Abstract
Adenoid cystic carcinoma (ACC) is a rare type of cancer that typically originates in the salivary glands. Surgical removal can lead to functional loss and psychological distress. Viscum album extract (VAE) is a herbal remedy with dose-dependent cytotoxic, apoptogenic and immunological effects. In some case reports, tumour regression has been observed following high-dose local applications of VAE. An active 88-year-old man with fast-growing ACC of the hard palate refused surgical removal and received high-dose intratumoural injections of VAE (alone) over a 10-month period. The tumour decreased in size, softened and loosened from its surroundings. A biopsy during the course showed inflammation. The patient remained well and without functional limitations during the therapy and follow-up period (5 months). VAE produced no reported side effects. This aged patient exemplifies a satisfying course of ACC under VAE resulting in good quality of life and partial tumour regression.
Background
Adenoid cystic carcinoma (ACC) is a rare cancer (estimated incidence 0.04–0.12/100 000/year1) mostly seen in patients in the fifth life decade, primarily affecting the salivary glands.1 2 Patients with ACC show a 5-year, 10-year and 15-year survival rate of 89%, 65% and 40%, respectively.3 ACC is usually slow-growing, per continuitatem and perineural. While it rarely metastasises to the regional lymph nodes, it is characterised by frequent late recurrences or distant metastases.2 Occasionally rapid progression is observed.4
Wide margin surgery is recommended wherever possible,2 followed by radiotherapy in cases of higher T status or narrow margins.1 Depending on the localisation of the tumour, maxillectomy may be necessary. This surgery often leads to eating problems, xerostomia, speech problems and psychological distress (change of self-image, interpersonal relations, social stigmatisation, social isolation, depression) which limit the patient's quality of life (QoL).5
Therapies using chemotherapeutic and biological agents are being investigated but have not proven effective to date.6
Owing to the rarity of ACC, most of the reported evidence is based on case reports, case series and small studies or data from databases of cases collected over a long period.6
Spontaneous remission of ACC is rare, with only four cases reported in the medical literature (personal comment, Dr JY Suen, University of Arkansas for Medical Sciences, 2011).7 8
Viscum album extracts (VAEs) are commonly used as supportive therapy in patients with cancer, especially in German-speaking European countries.9 They are usually applied subcutaneously as low-dose injections, but oral, intravenous and intratumoural applications, along with instillations in visceral cavities in even higher doses have been reported.10 Preclinical tests have presented the immune stimulatory, cytotoxic and apoptogenic effects of VAEs, especially of its lectins.11 12 Clinical trials have exhibited QoL improvement and potential effects on survival,13 14 while case reports and series have reported regression and remission of different tumour types after high-dose local application of VAEs.10 11 15–18 Frequently reported side effects include local skin reactions and flu-like symptoms and some allergic reactions have been reported, but otherwise VAE therapy remains safe, even at higher doses.19 To the best of our knowledge, no clinical data have been published on the use of VAEs in treating ACC; therefore, we present the following case, reported in accordance with the CARE (CAse REporting) guidelines.20
Case presentation
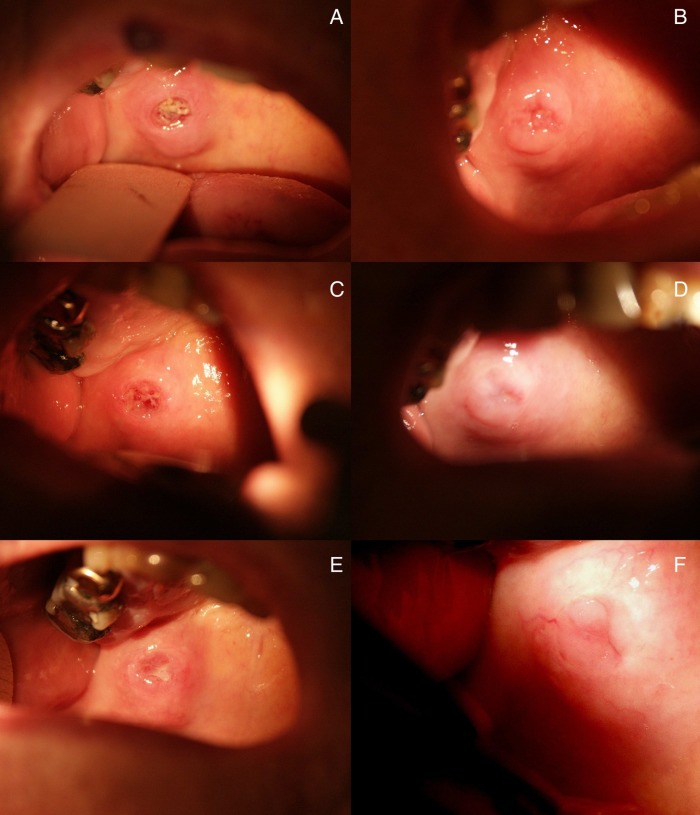
An active 88-year-old patient presented with a painless tumour approximately 2 cm in diameter. The tumour had a firm consistency and was attached to the underlying structures based on the clinical examination. It was localised on the right side of the hard palate (figure 1A, for further course of the lesion during VAE therapy see figure 1B–F). The surface of the tumour showed ulcerations. The mass had not been noticed 4 weeks prior at a dentist appointment. This was therefore interpreted as rapid tumour growth. The clinical examination could not distinguish between an inflammatory lesion, such as Wegener’s granulomatosis or a neoplasia.
Figure 1.
Patient's tumour (A) before therapy, (B) on day 12 of Viscum album extract therapy, (C) at the beginning of the 2nd month, (D) in the 3rd month, (E) in the 5th month and (F) in the 10th month.
Investigations
CT of the head showed a single mass of 2×1.5 cm in the density of soft tissue, located at the right palate without bone destruction or node involvement. A punch biopsy of the lesion confirmed the ACC diagnosis. According to these findings, the tumour was classified as T1, N0, MX (stage I). Further staging investigations and wide margin surgery were proposed to the patient. The patient was informed that after surgery the substantial defect between the nasal and oral cavities would be closed by wearing an obturator prosthesis. In view of these consequences and his advanced age, the patient refused surgery as well as further staging investigations and asked his general practitioner (specialising in phytotherapy and with 4.5 years of surgical training) for an alternative cancer treatment.
Treatment
The patient received intratumoural high-dose injections of ABNOBAviscum Quercus and ABNOBAviscum Fraxini, both of which are endotoxin-free plant extracts from defined parts of European VAE. ABNOBAviscum Quercus is derived from the host oak tree and ABNOBAviscum Fraxini from the host ash tree. A 20 mg dose contains about 8000 and 14 000 ng/mL mistletoe lectins, respectively.
The injections were started 18 days after the initial diagnosis of the tumour with a low first dose to prevent a possible local inflammatory response in this sensitive area. The dose was then increased and later changed to a lectin-rich VAE preparation (for amount of injections per month table 1, for course of injections figure 2). The injections were inserted in three different places across the tumour without prior anaesthesia. Irregularities in the intervals of injections were due to organisational difficulties in visiting the general practitioner. The treatment was conducted over 10 months.
Table 1.
Viscum album extract (VAE) injections per month
| Month | VAE Quercus 2 mg |
VAE Quercus 20 mg |
VAE Fraxini 20 mg |
|---|---|---|---|
| 1 | 1 | 3 | |
| 2 | 4 | ||
| 3 | 4 | ||
| 4 | 4 | ||
| 5 | 7 | ||
| 6 | 4 | ||
| 7 | 8 | ||
| 8 | 1 | 2 | |
| 9 | 2 | ||
| 10 | 4 |
Figure 2.
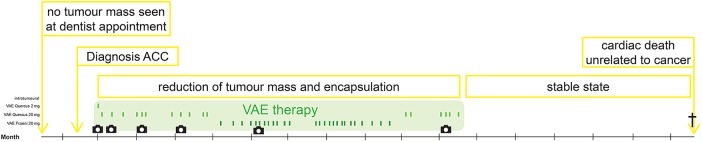
Timeline: VAE treatment and course of disease (ACC, Adenoid cystic carcinoma; VAE, Viscum album extract).
Outcome and follow-up
After 2 weeks of treatment (three applications), the tumour had become softer, decreased in size and the ulceration had healed (figure 1B). In the sixth month of treatment, a fine-needle aspiration biopsy confirmed the ACC diagnosis with a cribriform pattern and found intense inflammatory infiltration of lymphocytes, plasma cells and eosinophils (figure 3).
Figure 3.
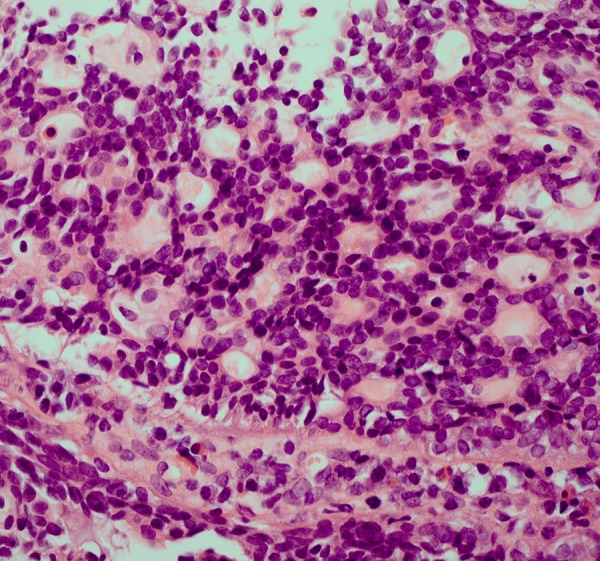
Punch biopsy of the patient's tumour after 6 months of Viscum album extract therapy. Adenoid cystic carcinoma with a cribriform pattern and inflammatory infiltration (lymphocytes, plasma cells, eosinophils), H&E stain.
After 10 months of treatment, the tumour was more mobile underneath the mucosa on clinical palpation and had decreased in size to approximately 1 cm in diameter which was interpreted as tumour regression, encapsulation and a reduction of invasive growth. Excision or further biopsy of the tumour was not carried out.
The therapy was well tolerated and side effects such as fever, local inflammation, swelling and pain did not occur during treatment. During therapy the patient was in a good condition. He experienced fatigue but was able to manage the household and care for his wife who, due to illness, was limited in her daily activities.
During the 5-month follow-up period following the termination of VAE therapy, no tumour growth was observed and the patient's QoL remained good. After 5 months of follow-up, the patient died of a sudden cardiac arrest unrelated to cancer or its therapy (for a timeline of the course figure 2). No autopsy was conducted.
Antecedent and further therapies
At the age of 80, the patient had had a tumour of the prostate excised. He was subsequently treated with an androgen ablative therapy and subcutaneous VAE injections (ISCADOR Quercus cum Argento). There were no relapses or metastases of this tumour during the follow-up period.
Further diagnoses were hypertension, hypercholesterolaemia, intermittent absolute arrhythmia, atrial fibrillation, coronary heart disease and heart failure. Medications included acetylsalicylic acid, torasemide, molsidomin, isosorbide mononitrate, digitalis, verapamil, lisinopril and the complementary and alternative medicine medications Cardiodoron, Galacordin and Oleum Strophanthi.
VAE was the only tumour-specific therapy used.
Discussion
To the best of our knowledge, this is the first case report describing the use of VAE to treat ACC. In vitro studies have shown mistletoe alkali of Viscum coloratum (an Asian species of the genus Viscum) to have dose-dependent inhibitory effects on the growth of ACC cells.21
Although cytotoxic effects of VAE have been observed in head and neck squamous cell carcinoma (SCC) cell lines,22 low-dose adjunct VAE treatment in patients with head and neck cancer shows no effect on QoL or overall survival.23
Tumour response under VAE therapy of head and neck cancer has been reported in five cases (for details table 2). In all of the cases the tumour entity was a SCC. Treatment lead to complete remission in three and a tumour response in two cases. In four of the five cases, VAE caused inflammatory reactions indicating that immunological stimulation plays a role in VAE's mechanism of action. In addition to the immunological effects, the regression may have also resulted from the strong cytotoxic and apoptotic effects of VAE and its constituents. The cytotoxic and apoptotic effects of VAE are presumably restricted to local application given that the cytotoxicity of the lectins is inhibited by serum proteins and antibodies when applied systemically.11 22 24
Table 2.
Case reports of VAE treatment in head and neck cancer
| Cases | Geir25 | Scheffler et al15 | Orange et al26 | Metelmann et al27 | Werthmann et al18 |
|---|---|---|---|---|---|
| Patient characteristics | 30-year-old Caucasian male | 68-year-old Caucasian male | 54-year-old Caucasian male | 66-year-old Caucasian male | 78-year-old Caucasian male |
| Tumour type | SCC of the larynx | SCC of the oral cavity | SCC of the tongue | SCC of the tongue | Cutaneous SCC |
| Localisation | Left side of the larynx, during the course relapse at the site of excision and lymph node metastases | Right side of the hard palate | Two neck and one submandibular lymph node with unknown primary tumour, during course of treatment tumour of the tongue | Exophytic tumour of the tongue, node involvement at both sides of the neck, during the course of treatment metastasis of the right kidney | Inner edge of the right eye |
| TNM status | Not specified | T1,Nx,M0 | Tx,N2,M1; T4,N2,cM0 | cT4a-b,cN2b,cM0 | cT1,cN0,cM0 |
| Cancer treatment other than VAE | Partial laryngectomy, neck dissection | None | Excision of one node, in the further course after termination of VAE therapy: chemoradiation treatment | None | None |
| VAE treatment | Isorel, treatment regimen not further specified | ABNOBAviscum Quercus 20 mg, intratumoural application, week 1–3: 2 applications/week, week 4–12: 1 application/week | ABNOBAviscum Fraxini 2–350 mg, intratumoural application, intravenous and subcutaneous application, daily application on days 1–3, 5, 8, 11 then weekly until day 60, then twice weekly, application time and dose were thoroughly orientated at immune reactions of the patient | ABNOBAviscum Abietis 0.2 mg, peritumoural injections every 2 weeks | ABNOBAviscum Fraxini 0.02–2 mg, peritumoural application, week 1–24: 1–2 applications/week with 1 intermission of 1 week, ABNOBAviscum Betulae 2–20 mg, peritumoural application, week 25–44: 1–2 applications/week with 3 intermissions of 1 week |
| VAE treatment period | approximately 1 year | 12 weeks | 1 year | 3 years | 1 year |
| Effect on the tumour | Complete remission of relapsed tumour | Complete remission | Clinical regression of lymph node metastases of about 40%, during further course tumour relapse with primary tumour of the tongue and growing node involvement, clinical response under chemoradiation treatment | Partial remission of the primary tumour, no progression of lymph node involvement, during the course growing metastasis of the right kidney | Complete remission |
| Inflammatory response to VAE treatment | Not specified |
|
|
▸ Follow-up of the primary tumour revealed a local inflammatory reaction |
|
SCC, squamous cell carcinoma; VAE, Viscum album extract.
The present case exhibits a clinical response to VAE therapy in a patient with ACC. During the treatment, which was well tolerated, the tumour decreased in size, became softer and appeared to loosen from its surrounding structures. Histological examination showed an intense infiltration of immune cells in the VAE-treated tumour tissue. After termination of the therapy, no further growth occurred during the follow-up period of 5 months (the follow-up period was restricted to 5 months because the patient suffered a sudden cardiac death).
The course of disease was of positive significance for the discussed patient, given his advanced age and the functional loss that would have resulted from surgical excision. The therapy enabled a consistent, high QoL and full functional capacity. Considering that no other tumour-specific therapy—pharmacological or surgical—was used, and the rarity of spontaneous remission of ACC, it is presumed that the tumour regression was induced by the VAE therapy.
We believe this case report is of great significance given it demonstrates an alternative treatment of ACC that does not disfigure or reduce patients’ QoL. Furthermore, investigations on chemotherapeutic agents and targeted therapies for progressive disease have not shown satisfying results to date.28
Given the singularity of the reported evidence and the lack of further study investigations, no conclusions can be drawn from this case regarding the long-term effects of high-dose local treatment. It is important to recognise that other treatment regimens showed effectiveness in ACC but could not improve long-term outcomes.29 30
Regarding the current state of evidence, VAE injections cannot replace surgery or other effective conventional anticancer treatments. Given the difficulties in conducting clinical trials in rare diseases, high-quality case reports and series should systematically and transparently report the course of disease under the application of VAE, including long-term follow-up, histological changes at the tumour site and, if possible, the patient's considerations and motives related to their choice of therapy. These case reports could eventually aid in the decision-making process regarding carrying out clinical trials.
Learning points.
Although radical surgical excision is recommended for local disease control of adenoid cystic carcinoma (ACC), effective conservative treatment and systemic therapy for relapsing or metastatic disease is lacking.
Viscum album extract (VAE) may positively affect ACC growth, however, further investigations are needed. Currently, VAE cannot replace recommended surgical treatment, because of lacking evidence.
Tumour response under VAE treatment—as in the presented case—may be explained through immunological and cytotoxic effects.
In rare malignant disease that lack treatment options like ACC, high-quality case reports and series should systematically and transparently report treatment regimens, histological changes at the tumour site, long-term follow-up and, if possible, the patient's considerations and motives related to therapy choice.
Acknowledgments
Thanks to Dr Cramer from the Pathological Institute Koblenz for the histological images and the Christopherus-Fond, Stuttgart for financial support.
Footnotes
Contributors: PGW, PH and GSK contributed to the case report design. DH was the physician in charge who provided the patient's information and arranged the photo documentation. PGW and DH collected and provided the data. PGW was the principle author of the paper, had full access to all data and is the guarantor. GSK supervised the report and the publication process.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tian Z, Li L, Wang L, et al. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg 2010;39:235–42 [DOI] [PubMed] [Google Scholar]
- 2.Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg 2004;12:127–32 [DOI] [PubMed] [Google Scholar]
- 3.Garden AS, Weber RS, Ang KK, et al. Postoperative radiation therapy for malignant tumors of minor salivary glands. Outcome and patterns of failure. Cancer 1994;73:2563–9 [DOI] [PubMed] [Google Scholar]
- 4.Oates JA, Kinsella DC, Gutowski NJ. Neurological picture. Fast-growing adenoid cystic carcinoma: a neurotropic malignancy. J Neurol Neurosurg Psychiatry 2006;77:1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irish J, Sandhu N, Simpson C, et al. Quality of life in patients with maxillectomy prostheses. Head Neck 2009;31:813–21 [DOI] [PubMed] [Google Scholar]
- 6.Kaufman J. A guide to systemic therapy for patients with progressive adenoid cystic carcinoma. Adenoid Cystic Carcinoma Research Foundation, 2010 [Google Scholar]
- 7.O'Regan B, Hirshberg C. Spontaneous remission—an annonted bibliography. Institute of Noetic Sciences, 1993 [Google Scholar]
- 8.Grillet B, Demedts M, Roelens J, et al. Spontaneous regression of lung metastases of adenoid cystic carcinoma. Chest 1984;85:289–91 [DOI] [PubMed] [Google Scholar]
- 9.Molassiotis A, Fernadez-Ortega P, Pud D, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol 2005;16:655–63 [DOI] [PubMed] [Google Scholar]
- 10.Kienle GS, Kiene H. Complementary cancer therapy: a systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur J Med Res 2007;12:103–19 [PubMed] [Google Scholar]
- 11.Kienle GS, Kiene H. Die Mistel in der Onkologie—Fakten und konzeptionelle Grundlagen. Stuttgart, New York: Schattauer Verlag, 2003 [Google Scholar]
- 12.Büssing A, ed. Mistletoe. The genus Viscum. Amsterdam: Hardwood Academic Publishers, 2000 [Google Scholar]
- 13.Kienle GS, Kiene H. Influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther 2010;9:142–57 [DOI] [PubMed] [Google Scholar]
- 14.Troger W, Galun D, Reif M, et al. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: a randomised clinical trial on overall survival. Eur J Cancer 2013;49:3788–97 [DOI] [PubMed] [Google Scholar]
- 15.Scheffler A, Mast H, Fischer S, et al. Komplette Remission eines Mundhöhlenkarzinoms nach alleiniger Mistelbehandlung. In: Scheer R, Becker H, Berg PA, eds. Grundlagen der Misteltherapie. Aktueller Stand der Forschung und klinische Anwendung. Stuttgart: Hippokrates Verlag GmbH, 1996:453–66 [Google Scholar]
- 16.Orange M, Fonseca M, Lace A, et al. Durable tumour responses following primary high-dose induction with mistletoe extracts: two case reports. Eur J Integr Med 2010;1:227 [Google Scholar]
- 17.Orange M, Lace A, Fonseca M, et al. Durable regression of primary cutaneous B-cell lymphoma following fever-inducing mistletoe treatment—two case reports. Glob Adv Health Med 2012;1:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werthmann PG, Strater G, Friesland H, et al. Durable response of cutaneous squamous cell carcinoma following high-dose peri-lesional injections of Viscum album extracts—a case report. Phytomedicine 2013;20:324–7 [DOI] [PubMed] [Google Scholar]
- 19.Kienle GS, Grugel R, Kiene H. Safety of higher dosages of Viscum album L. in animals and humans—systematic review of immune changes and safety parameters. BMC Complement Altern Med 2011;11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagnier JJ, Riley D, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Dtsch Arztebl Int 2013;110:603–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ya-Juan L, Hong-Lan Z. Inhibitory effect of mistletoe alkali on adenoid cystic carcinoma cells. J Jilin Univ 2008;34:601–4 [Google Scholar]
- 22.Klingbeil MF, Xavier FC, Sardinha LR, et al. Cytotoxic effects of mistletoe (Viscum album L.) in head and neck squamous cell carcinoma cell lines. Oncol Rep 2013;30:2316–22 [DOI] [PubMed] [Google Scholar]
- 23.Steuer-Vogt MK, Bonkowsky V, Scholz M, et al. [Influence of ML-1 standardized mistletoe extract on the quality of life in head and neck cancer patients]. HNO 2006;54:277–86 [DOI] [PubMed] [Google Scholar]
- 24.Kienle GS, Glockmann A, Schink M, et al. Viscum album L. extracts in breast and gynaecological cancers: a systematic review of clinical and preclinical research. J Exp Clin Cancer Res 2009;28:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geir W. [The mistletoe preparation ISOREL in the treatment of laryngeal cancer—case report of a complete remission] Das Mistelpräparat ISOREL bei Larynxkarzinom—Fallbericht von einer Totalremission. Krebsgeschehen 1982;1982:1–12 [Google Scholar]
- 26.Orange M, Lace A, von Laue B. The importance of the primary dosage in mistletoe therapy. In: Scheer R, Alban S, Becker H, Holzgrabe U, Kemper FH, Kreis W, et al., eds. Die Mistel in der Tumortherapie 2. Essen: KVC Verlag, 2009:385–400 [Google Scholar]
- 27.Metelmann HR, Hyckel P, Podmelle F. Oral cancer treatment and immune targets—a role for dendritic cells? J Craniomaxillofac Surg 2012;40:103–4 [DOI] [PubMed] [Google Scholar]
- 28.Papaspyrou G, Hoch S, Rinaldo A, et al. Chemotherapy and targeted therapy in adenoid cystic carcinoma of the head and neck: a review. Head Neck 2011;33:905–11 [DOI] [PubMed] [Google Scholar]
- 29.Vikram B, Strong EW, Shah JP, et al. Radiation therapy in adenoid-cystic carcinoma. Int J Radiat Oncol Biol Phys 1984;10:221–3 [DOI] [PubMed] [Google Scholar]
- 30.Jensen AD, Krauss J, Weichert W, et al. RadioImmunotherapy for adenoid cystic carcinoma: a single-institution series of combined treatment with cetuximab. Radiat Oncol 2010;5:102. [DOI] [PMC free article] [PubMed] [Google Scholar]