | 0.002 | 0.15 | 0.28 | 0.0000 | 0.002 | 0.15 | 0.74 | 0.04 |
| Sigma | 0.0008 | 0.19 | 0.03 | 0.20 | 0.002 | 0.19 | 0.12 | 0.008 | 0.002 | 0.16 | 0.10 | 0.04 |
| C4/A1 and O1/A2 | ||||||||||||
| Low Delta | 0.002 | 0.19 | 0.04 | 0.12 | 0.004 | 0.16 | 0.15 | 0.003 | 0.002 | 0.15 | 0.22 | 0.0002 |
| Delta | 0.001 | 0.17 | 0.02 | 0.25 | 0.002 | 0.15 | 0.09 | 0.02 | 0.002 | 0.15 | 0.18 | 0.001 |
| Theta | 0.0003 | 0.18 | 0.002 | 0.71 | 0.002 | 0.16 | 0.10 | 0.01 | 0.0008 | 0.16 | 0.07 | 0.04 |
| Alpha | 0.0005 | 0.17 | 0.01 | 0.42 | 0.002 | 0.15 | 0.16 | 0.002 | 0.001 | 0.16 | 0.05 | 0.09 |
| Sigma | 0.001 | 0.18 | 0.06 | 0.07 | 0.002 | 0.19 | 0.11 | 0.009 | 0.002 | 0.15 | 0.10 | 0.01 |
Our analysis of diagonal electrode pairs, revealed a linear increase in coherence between the left central and right occipital electrodes in all frequency bands except the delta band in stage 2 sleep. In addition, a linear trend was detected during REM sleep in the two lowest frequency bands. We found the same effect during REM sleep between the right central and left occipital electrodes. The only other effect between this electrode pair (C4/A1 and O1/A2) was in the alpha band during stage 2 sleep. No linear trend was observed in interhemispheric coherence.
3.5 Spindle Frequency Peak
Visual inspection of the coherence spectrum revealed an increase in the frequency at which the peak in the spindle range occurred. To test whether this increase was significant, we determined the frequency of the spindle peak in the coherence spectrum for central channels (C3/A2 and C4/A1) for each participant. We performed a paired t-test comparing the Initial to the Follow-Up session for the children and teen cohorts (Table 1). We found a significant increase in the frequency of the peak for children (p = 0.01) and teens (p = 0.002). To examine this trend across cohorts, we performed a linear regression and observed a significant linear increase in frequency (slope = 0.08 Hz/year, intercept = 11.68 Hz, R2 = 0.17, p = 0.001).
4. Discussion
The use of sleep EEG to examine developmental changes in brain coherence in this study takes advantage of several methodological differences from traditional waking EEG coherence measure. In the first place, our approach includes a fixed sleep schedule before in-lab sessions; second the EEG recordings were performed in a laboratory setting that excluded such potentially confounding variables as exposure to light, noise, and fluctuations in temperature. Third, in contrast to waking EEG studies that estimate coherence from several minutes of data collected with either eyes open or eyes closed, sleep data provides a stable measure comprising several hours of EEG recording.
Finally, within sleep we can examine three distinct brain milieux (SWS, stage 2 and REM sleep) each of which has a unique neural substrate. An advantage of recording across distinct brain states arises in interpreting developmental changes. For example, age-related differences that are consistent for all three states likely represent increased quantity or strength of connections associated with brain maturation; by contrast, effects limited to a single state may indicate developmental changes affecting the neural substrate of that state.
4.1 A Framework for Coherence
Our findings can be interpreted in the context of current theories on the functional (neural) framework for EEG coherence. The cerebral cortex is a distributed system in which function arises from the successful integration of activity in anatomically distributed populations of neurons. Singer (Singer, 2009) and others have proposed an elegant solution to the problem of functional integration by showing that populations of neurons in distal regions connected by axons synchronize their responses temporally by oscillating at the same frequency. In this way, temporary neural networks can be formed to serve a specific function and then dissolved to form new networks in seconds. A property of such networks is that synchronization among remote groups of neurons or among large assemblies of neurons occurs at slow frequencies, while local synchronizations can occur at both slow and fast frequencies (Buzsaki and Draguhn, 2004, Singer, 2009, von Stein and Sarnthein, 2000) (Nir et al., 2008).
This framework guides the interpretation of our findings in the following ways. Where we see enhanced low-frequency coherence between distal electrodes, we presume long-range integration either between cortico-cortical regions or driven by a common third region; where we see greater distal coherence in the higher-frequency range (e.g., spindle activity) we presume a common third region, such as the thalamus, must be driving both regions. On the other hand, coherence between proximal electrodes at low and high frequencies can result from cortico-cortico coupling or be driven by a common third region. We make use of this framework to interpret our data with the caveat that without making direct measurements the underlying neural mechanisms remain unknown.
4.2 Development of Long-Range Connectivity over the Right Hemisphere
The greatest developmental change for both the children and teen cohorts was found for coherence within the right hemisphere. This finding aligns with previous data showing that the two hemispheres develop at different rates. For example, Thatcher (Thatcher et al., 1987) using cross-sectional data showed that the developmental trajectory of anterior to posterior (frontal to occipital) waking EEG coherence differs in the right and left hemispheres. In particular, he showed a marked increase in coherence from ages 4 to 6 in the left hemisphere against a background of steadily increasing coherence. This report also identified a similar growth spurt in the right hemisphere between the ages of 8 and 10 years. In the Thatcher study, the data from participants ages 16 to 26 were averaged into one group, making it difficult to determine how coherence evolves during this age range from that report. Our findings indicate a significant increase in right intrahemispheric coherence between distal electrodes (C4/A1 to O2/A1) across ages 15/16 to 17 - 19 years. Furthermore, in Figure 2 we demonstrate that this increase is found in most individuals across frequencies and is not driven by a few participants.
The increase in the children was confined to the two lowest frequency bands (low-delta and delta), indicating that slow waves become more synchronized during SWS and REM sleep, but not during stage 2 sleep. Because this finding was not found across all states and was limited to the lowest frequencies, we believe it indicates increased coupling of distal connections, perhaps due to structural changes (e.g., mylenation or pruning) and may have unknown functional significance. On the other hand, the developmental increase of right-hemisphere coherence in the teens was state and frequency non-specific. Such an overall increase in coherence during later adolescence can reflect increased anatomical coupling between cortico-cortical (low-frequency) and thalamocortical (high-frequency) circuits perhaps as a result of increased mylenation.
4.3 Short-Range Connectivity Over Posterior Regions
The other electrode pair that showed significant developmental changes in coherence was the occipital pair O1/A2 and O2/A1. Since this electrode pair is separated by less than 10 cm, volume conduction effects likely contribute to the coherence estimates (Nunez et al., 1997). The only instance of a decline in coherence across assessments occurred for this pair for the spindle band during SWS and stage 2 sleep in the children cohort. NREM sleep spindles have a known thalamocortical origin, and thus we attribute this developmental decline to a weakening of this circuit with the occipital lobes, during states when sleep spindles occur.
The teen cohort, in contrast, showed an increase in coherence measured between the occipital electrodes for the sigma band in all states. Because the developmental change was state nonspecific, we cannot discern whether this increase was due to an increase in cortico-cortical coupling between the two regions or subcortical coupling. On the other hand, its presence in all states increases the likelihood that this coherence increase signals anatomical development (e.g., increased mylenation). Our other teen developmental finding of increased occipital coherence in the low-delta and delta bands during SWS points to greater posterior slow wave synchrony as a function of development.
4.4 Intrahemispheric and Diagonal Coherence Increases Linearly From 9 – 23 Years
Combining our data to look across all age cohorts (children, teens, and adults) revealed a linear increase in left and right intrahemsipheric coherence similar to that shown in previous waking EEG studies (Barry et al., 2004) (Thatcher et al., 1987). This effect was state independent, occurring during slow wave, stage 2, and REM sleep and for most frequency bands. Thatcher and colleagues (Thatcher et al., 1987) waking EEG study of 557 participants ranging in age from two months to 26.4 years found that coherence increases with age; however, they did not report frequencies and examined only intrahemispheric coherence. A subsequent cross-sectional study of 8 to 12-year olds by Barry et al., (Barry et al., 2004) examined both inter- and intra- hemispheric coherence during eyes-closed resting EEG recordings. Similar to our data, they found a significant linear increase in intrahemispheric coherence between the frontal and occipital lobes in the delta (1.5 – 3.5 Hz), theta (3.5 – 7.5 Hz), and alpha (7.5 – 12.5 Hz) bands, but not in the beta (12.5 – 25 Hz) band. We extend their finding to a wider age range, and show a linear increase that extends to early adulthood (ages 20 – 23 years).
We hypothesize that the increased coherence in intrahemispheric coherence reflects increases in mylenation across adolescent development. Anatomical analyses in adults show that approximately 95% of cortical white matter consists of axons connecting cortico-cortical regions within a hemisphere (Nunez, 1981). Postmortem studies show that axonal diameter increases and myelin sheath grows from birth to adulthood in the parahippocampal region (Benes et al., 1994). Structural MRI studies of adolescent development suggest that mylenation continues into early adulthood in many cortical areas (for a review see Paus et al., 2001). One consistent finding from diffusion tensor imaging studies (Ashtari et al., 2007) is an increase in fractional anisotropy in the left arcuate fasciculus, which connects posterior receptive areas (such as the occipital lobes corresponding to EEG electrode locations O1/A2 and O2/A1) with premotor/motor areas (corresponding to EEG electrode locations C3/A2 and C4/A1).
In addition to the age-related linear increase in intrahemispheric coherence, we found a significant linear increase in coherence between the diagonal channels (C3/A2 and C4/A1; C4/A1 and O1/A2) during stage 2 and REM sleep. Absolute coherence values for coherence pairs, however, are much lower than the coherence values observed at other channel pairs, most likely due to the scarcity of axonal connections between diagonal hemispheres. Despite the low coherence values overall, a peak of coherence is visible in the sigma band.
4.5 Interhemispheric Coherence Does Not Show An Age-Related Linear Trend
We found no age-related linear trends in interhemispheric coherence for any state or frequency, which is congruent with the findings of Barry et al. (Barry et al., 2004), who examined waking interhemispheric coherence between central, parietal, and occipital areas in children. The lack of developmental changes in interhemispheric coherence may be accounted for by cortical architecture: only about 2 – 3 % of cortical white matter connects the hemispheres compared to 95% of white matter tracts that run within a hemisphere (Braitenberg, 1978) (Schultz and Braintenberg, 2002) (Nunez, 1981). Coherent activity between the hemispheres most likely depends on the corpus callosum. This finding also aligns with structural MRI studies that show significant age-related increases in white matter in a number of cortical regions but no difference in the genu of the corpus callosum (Kim et al., 2007).
4.6 General Observations About the Spectrum
A prominent spindle peak appears in the coherence spectrum of all electrode pairs during SWS and stage 2 sleep. Achermann and Borbély (Achermann and Borbely, 1998) have previously shown that spindles in the human sleep EEG manifest strong coherent activity throughout the cortex. This finding is corroborated by animal studies showing that sleep spindles are synchronous over large cortical areas, and the synchrony is driven by corticothalamic projections (Contreras et al., 1996). We observed an increase in the frequency of the spindle peak in the coherence spectrum with increasing age. We observe a similar increase in the power spectrum of the sleep EEG and we speculate that this increase may be due to the increases in mylenation, which would result in faster EEG frequencies (Nunez, 2000).
Another finding of the Achermann and Borbély (Achermann and Borbely, 1998) was a resemblance of interhemispheric coherence of the sleep EEG to the 1/f-like decline observed in a typical EEG power spectrum; intrahemispheric coherence in the sleep EEG, by contrast, did not show such a pattern. A local field potential (LFP) study of interhemispheric coherence during restful waking, stage 2 sleep and REM sleep between two mirror auditory sites found the same 1/f feature of the coherence spectrum (Nir et al., 2008). Achermann and Borbély (1998a) postulate that this pattern may be due to transcallosal connectivity, which may act as a low pass filter. Indeed, in acallosal children, low-frequency waking EEG coherence is reduced compared to controls (Koeda et al., 1995) as it is in acallosal mice during sleep (Vyazovskiy et al., 2004). Our data provide further support for this notion, given that we find the same pattern across a broad age range (9 to 23 years). Unlike Achermann and Borbély (Achermann and Borbely, 1998), we observe a dual peak in the low frequencies during REM sleep between C3/A2 and C4/A1 in all cohorts. This finding may be due to the use of referential derivations in this study and bipolar derivations in the study of Achermann and Borbély (Achermann and Borbely, 1998).
4.7 Coherence, Power, Skull Size and Electrode Separation
We highlight here a few key points about the relation among coherence, power, electrode separation, and changes in skull size. Although coherence and power are not independent of one another (Nunez and Srinivasan, 2006), Achermann and Borbély (1998b), have shown that sleep EEG power in the low delta band (1 - 2 Hz) decreases over the course of sleep, while coherence does not change. Thus over the ages examined in this report (9 - 23 years), sleep EEG power decreases (Tarokh and Carskadon, 2010, Tarokh et al., 2009), while coherence increases.
Coherence between electrode pairs depends in part on the distance between the electrodes, and coherence estimates of electrode pairs separated by less than 10-12 cm are inflated due to the effects of volume conduction (Nunez et al., 1997) (Srinivasan et al., 1998). In the current study, the distance between the occipital EEG leads is significantly smaller than between the other electrode pairs, therefore we observed higher coherence between these channels. Our focus is not on absolute coherence values, rather on developmental changes in coherence; we found no evidence that the distance between electrodes changes as a function of the developmental range we examined. For example, intracranial volume reaches adult values by age 10 years (Pfefferbaum et al., 1994). Furthermore, Karbowski has shown that connectivity is either only weakly dependent on or independent of brain size (Karbowski, 2003). Children's brain volume is about 90% and 98% of adult scales at ages 2 and 5 years, respectively (Blinkov and Glezer, 1968) (Nunez, 1995). Thus, size contributions to coherence changes are expected to be small across adolescence (Nunez, 2000).
4.8 Limitations
One of the limitations of this study is the small number of electrode pairs examined, which limits our ability to reach conclusions about the locality of the observed effects. Second, we were underpowered to examine whether changes in coherence differed for males and females. Third, coherence depends on the choice of the reference electrode, and signals that share a common reference have elevated coherence values, as was the case in our calculation of intrahemispheric coherence (Nunez, 1981). The aim of the present study, however, was to examine coherence as a function of development; by recording and analyzing signals in the same way across cohorts, we were able to compare coherence values across cohorts. Fourth, waking data are necessary to confirm state-independent changes that support strong conclusions about anatomical changes in the brain. Fifth, brain development may not be complete in our young adult group since evidence from MRI studies indicates that brain development continues well into the twenties ((e.g., Lebel et al., 2008). This limitation resulted from using control data from another study (unpublished data). Finally, as noted by Thatcher (Thatcher, 1992) “The neurophysiological mechanisms responsible for the changes in the numbers or strengths of connections may include axonal sprouting, synaptogenesis, mylenation, expansion of existing synaptic terminals, pruning of synaptic connections, presynaptic changes in the amount of neurotransmitter and changes in the postsynaptic response to a given neurotransmitter (see discussions by Purves, 1988; and Huttenlocher, 1984). Currently, measures of EEG coherence cannot discern between these various possibilities.”
Adolescence is a time of significant cognitive, motor, and sensory development, functions that depend in large part on the structural and functional maturation of neural pathways connecting brain regions. A concurrent decline in the number of synapses and increased connectivity might provide an increase in the signal-to-noise ratio, which would allow for more efficient processing of information. Some have hypothesized that a number of psychiatric disorders (e.g. schizophrenia) that arise during adolescence are the result of these processes going awry (Feinberg, 1982, Vidal et al., 2006). Therefore, an understanding of the normal developmental trajectory of brain connectivity in this developmental stage is instructive. We believe that the sleep EEG provides a valuable avenue to examine such changes.
Figure 3. Exemplary figure of coherence as a function of age.
Plot of intrahemispheric coherence for the left and right hemispheres for all participants at every age assessment during slow wave sleep (SWS) in the low delta band (0.8 to 1.6 Hz). On the left is the intrahemispheric coherence between the left central (C3/A2) and left occipital (O1/A2) electrodes and on the right is coherence between the right central (C4/A1) and right occipital (O2/A1) electrodes. A significant linear trend was detected for both these channels during SWS (slope = 0.01 Hz/Year, Intercept = 0.22, R2 = 0.20, and p-value = 0.0004).
Acknowledgments
The authors would like to thank Drs. Ronald Seifer, Christine Acebo, Tracy Rupp, Oskar Jenni, Monique LeBourgeois, Eliza Van Reen, Gahan Fallone, and Margaret Borkowski for technical assistance. The authors are grateful to Drs. Elizabeth Forbes and Judith Owens for performing the Tanner staging and William Coon and Henry Arantes for sleep stage scoring. We also thank our research staff, laboratory technicians, and participants. This work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA07459-21 to L.T. and AA13252 to M.A.C) and the Swiss National Science Foundation (320000-112674 and 320030-130766 to P.A.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achermann P, Borbely AA. Coherence analysis of the human sleep electroencephalogram. Neuroscience. 1998;85:1195–1208. doi: 10.1016/s0306-4522(97)00692-1. [DOI] [PubMed] [Google Scholar]
- Anokhin AP, Lutzenberger W, Birbaumer N. Spatiotemporal organization of brain dynamics and intelligence: an EEG study in adolescents. Int J Psychophysiol. 1999;33:259–273. doi: 10.1016/s0167-8760(99)00064-1. [DOI] [PubMed] [Google Scholar]
- Armitage P, Parmar M. Some approaches to the problem of multiplicity in clinical trials.. Proceedings of the XIIthe International Biometrics Conference..1986. [Google Scholar]
- Armitage R, Hoffmann R, Emslie G, Rintelmann J, Robert J. Sleep microarchitecture in childhood and adolescent depression: temporal coherence. Clin EEG Neurosci. 2006;37:1–9. doi: 10.1177/155005940603700103. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione KL, Hasan KM, Wu J, McIlree C, Kester H, Ardekani BA, Roofeh D, Szeszko PR, Kumra S. White matter development during late adolescence in healthy males: a cross-sectional diffusion tensor imaging study. NeuroImage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, McCarthy R, Selikowitz M, Johnstone SJ, Rushby JA. Age and gender effects in EEG coherence: I. Developmental trends in normal children. Clin Neurophysiol. 2004;115:2252–2258. doi: 10.1016/j.clinph.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of general psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Blinkov SM, Glezer II. The Human Brain in Figures and Tables: A Quantitative Handbook. Plenum Press; New York: 1968. [Google Scholar]
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. Journal of biological rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Cortical architectonics: general and areal. In: Brazier M, Petsche H, editors. Architectonics of the Cerebral Cortex. Raven Press; New York: 1978. pp. 443 – 465. [Google Scholar]
- Buckelmuller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–356. doi: 10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Science. Vol. 304. New York, NY: 2004. Neuronal oscillations in cortical networks. pp. 1926–1929. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I. The onset of the adolescent delta power decline occurs after age 11 years: a comment on Tarokh and Carskadon. SLEEP. 2010 doi: 10.1093/sleep/33.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Science. Vol. 274. New York, NY: 1996. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. pp. 771–774. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep medicine reviews. 2003;7:423–440. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Ferlazzo F, Bertini M. Slow eye movements and EEG power spectra during wake-sleep transition. Clin Neurophysiol. 2000;111:2107–2115. doi: 10.1016/s1388-2457(00)00476-4. [DOI] [PubMed] [Google Scholar]
- Dijk DJ. Circadian variation of EEG power spectra in NREM and REM sleep in humans: dissociation from body temperature. Journal of sleep research. 1999;8:189–195. doi: 10.1046/j.1365-2869.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey SD. Adjustment of p-values for multiplicities of intercorrelating symptoms. Proceedings of the VIth International Society for Clinical Biostatisticians. 1985 [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of psychiatric research. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Gasser T, Verleger R, Bacher P, Sroka L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalography and clinical neurophysiology. 1988;69:91–99. doi: 10.1016/0013-4694(88)90204-0. [DOI] [PubMed] [Google Scholar]
- Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: from childhood to middle age. Journal of sleep research. 2001;10:165–172. doi: 10.1046/j.1365-2869.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroenceph Clin Neurophysiol. 1958;10:370–371. [Google Scholar]
- Jenni OG, Carskadon MA. Spectral analysis of the sleep electroencephalogram during adolescence. Sleep. 2004;27:774–783. [PubMed] [Google Scholar]
- Karbowski J. How does connectivity between cortical areas depend on brain size? Implications for efficient computation. Journal of computational neuroscience. 2003;15:347–356. doi: 10.1023/a:1027467911225. [DOI] [PubMed] [Google Scholar]
- Kim EY, Kim DH, Yoo E, Park HJ, Golay X, Lee SK, Kim DJ, Kim J, Kim DI. Visualization of maturation of the corpus callosum during childhood and adolescence using T2 relaxometry. Int J Dev Neurosci. 2007;25:409–414. doi: 10.1016/j.ijdevneu.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Koeda T, Knyazeva M, Njiokiktjien C, Jonkman EJ, De Sonneville L, Vildavsky V. The EEG in acallosal children. Coherence values in the resting state: left hemisphere compensatory mechanism? Electroencephalography and clinical neurophysiology. 1995;95:397–407. doi: 10.1016/0013-4694(95)00171-9. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of neuroscience methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Arieli A, Fried I, Malach R. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nature neuroscience. 2008;11:1100–1108. doi: 10.1038/nn.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL. Electric Fields of the Brain: The Neurophysics of EEG. Oxford University Press; New York: 1981. [Google Scholar]
- Nunez PL. Neocortical Dynamics and Human EEG Rhythms. Oxford University Press; New York: 1995. [Google Scholar]
- Nunez PL. Toward a quantitative description of large-scale neocortical dynamic function and EEG. The Behavioral and brain sciences. 2000;23:371–398. doi: 10.1017/s0140525x00003253. discussion 399-437. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. Second Edition Oxford Univeristy Press; New York: 2006. [Google Scholar]
- Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, Cadusch PJ. EEG coherency. I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalography and clinical neurophysiology. 1997;103:499–515. doi: 10.1016/s0013-4694(97)00066-7. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain research bulletin. 2001;54:255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. The Behavioral and brain sciences. 1997;20:537–556. doi: 10.1017/s0140525x97001581. discussion 556-596. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. UCLA Brain Information Service/Brain Research Institute; Los Angeles: 1968. [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Development of effective connectivity for narrative comprehension in children. Neuroreport. 2007;18:1411–1415. doi: 10.1097/WNR.0b013e3282e9a4ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A, Braintenberg V. The human cortical white matter: quantitative aspects of cortico-cortical long-range connectivity. In: Schultz A, Miller R, editors. Cortical Areas: Unity and Diversity. Vol. 5. Taylor and Francis; London: 2002. pp. 377–386. [Google Scholar]
- Segalowitz SJ, Santesso DL, Jetha MK. Electrophysiological changes during adolescence: a review. Brain and cognition. 72:86–100. doi: 10.1016/j.bandc.2009.10.003. In Press. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Distributed processing and temporal codes in neuronal networks. Cognitive neurodynamics. 2009;3:189–196. doi: 10.1007/s11571-009-9087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Srinivasan R, Nunez PL, Silberstein RB. Spatial filtering and neocortical dynamics: estimates of EEG coherence. IEEE transactions on bio-medical engineering. 1998;45:814–826. doi: 10.1109/10.686789. [DOI] [PubMed] [Google Scholar]
- Steen RG, Ogg RJ, Reddick WE, Kingsley PB. Age-related changes in the pediatric brain: quantitative MR evidence of maturational changes during adolescence. Ajnr. 1997;18:819–828. [PMC free article] [PubMed] [Google Scholar]
- Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain and cognition. 2009;70:1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Tanner J. Growth at Adolescence. Blackwell; Oxford: 1962. [Google Scholar]
- Tarokh L, Carskadon MA. Sleep electroencephalogram in children with a parental history of alcohol abuse/dependence. Journal of sleep research. 2009 doi: 10.1111/j.1365-2869.2009.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Carskadon MA. Developmental Changes in the Human Sleep EEG During Early Adolescence. SLEEP. 2010 doi: 10.1093/sleep/33.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarokh L, Coon WG, Carskadon MA. Developmental Changes of the Sleep EEG in Post-Pubertal Adolescents. SLEEP. 2009;32:A5 – A6. [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization during early childhood. Brain and cognition. 1992;20:24–50. doi: 10.1016/0278-2626(92)90060-y. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, Walker RA, Giudice S. Science. Vol. 236. New York, NY: 1987. Human cerebral hemispheres develop at different rates and ages. pp. 1110–1113. [DOI] [PubMed] [Google Scholar]
- Vidal CN, Rapoport JL, Hayashi KM, Geaga JA, Sui Y, McLemore LE, Alaghband Y, Giedd JN, Gochman P, Blumenthal J, Gogtay N, Nicolson R, Toga AW, Thompson PM. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Archives of general psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38:301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Achermann P, Borbely AA, Tobler I. Interhemispheric coherence of the sleep electroencephalogram in mice with congenital callosal dysgenesis. Neuroscience. 2004;124:481–488. doi: 10.1016/j.neuroscience.2003.12.018. [DOI] [PubMed] [Google Scholar]